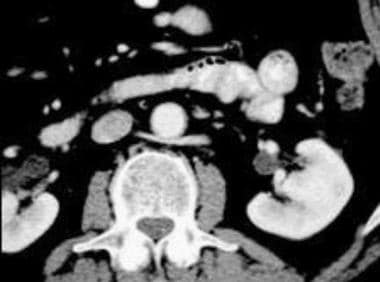
Practice Essentials
The preferred method of imaging renal cell carcinomas is dedicated renal computed tomography (CT). In most cases, this single examination can detect and stage RCC and provide information for surgical planning. [1, 2, 3] Worldwide, renal cell carcinoma (RCC) represents the sixth most frequently diagnosed cancer in men and the 10th in women, accounting for 5% and 3% of all oncologic diagnoses, respectively. [4] In the United States, there are approximately 65,000 new cases and almost 15,000 deaths from RCC each year. It most often occurs in patients aged 55 years or older (average age, 64 yr). [5] One fourth to one third of patients present with metastatic disease. In only approximately 2% of cases are bilateral tumors seen at presentation. Because of improvements in diagnostic tests such as CT and MRI, the incidence of RCC has increased, with the most common type of RCC being clear cell carcinoma. [6]
(See the images below.)
Risk factors include increased age; male sex; smoking; cadmium, benzene, trichloroethylene, and asbestos exposure; excessive weight; chronic dialysis use; and several genetic syndromes (familial RCC, hereditary papillary RCC, von Hippel–Lindau syndrome, and tuberous sclerosis). [7, 8]
RCCs can be staged by using the American Joint Committee on Cancer TNM (Tumor, Node, Metastases) classification, as follows:
-
Stage 1 RCCs are 7 cm or smaller and confined to the kidney.
-
Stage 2 RCCs are larger than 7 cm but still organ confined.
-
Stage 3 tumors extend into the renal vein or vena cava, involve the ipsilateral adrenal gland and/or perinephric fat, or have spread to local lymph nodes.
-
Stage 4 tumors extend beyond the Gerota fascia, have spread to local or distant nodes, or have distant metastases.
Guidelines
The American College of Radiology (ACR) Appropriateness Criteria for RCC staging recommends contrast-enhanced multiphasic CT scanning of the abdomen as the diagnostic modality of choice for staging a primary renal tumor. MRI of the abdomen is a suitable substitute when contrast-enhanced CT is contraindicated. If the status of the renal veins and IVC cannot be determined on CT, contrast-enhanced multiphasic 3-D MR venography can be performed. CT of the chest should be used to detect pulmonary metastasis in patients with large or locally advanced tumors. Chest radiography may be sufficient in patients with small primary tumors. In patients with suspicion of metastatic disease based on symptoms, other sites of metastases, or abnormal laboratory findings, brain MRI and bone scans can be performed. [9]
The National Comprehensive Cancer Network (NCCN) guidelines for kidney cancer recommend the following as part of the initial workup [10] :
-
Abdominal/pelvic CT or abdominal MRI with or without contrast, depending on renal insufficiency
-
Chest imaging
-
Brain MRI, if clinically indicated
The NCCN guideline recommends abdominal MRI to assess suspected tumor involvement in the inferior vena cava, or as an alternative to CT for renal mass detection and staging in cases where the use of contrast is contraindicated because of allergy or renal insufficiency. [10]
The American Urological Association (AUA) guideline for the management of the clinical T1 renal mass recommends a high-quality cross-sectional CT or MRI, first without and then with intravenous contrast if kidney function is adequate. The objectives are as follows [11] :
-
Rule out angiomyolipoma radiographically if possible
-
Evaluate for locally invasive features
-
Study the involved anatomy
-
Determine the status of the uninvolved kidney and its vasculature
Imaging modalities
Although a variety of examinations (ultrasound [US], magnetic resonance imaging [MRI], angiography) can be used in the workup of patients with suspected RCC, the preferred method of imaging is dedicated renal computed tomography (CT). In most cases, this single examination can be used to detect and stage RCC and to provide information for surgical planning without additional imaging. [1, 2, 3]
Noninvasive cross-sectional imaging (CT, MRI, US) has replaced angiography in the workup of patients with known or suspected RCC. Advances in CT angiography and magnetic resonance angiography have diminished the need for preoperative conventional angiographic mapping of the renal vasculature prior to nephron-sparing or minimally invasive surgery. Angiography is still occasionally used if the origin of a tumor (eg, renal vs adrenal) is not certain. In these patients, selective injection of the renal and adrenal arteries, as well as additional vessels, may be necessary.
High resolution, reproducibility, reasonable preparation and acquisition time, and acceptable cost allow CT to remain as the primary choice for radiologic imaging. MRI is an important alternative in patients requiring further imaging and in cases of allergies, pregnancy, or surveillance. Because of concern over radiation exposure, there has been a trend toward more use of MRI. [12, 13]
Kutluhan et al reported that renal capsular invasion and perirenal fat invasion are reliable signs of locally advanced RCC in both CT and MRI [14]
Preliminary studies have reported a promising role for positron emission tomography (PET)/CT with radiolabeled molecules targeting prostate-specific membrane antigen (PSMA) in patients with RCC. [15]
A systematic review, by Vogel et al, of 40 studies with a combined 4354 patients compared CT, MRI, positron emission tomography (PET)/CT, and ultrasonography for diagnosing and staging RCC in adults. For CT, median sensitivity and specificity were 88% and 75%, respectively, and for MRI, 87.5% and 89%. Staging sensitivity and specificity for CT were 87% and 74.5%, while MRI showed a median sensitivity of 90% and specificity of 75%. Contrast-enhanced US had a median diagnostic sensitivity of 93% and mediocre specificity, and the diagnostic performance of unenhanced US was poor. [16]
Oliva et al compared the characteristics on MRI of papillary renal cell tumors and clear cell tumors and noted that they had a similar appearance and signal intensity ratio on T1-weighted images, but on T2-weighted images, most papillary tumors were hypointense and most clear cell tumors were hyperintense. A tumor T2 signal intensity ratio of 0.66 or less had a specificity of 100% and a sensitivity of 54% for papillary tumors. [17]
A meta-analysis, by Chiarello et al, of 13 studies involving 275 papillary RCC lesions and 758 other renal masses that used MRI to differentiate papillary RCC from other renal lesions found moderate sensitivity and excellent specificity of quantitative enhancement in the corticomedullary phase (sensitivity of 79.6% and specificity of 88.1%). [13]
Taouli et al compared diffusion-weighted MRI with contrast-enhanced MRI to diagnose renal lesions. They found that although diffusion-weighted images can be used to characterize renal lesions (eg, differentiate solid tumors from oncocytomas and characterize histologic subtype), such images are less accurate than contrast-enhanced images. The area under the curve (AUC), sensitivity, and specificity of diffusion-weighted imaging were 0.856, 86%, and 80%, respectively, whereas the AUC, sensitivity, and specificity of contrast-enhanced MR imaging were 0.944, 100%, and 89%, respectively. [18] MRI findings correlate well with aggressive histology in the progression of RCC. [19]
In the few patients in whom CT findings are equivocal, MRI or US can be useful. Some studies have suggested a use for contrast-enhanced Doppler US for lesions that show equivocal enhancement at CT. Angiography is rarely used in the workup of suggested RCC, but it can provide information about the origin of the tumor in troublesome cases. Patients with a hereditary predisposition for RCC should be periodically examined by using dedicated renal CT.
