Practice Essentials
Prostate cancer is the most common noncutaneous cancer in men and accounts for almost 10% of cancer-related deaths in males. Adenocarcinomas constitute 95% of prostate cancers are. Seventy percent of prostate cancers arise in the peripheral zone, 15-20% in the central zone, and 10-15% in the transition zone. The prostate can be divided into 5 zonal components (ie, the nonglandular anterior fibromuscular stroma) and 4 glandular components (ie, the peripheral zone [about 70% of the glandular prostate], central zone [25%], transition zones [5%], and periurethral glandular tissue [< 1%]). [1] With aging, the periurethral glandular tissue and the transition zones may considerably hypertrophy, gradually compressing the central zone and stretching the peripheral zone. This hyperplasia does not involve the peripheral zone, and, therefore, only 2 areas are considered from a radiologic point of view: the peripheral zone and the central gland. [2, 3, 4, 5, 6]
Prostate cancer staging traditionally includes computed tomography for assessment of lymph nodes and technetium-99m bone scintigraphy for assessment of bone metastases. Multiparametric magnetic resonance imaging (mpMRI) has been increasingly used. In a study by Eyrich et al using the Michigan Urological Surgery Improvement Collaborative registry data, mpMRI appeared superior to CT and comparable to bone scintigraphy for detection of lymph node and bone metastases, respectively. The authors noted that pretreatment mpMRI may obviate the need for additional staging imaging. [7]
Gallium-68 prostate-specific membrane antigen (PSMA) PET/CT has been shown in studies to more accurately stage cancer recurrences and facilitate personalized treatment. [8, 9, 10] Imaging agents targeting PSMA, such as gallium-68-labeled PSMA-targeted compounds and 18F-labeled radiotracers, have been shown to provide superior diagnostic performance, with a positive predictive value exceeding 95%. [11, 12, 13, 14]
According to the American Society of Clinical Oncology (ASCO), one or more of the following imaging modalities should be used for patients with advanced prostate cancer: conventional imaging (CT, bone scan, and/or prostate MRI) and/or next-generation imaging (NGI) (PET, PET/CT, PET/MRI, whole-body MRI). In cases in which salvage radiotherapy is being considered, NGI should be offered (PSMA imaging, 11C choline or 18F fluciclovine PET/CT or PET/MRI, whole-body MRI, and/or 18F sodium fluoride [NaF] PET/CT) because they have been shown to have superior disease detection performance characteristics and may alter patient management. [10]
Goals of imaging
The standard approach to the diagnosis of prostate cancer consists of prostate-specific antigen (PSA) screening, digital rectal examination, and random transrectal biopsy. This approach, however, does not detect all prostate cancers, incorrectly grades a substantial amount of them (both overgrading and undergrading), and unwantedly detects a significant number of indolent cancers. [15] Furthermore, different treatment options, ranging from early aggressive treatments, such as radical prostatectomy and radical radiotherapy, to deferred treatments (ie, treating men if and when the disease progresses and becomes symptomatic), are dependent on parameters such as tumor grade and tumor stage.
Imaging plays an important role in the noninvasive detection, localization, grading, and staging of prostate carcinoma and in carrying out biopsies for histopathologic analysis of the tumor. Particularly, MRI has become a powerful tool to achieve all of these goals. [16, 17, 18, 19, 20] The combination of T2-weighted imaging with functional techniques such as diffusion-weighted imaging, dynamic contrast-enhanced imaging, and spectroscopy is helpful in detecting and localizing a prostatic lesion that can serve as a target for subsequent core needle sampling under ultrasound- or MRI-guidance.
Various grading systems have been proposed for assessing the aggressiveness of prostate cancer, but the Gleason system is one of the most widely used internationally. It recognizes a primary and a secondary pattern, as well