(See the renal cell carcinoma images below.)
According to Guzzo et al, multidetector CT with 3-D mapping is effective in accurately characterizing the level of venous thrombus in patients with renal cell carcinoma. When excluding patients with segmental venous involvement only, the concordance rate between multidetector CT and pathologic findings was 84%, and multidetector CT predicted the level of tumor thrombus in 26 of 27 patients (96%). The investigators noted that in patients with renal cell carcinoma in whom multidetector CT fails to detect tumor thrombus, it is unlikely that a tumor thrombus will be found at surgery that would change the surgical approach. [1]
After studying 298 cases of RCC and oncocytoma using preoperative multiphasic multidetector CT, Young et al concluded that this approach can assist in discriminating clear cell RCC from other forms of RCC. [20]
Radiogenomics is a field of translational radiology that aims to associate a disease's radiologic phenotype with its underlying genotype, thus offering a novel class of noninvasive biomarkers with diagnostic, prognostic, and therapeutic potential. [21, 22]
Dual-energy CT (DECT) can be used to measure iodine and calcium concentrations and increase the iodine signal to help differentiate pathologic processes and clarify the internal structure of mass lesions. DECT can also potentially reduce the radiation dose by applying virtual noncontrast images, eliminating the need for precontrast CT. [23]
Limitations of techniques
The primary limitation of CT is the characterization of hypoattenuation in masses smaller than 8-10 mm, in which pseudoenhancement may be a problem. In these cases, US may be of some use in characterizing the lesions as cysts. In addition, spread to regional lymph nodes in the absence of lymph node enlargement can be missed. If contrast material cannot be intravenously administered, CT is a poor choice for evaluating renal masses. MRI should be performed instead.
The primary limitations of US include problems related to incomplete staging (bones, lungs, regional nodes) and to the detection of small non–contour-deforming masses. In addition, large patients are not good candidates for US because of technical difficulties in obtaining adequate images.
MRI is limited by patient cooperation because MRI is more sensitive to motion artifact than CT. However, advances in techniques for limiting motion, as well as techniques that allow free breathing, may obviate these limitations. However, MRI is still more expensive and less readily available than CT. Furthermore, patients with pacemakers, certain types of medical implants, or severe claustrophobia are excluded from undergoing MRI.
Pregnancy
When RCC is suggested in a pregnant patient, US should be considered first for imaging, especially in the first trimester. CT also can be useful, and the radiation exposure to the fetus is justifiable, especially if the clinical picture is confusing; any fetal damage is unlikely at the radiation doses typically used. The dose should be kept to a minimum by increasing the pitch and decreasing the microamperes and avoiding scanning the pelvis if possible. MRI is good for detecting, characterizing, and staging renal masses and avoids the exposure to ionizing radiation; however, intravenous gadolinium contrast cannot be used.
The use of the most appropriate and accurate diagnostic method (contrast-enhanced CT or MRI) and the most appropriate treatment of the mother is most likely to result in long-term benefit to the fetus. Nephrectomy can be performed with the least morbidity to the mother and fetus in the second trimester and is probably preferable to leaving a malignancy untreated throughout pregnancy.
Contrast agent allergy
If contrast enhancement is needed, actions can be taken to decrease the risk of an adverse reaction in patients with an allergy to iodinated contrast material. The patient can be premedicated with steroids and histamine blockers. Use of low-osmolar contrast may also help.
In patients who have had previous life-threatening reactions, the use of iodinated contrast material should be avoided.
Renal insufficiency
In patients with renal insufficiency, avoiding or limiting intravenous iodinated contrast material and ensuring adequate hydration is best if the creatinine levels are above 2 and if the patient is not receiving long-term dialysis. Poor renal function also results in failure to opacify the kidneys and collecting system, limiting evaluation of the kidneys. In patients with renal insufficiency, MRI is an excellent alternative to CT.
Another consideration in patients with abnormal renal function not yet requiring dialysis is that nephrectomy will likely result in dialysis dependence. In these patients, accurate diagnosis and staging is imperative and is probably best accomplished by using contrast-enhanced MRI.
In patients already undergoing dialysis, iodinated contrast material does not need to be avoided, and in fact, CT is preferable to MRI because of the small but documented risk of nephrogenic systemic fibrosis (NSF).
Radiography
Plain radiographic findings often are unrevealing in patients with renal cell carcinoma, unless the mass contains detectable calcification or is large enough to distort the normal renal contour. Plain radiography has no role in the primary search for RCC or in the follow-up observation of patients with RCC because of its limited sensitivity and specificity.
Intravenous urography (IVU) is also limited in depicting RCCs. Large lesions, which can distort the renal contour or the collecting system, may be detected with IVU. If RCC is suggested, further imaging with CT or MRI is necessary to confirm a solid mass and to stage the disease. If the lesion appears to be a cyst, US is the next best test in the patient's workup.
Findings of RCC are nonspecific and include mass effect on the collecting system, distortion of the renal contour, enlargement of a portion of the kidney, and calcifications. If good nephrotomograms are obtained at peak renal enhancement, most RCCs are less attenuating than surrounding renal parenchyma. Renal vein invasion may be inferred if contrast material excretion by the affected kidney is poor or absent. Alternatively, this finding may result from extensive involvement of the kidney or ureteral obstruction caused by mass effect.
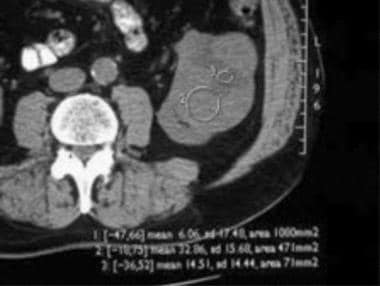
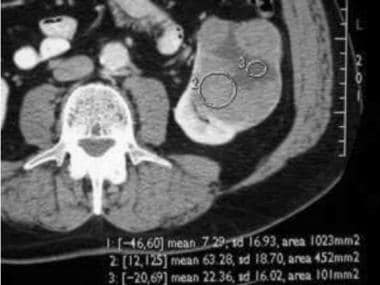
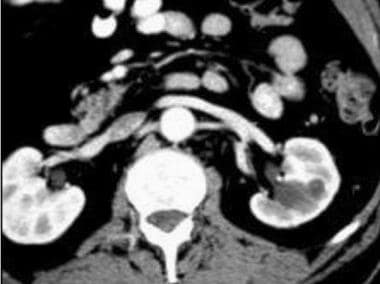
Computed Tomography
A dedicated renal CT examination consists of thin-section (2.5-5 mm) helical imaging of the kidneys before the intravenous administration of contrast agent, followed by imaging 60-70 seconds and 3-5 minutes after administration of the contrast agent.
The imaging parameters (kilovoltage, microamperage, field of view, section thickness) should be kept constant for all phases of imaging to enable comparison of the attenuation measurements. The addition of an arterial phase CT (either with bolus tracking or after a 20-25 second delay) with thin slices (1-2 mm) may be helpful to evaluate arterial anatomy, especially if partial resection is contemplated or if renal parenchymal or vascular anomalies are suspected. [1, 24, 25, 26, 27, 28, 29]
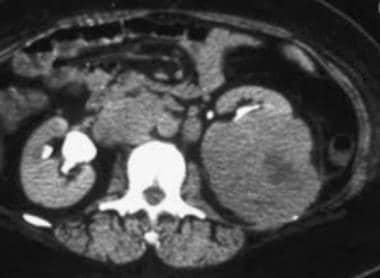
(See the CT scans of RCC below.)
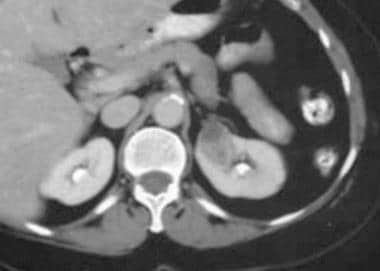
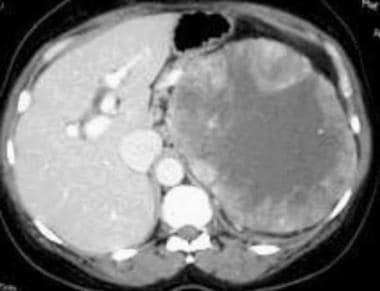
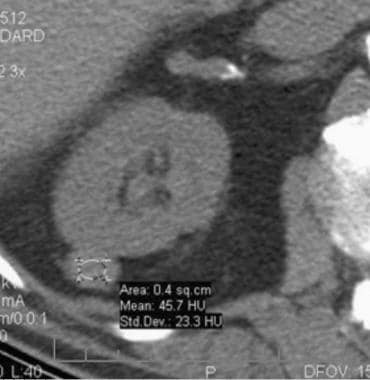 Case 4. Renal cell carcinoma. Dedicated renal CT scan obtained before contrast enhancement. Right kidney has an attenuation measurement of 45.7 HU.
Case 4. Renal cell carcinoma. Dedicated renal CT scan obtained before contrast enhancement. Right kidney has an attenuation measurement of 45.7 HU.
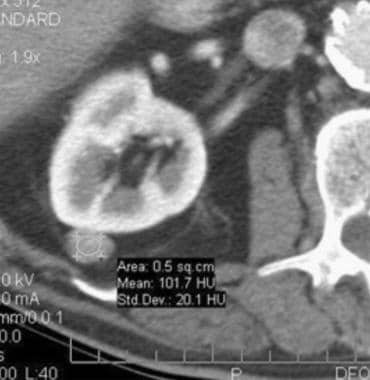 Case 4. Renal cell carcinoma. Contrast-enhanced dedicated renal CT scan with an attenuation measurement of 101.7 HU.
Case 4. Renal cell carcinoma. Contrast-enhanced dedicated renal CT scan with an attenuation measurement of 101.7 HU.
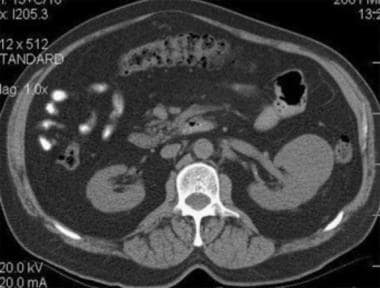 Case 5. Typical renal cell carcinoma. CT scan obtained before contrast enhancement has an attenuation measurement of 33.9 HU.
Case 5. Typical renal cell carcinoma. CT scan obtained before contrast enhancement has an attenuation measurement of 33.9 HU.
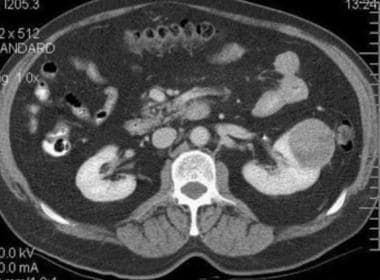 Case 5. Typical renal cell carcinoma. Contrast-enhanced CT scan has an attenuation measurement of 75.8 HU.
Case 5. Typical renal cell carcinoma. Contrast-enhanced CT scan has an attenuation measurement of 75.8 HU.
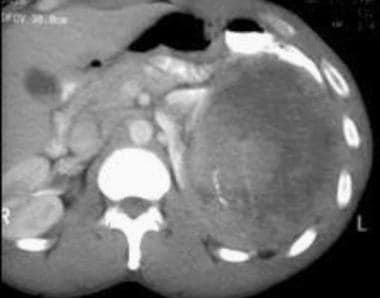
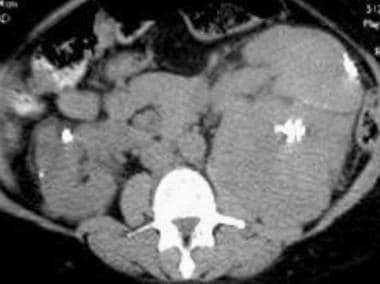
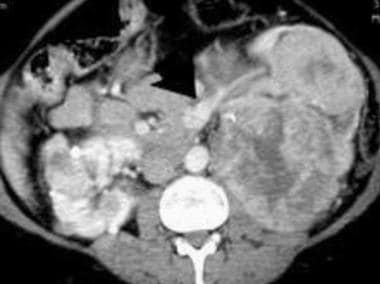
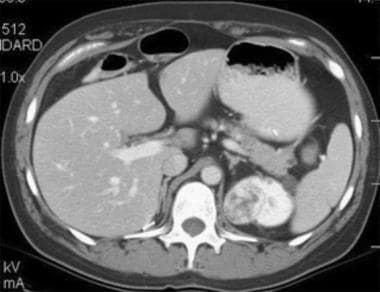 Case 6. Multifocal renal cell carcinoma in a patient with Von Hippel-Lindau disease. Contrast-enhanced CT scan.
Case 6. Multifocal renal cell carcinoma in a patient with Von Hippel-Lindau disease. Contrast-enhanced CT scan.
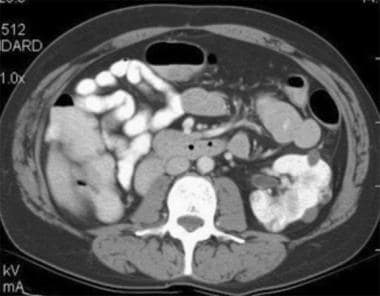 Case 6. Multifocal renal cell carcinoma in a patient with Von Hippel Lindau disease. Patient had already undergone a right nephrectomy. Contrast-enhanced CT scan.
Case 6. Multifocal renal cell carcinoma in a patient with Von Hippel Lindau disease. Patient had already undergone a right nephrectomy. Contrast-enhanced CT scan.
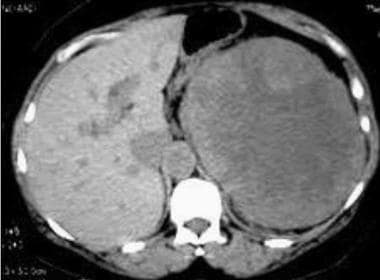 Case 7. Multifocal renal cell carcinoma in patient presenting with palpable mass. Nonenhanced CT scan.
Case 7. Multifocal renal cell carcinoma in patient presenting with palpable mass. Nonenhanced CT scan.
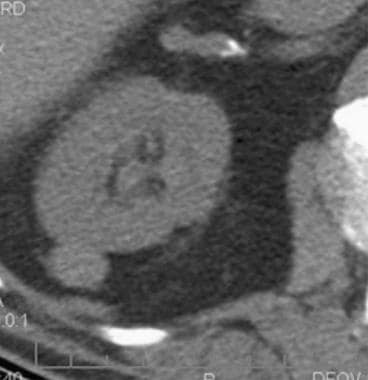
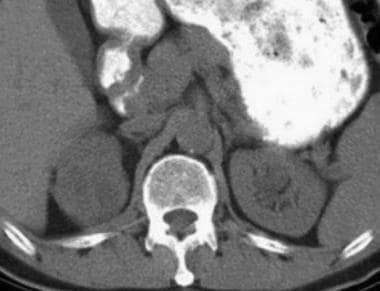 Case 8. Cystic renal cell carcinoma. Nonenhanced CT scan with an attenuation measurement of 25.8 HU.
Case 8. Cystic renal cell carcinoma. Nonenhanced CT scan with an attenuation measurement of 25.8 HU.
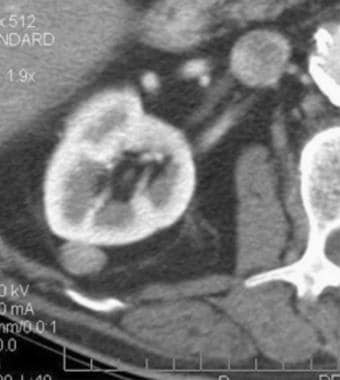
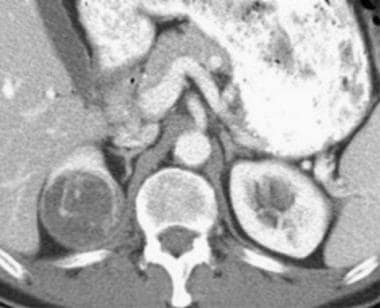 Case 8. Cystic renal cell carcinoma. Contrast-enhanced CT scan with an attenuation measurement of 47.1 HU.
Case 8. Cystic renal cell carcinoma. Contrast-enhanced CT scan with an attenuation measurement of 47.1 HU.
Historically, enhancement was considered present if the attenuation of the lesion increased by more than 10 HU from baseline.
On initial nonenhanced CT scans, RCCs may appear as isoattenuating, hypoattenuating, or hyperattenuating relative to the remainder of the kidney. Calcifications may be present and are usually amorphous and internal, although rimlike calcifications can also be present.
On contrast-enhanced CT scans, RCC is usually solid, and decreased attenuation suggestive of necrosis is often present. Sometimes, RCC is a predominantly cystic mass, with thick septa and wall nodularity.
RCC may also appear as a completely solid and highly enhancing mass.
Staging of RCC, which can be performed by using CT or MRI, includes the assessment of ipsilateral or contralateral adrenal involvement, direct extension into adjacent organs, enlargement of retroperitoneal lymph nodes, invasion of the ipsilateral renal vein (with or without extension into the inferior vena cava), and distant metastatic disease (liver, bone, lungs). Retrocrural, subcarinal, or mediastinal lymph nodes can also be enlarged.
If a solitary mass is enhancing, the degree of confidence in diagnosing RCC is high. When masses are multiple, metastatic disease and lymphoma must be considered, especially if the patient has a history of a primary malignancy. When a mass is predominantly cystic, the confidence level decreases. In these patients, US may be useful.
A false-positive diagnosis of enhancement may occur in small masses. This so-called pseudoenhancement may be the result of reconstruction algorithms, beam-hardening artifact, or cupping artifact. It is more pronounced on multidetector spiral CT scans than on single-detector spiral CT scans. In patients with these findings, MRI or US is helpful in proving that the lesions are cysts. In patients in whom nonenhanced imaging was not obtained, differentiation of solid masses from hyperattenuating cysts (Bosniak class 2 lesions) may be difficult. In one series, RCCs were significantly larger, had greater mean attenuation (>70 HU), and had increased central heterogeneity compared to hyperattenuating cysts.
Occasionally, masses are predominantly cystic but indeterminate because of septa or nodularity (Bosniak class 3 lesions). The lesions are often complex cysts, but they may be removed because of their suggestive appearance.
Briefly, the Bosniak classification of renal masses is as follows: class I includes simple cysts; class II, minimally complicated but overwhelmingly benign masses with thin septa, hyperattenuation, or small amounts of mural or septal calcification; class III, moderately complicated masses with mural nodularity, thick septa, or irregular or thick calcifications that often require surgical exploration; and class IV, significantly complicated and generally malignant masses with thick and irregular enhancing regions and definite solid components.
Oncocytomas cannot be reliably differentiated from RCCs without pathologic analysis. Macroscopic areas of fat in the tumor mass are reported in RCC, but they are extremely rare. Almost all renal tumors with measurable areas of fat are angiomyolipomas (AMLs); however, some AMLs do not contain visible fat and may be mistaken for RCCs. In one series, homogeneous and prolonged enhancement were valuable predictors for differentiation of AML with minimal fat from RCC. High attenuation on nonenhanced CT scans and the degree of enhancement were helpful but less valuable.
A false-negative diagnosis can occur if the attenuation is not carefully measured before and after the administration of contrast material, if cystic masses are not carefully examined for septa or nodularity, if the enhancement of a lesion is missed (as a result of lack of enhancement at the time of scanning), or if the masses are too small for adequate characterization at the time of their discovery.
In patients with renal failure and long-standing dialysis dependence, detection of an RCC (especially the papillary type) is increased when imaging is performed soon after the contrast bolus passes (in the arterial phase). In patients with normal renal function, imaging during the arterial or cortical phases may make the lesions less visible because the typical hypoenhancement of the tumors cannot be distinguished from the nonenhancement of the adjacent medulla.
Magnetic Resonance Imaging
MRI findings are similar to those of CT, with masses ranging from predominantly cystic with septa or nodularity to solid with enhancement. The numeric criteria for enhancement are not defined for MRI as they are for CT, but MRI signal intensity changes can be measured. [30, 31, 32, 33, 34]
(See the MRIs of RCC below.)
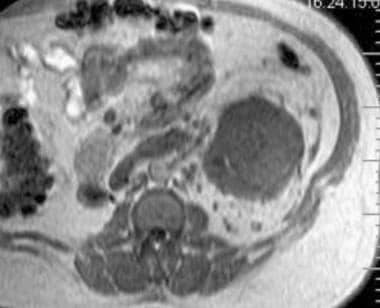 Case 16. Left renal cell carcinoma in patient who underwent prior right nephrectomy for renal cell carcinoma. T1-weighted axial magnetic resonance image (MRI).
Case 16. Left renal cell carcinoma in patient who underwent prior right nephrectomy for renal cell carcinoma. T1-weighted axial magnetic resonance image (MRI).
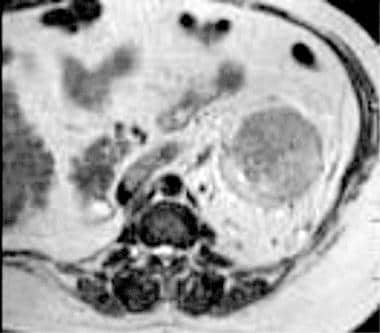 Case 16. Left renal cell carcinoma in patient with prior right nephrectomy for renal cell carcinoma. T2-weighted axial MRI with renal vein invasion and extension of tumor into the inferior vena cava.
Case 16. Left renal cell carcinoma in patient with prior right nephrectomy for renal cell carcinoma. T2-weighted axial MRI with renal vein invasion and extension of tumor into the inferior vena cava.
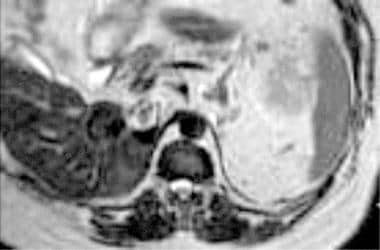 Case 16. Left renal cell carcinoma in patient with prior right nephrectomy for renal cell carcinoma. T2-weighted axial MRI obtained above the level in image 38. Tumor extends into the intrahepatic inferior vena cava.
Case 16. Left renal cell carcinoma in patient with prior right nephrectomy for renal cell carcinoma. T2-weighted axial MRI obtained above the level in image 38. Tumor extends into the intrahepatic inferior vena cava.
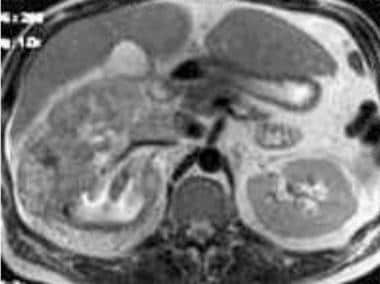 Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
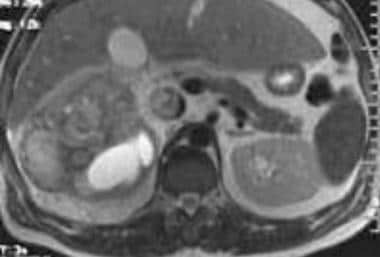 Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
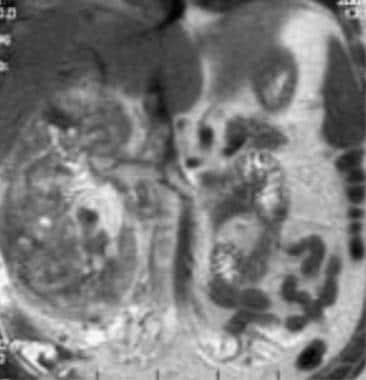 Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted coronal MRI.
Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted coronal MRI.
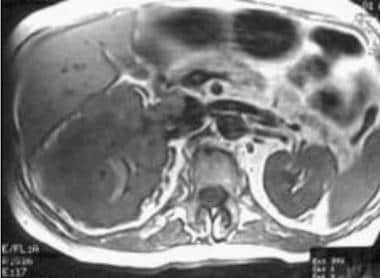 Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T1-weighted axial MRI before contrast enhancement.
Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T1-weighted axial MRI before contrast enhancement.
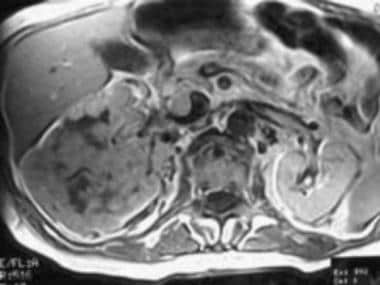 Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T1-weighted contrast-enhanced axial MRI.
Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T1-weighted contrast-enhanced axial MRI.
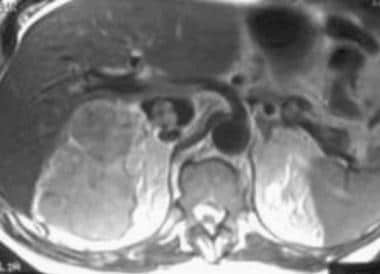 Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
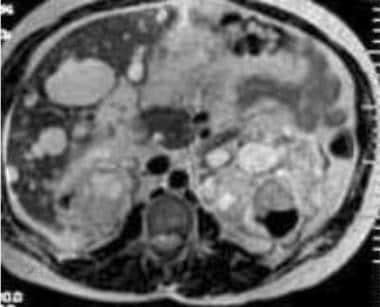 Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T2-weighted MRI.
Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T2-weighted MRI.
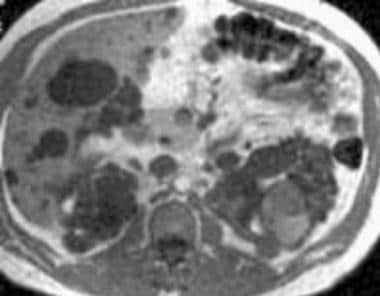 Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T1-weighted MRI before contrast enhancement.
Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T1-weighted MRI before contrast enhancement.
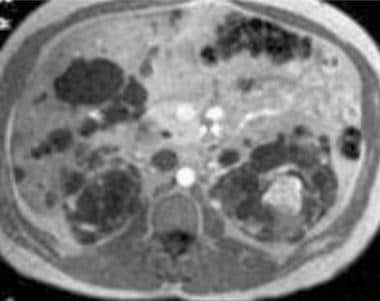 Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T1-weighted contrast-enhanced MRI.
Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T1-weighted contrast-enhanced MRI.
On nonenhanced T1-weighted images, RCCs usually appear isointense or hypointense relative to the remainder of the kidney. With chemical shift imaging, [35] some clear cell carcinomas show focal or diffuse loss of signal intensity. On T2-weighted images, RCCs are usually hyperintense. Most often, they are heterogeneous.
The presence of necrosis or hemorrhage may alter these signal intensity characteristics. MRI may be especially helpful in imaging the superior or inferior poles of the kidneys, in direct coronal or sagittal imaging, and in determining invasion of venous structures.
Degree of confidence
The degree of confidence in the diagnosis with MRI is similar to that of CT. That is, confidence is high for lesions showing enhancement and less for cystic lesions. Thus, MRI has no advantage compared to contrast-enhanced CT for the diagnosis of RCC, but MRI is superior to nonenhanced CT.
As with CT, false-positive findings of RCC can occur with other solid-enhancing masses (metastatic disease, lymphoma, oncocytoma, non–fat-containing AML), which are virtually indistinguishable from RCC when they are solitary and when the relevant history is absent.
As with CT, a false-negative diagnosis can occur if cystic masses are not carefully examined for septa or nodularity, if enhancement of a lesion is missed (as a result of lack of contrast enhancement at the time of scanning), or if the masses are too small for adequate characterization at the time of their discovery.
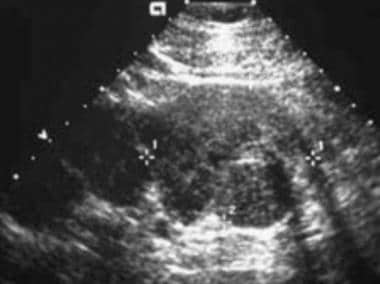
Ultrasonography
On sonograms, renal cell carcinoma can be isoechoic, hypoechoic, or hyperechoic relative to the remainder of the renal parenchyma. Smaller lesions with less necrosis are more likely to be hyperechoic and may be confused with AMLs. Isoechoic tumors are detected only by distortion of the renal contour, focal enlargement of a portion of the kidney, or distortion of the central sinus fat. [36, 37]
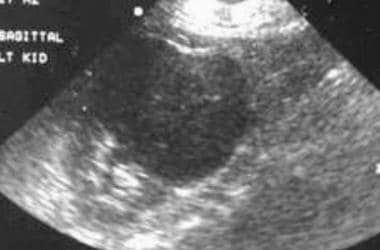
(See the sonograms of RCC below.)
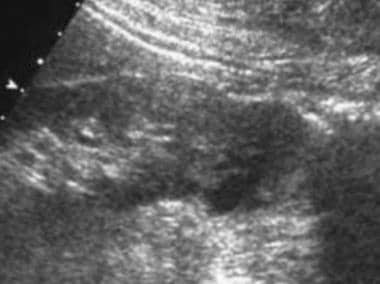
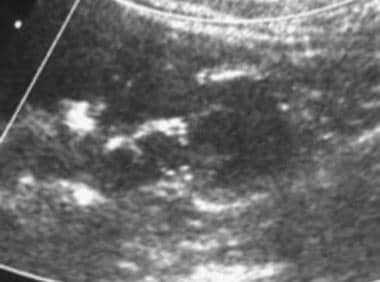 Case 21. Right renal cell carcinoma. Doppler ultrasonogram obtained prior to the intravenous administration of ultrasonographic contrast agent.
Case 21. Right renal cell carcinoma. Doppler ultrasonogram obtained prior to the intravenous administration of ultrasonographic contrast agent.
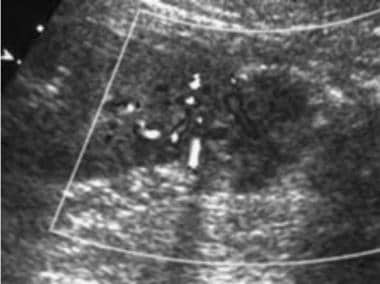
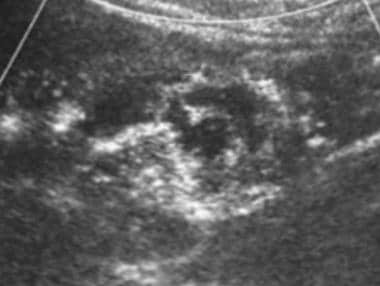 Case 21. Right renal cell carcinoma. Doppler ultrasonogram obtained after the intravenous administration of ultrasonographic contrast agent.
Case 21. Right renal cell carcinoma. Doppler ultrasonogram obtained after the intravenous administration of ultrasonographic contrast agent.
For the workup of RCC, US is used primarily to differentiate solid masses from simple cysts and to visualize the internal architecture of lesions more effectively than can be accomplished by using CT or MRI.
Studies suggest a role for contrast-enhanced Doppler US (CEUS) in the workup of masses showing poor enhancement at arterial phase CT. [36, 37, 38] In one series of 26 pathologically proven malignant masses, CT failed to show arterial enhancement in 5. In all 5, blood flow was confirmed with CEUS.
Cao et al retrospectively explored the value of contrast-enhanced ultrasound (CEUS) in differentiating small RCCs from angiomyolipomas (AMLs) and in distinguishing between clear cell RCC (ccRCC), papillary RCC (pRCC), and chromophobe RCC (chRCC). A total of 151 patients with small renal masses (110 ccRCCs, 12 pRCCs, 9 chRCCs, and 20 AMLs) were enrolled in this study. Sensitivity, specificity, and AUC of the characteristics combined for differentiating RCC from AML were 95.0%, 90.8%, and 0.920, respectively. [39]
Degree of confidence
Confidence in tumor detection is increased as lesions increase in size. Larger lesions are usually more heterogeneous and more often hypoechoic. In a reported series, a detection rate of 85% was seen with lesions larger than 3 cm. [40] A detection rate of less than 60% was seen with lesions smaller than 2 cm.
Confidence also increases if lesions are solid, lobulated, or well differentiated from the normal parenchyma; if they have poor through-transmission; and if they show flow with CEUS.
False-positive results are rare because US is seldom the sole imaging modality used before intervention.
A prominent column of Bertin or fetal lobulation may mimic a solid renal mass and can be resolved with a dedicated CT or MRI examination.
False-negative findings can occur if care is not taken to fully examine all aspects of the kidney, because US is highly operator-dependent. False-negative results are also possible if the RCC is small, is isoechoic to the parenchyma, and/or is not contour deforming.
Nuclear Imaging
Nuclear medicine scintigraphy is not used as the primary modality in the evaluation of suspected RCC. However, it can aid in confirming the presence of a pseudomass (eg, column of Bertin, dromedary hump, fetal lobulation). Nuclear medicine imaging combines the high sensitivity of radionuclides with the high resolution of structural imaging to visualize metabolic processes and specific targets of RCC for more accurate and reliable diagnosis, staging, prediction of prognosis, and response assessment. [41]
Scintigraphy with technetium dimethylsuccinic acid demonstrates normal uptake in the region of a pseudomass, whereas a real mass causes a focal photopenic defect.
Bone scanning with technetium methylene diphosphonate is indicated to confirm bony metastatic disease in a patient with RCC and symptoms referable to the skeleton.
The specificity of the technique is low in that all types of masses cause photopenic defects if they are large enough.
Angiography
Noninvasive cross-sectional imaging (CT, MRI, US) has replaced angiography in the workup of patients with known or suspected RCC. Advances in CT angiography and magnetic resonance angiography have diminished the need for preoperative conventional angiographic mapping of the renal vasculature prior to nephron-sparing or minimally invasive surgery. Angiography is still occasionally used if the origin of a tumor (eg, renal vs adrenal) is not certain. In these patients, selective injection of the renal and adrenal arteries, as well as additional vessels, may be necessary.
Questions & Answers
Overview
What is the preferred modality for renal cell carcinoma (RCC) imaging?
What is the prevalence of renal cell carcinoma (RCC)?
What are the risk factors for renal cell carcinoma (RCC)?
How is renal cell carcinoma (RCC) staged?
What is included in the imaging evaluation of renal cell carcinoma (RCC)?
What are the limitations of imaging to evaluate renal cell carcinoma (RCC)?
How is renal cell carcinoma (RCC) evaluated during pregnancy?
Which radiographic findings are characteristic of renal cell carcinoma (RCC)?
Which CT findings are characteristic of renal cell carcinoma (RCC)?
Which MRI findings are characteristic of renal cell carcinoma (RCC)?
How accurate is MRI in the diagnosis of renal cell carcinoma (RCC)?
Which ultrasonography findings are characteristic of renal cell carcinoma (RCC)?
How accurate is ultrasonography in the diagnosis of renal cell carcinoma (RCC)?
What is the role of nuclear medicine scintigraphy in renal cell carcinoma (RCC) imaging?
What is the role of angiography in renal cell carcinoma (RCC) imaging?
-
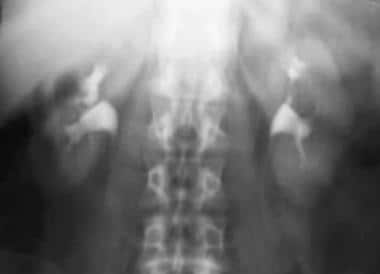
Case 1. Large renal cell carcinoma. Three-minute tomogram.
-
Case 1. Large renal cell carcinoma. Delayed intravenous urographic image.
-
Case 1. Large renal cell carcinoma. Contrast-enhanced computed tomography (CT) scan.
-
Case 2. Large renal cell carcinoma. Delayed tomogram.
-
Case 2. Large renal cell carcinoma. Sonogram.
-
Case 2. Large renal cell carcinoma. Contrast-enhanced CT scan.
-
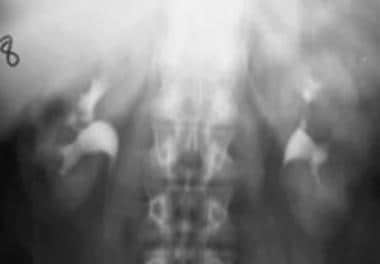
Case 3. Small left renal cell carcinoma is subtle on this intravenous urographic image.
-
Case 3. Small renal cell carcinoma. Tomogram.
-
Case 3. Small renal cell carcinoma. Contrast-enhanced CT scan.
-
Case 4. Renal cell carcinoma. Dedicated renal CT scan. Before contrast enhancement, right kidney.
-
Case 4. Renal cell carcinoma. Dedicated renal CT scan obtained before contrast enhancement. Right kidney has an attenuation measurement of 45.7 HU.
-
Case 4. Renal cell carcinoma. Contrast-enhanced dedicated renal CT scan. Right kidney.
-
Case 4. Renal cell carcinoma. Contrast-enhanced dedicated renal CT scan with an attenuation measurement of 101.7 HU.
-
Case 5. Typical renal cell carcinoma. CT scan obtained before contrast enhancement has an attenuation measurement of 33.9 HU.
-
Case 5. Typical renal cell carcinoma. Contrast-enhanced CT scan has an attenuation measurement of 75.8 HU.
-
Case 6. Multifocal renal cell carcinoma in a patient with Von Hippel-Lindau disease. Contrast-enhanced CT scan.
-
Case 6. Multifocal renal cell carcinoma in a patient with Von Hippel Lindau disease. Patient had already undergone a right nephrectomy. Contrast-enhanced CT scan.
-
Case 7. Multifocal renal cell carcinoma in patient presenting with palpable mass. Nonenhanced CT scan.
-
Case 7. Multifocal renal cell carcinoma. Nonenhanced CT scan.
-
Case 7. Multifocal renal cell carcinoma. Contrast-enhanced CT scan.
-
Case 7. Multifocal renal cell carcinoma. Contrast-enhanced CT scan.
-
Case 8. Cystic renal cell carcinoma. Nonenhanced CT scan with an attenuation measurement of 25.8 HU.
-
Case 8. Cystic renal cell carcinoma. Contrast-enhanced CT scan with an attenuation measurement of 47.1 HU.
-
Case 9. Cystic renal cell carcinoma. Contrast-enhanced CT scan with an enhancing rim of soft tissue.
-
Case 9. Renal cell carcinoma. Metastatic lesion to the thoracic spine. Loss of the anterior cortex of a midthoracic vertebral body, with the suggestion of an anterior soft-tissue mass is shown.
-
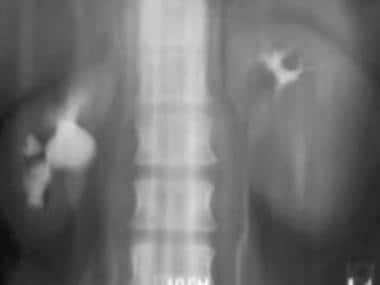
Case 10. Stage 4 renal cell carcinoma. Metastatic disease to the mediastinal nodes and left rib.
-
Case 10. Stage 4 renal cell carcinoma. Metastatic disease to the liver.
-
Case 10. Stage 4 renal cell carcinoma. Metastatic disease to the right iliac bone.
-
Case 11. Stage 3a renal cell carcinoma. Patient receiving long-term dialysis with a proven right-sided renal cell carcinoma. Image shows inferior vena cava invasion.
-
Case 11. Stage 3a renal cell carcinoma. Right atrial extension of a tumor thrombus.
-
Case 12. Recurrent renal cell carcinoma. Status post–right nephrectomy with local recurrence (arrow) in nephrectomy bed. Contrast-enhanced CT scan.
-
Case 13. Recurrent renal cell carcinoma. Status post–left nephrectomy with metastatic disease to mediastinal nodes. Contrast-enhanced CT scan.
-
Case 13. Recurrent renal cell carcinoma. Status post left nephrectomy with metastatic disease to the contralateral adrenal gland.
-
Case 14. Recurrent renal cell carcinoma. Status post right nephrectomy with metastatic disease to the ipsilateral psoas muscle and liver.
-
Case 14. Recurrent renal cell carcinoma. Status post right nephrectomy with metastatic disease to the liver.
-
Case 15. Renal cell carcinoma. Angiomyolipoma. Contrast-enhanced CT scan.
-
Case 16. Left renal cell carcinoma in patient who underwent prior right nephrectomy for renal cell carcinoma. T1-weighted axial magnetic resonance image (MRI).
-
Case 16. Left renal cell carcinoma in patient with prior right nephrectomy for renal cell carcinoma. T2-weighted axial MRI with renal vein invasion and extension of tumor into the inferior vena cava.
-
Case 16. Left renal cell carcinoma in patient with prior right nephrectomy for renal cell carcinoma. T2-weighted axial MRI obtained above the level in image 38. Tumor extends into the intrahepatic inferior vena cava.
-
Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
-
Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
-
Case 17. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted coronal MRI.
-
Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T1-weighted axial MRI before contrast enhancement.
-
Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T1-weighted contrast-enhanced axial MRI.
-
Case 18. Large right renal cell carcinoma with renal vein and inferior vena cava invasion. T2-weighted axial MRI.
-
Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T2-weighted MRI.
-
Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T1-weighted MRI before contrast enhancement.
-
Case 19. Left renal cell carcinoma in a patient with polycystic kidney disease. Axial T1-weighted contrast-enhanced MRI.
-
Case 20. Renal cell carcinoma. Oncocytoma. T2-weighted axial MRI.
-
Case 20. Renal cell carcinoma. Oncocytoma. Dynamic enhanced coronal T1-weighted MRI.
-
Case 21. Right renal cell carcinoma. Ultrasonogram.
-
Case 21. Right renal cell carcinoma. Doppler ultrasonogram.
-
Case 21. Right renal cell carcinoma. Doppler ultrasonogram obtained prior to the intravenous administration of ultrasonographic contrast agent.
-
Case 21. Right renal cell carcinoma. Doppler ultrasonogram obtained after the intravenous administration of ultrasonographic contrast agent.
-
Case 22. Left renal cell carcinoma. Ultrasonogram.
-
Case 22. Left renal cell carcinoma. Nonenhanced CT scan.
-
Case 22. Left renal cell carcinoma. Contrast-enhanced CT scan.
-
Case 22. Left renal cell carcinoma. Anterior component of circumaortic renal vein is normal.
-
Case 22. Left renal cell carcinoma. Posterior component of circumaortic renal vein is normal.
-
Case 23. Renal cell carcinoma. Complex cyst. Nonenhanced CT scan.
-
Case 23. Renal cell carcinoma. Complex cyst. Contrast-enhanced CT scan.
-
Case 23. Renal cell carcinoma. Complex cyst. Ultrasonogram with low-level echoes.
-
Case 23. Renal cell carcinoma. Complex cyst. Doppler ultrasonogram shows no internal flow.
-
Case 24 CT axial image from a CT angiogram on a patient with renal cell carcinoma in a horseshoe kidney.
-
Case 24 CT axial image slightly lower from a CT angiogram on a patient with renal cell carcinoma in a horseshoe kidney.
-
Case 24 Volume rendered image from a CT angiogram on a patient with renal cell carcinoma in a horseshoe kidney. The tumor has been colored to show relationship to the multiple renal arteries (two on the left and two on the right, one of which comes from below from the iliac artery).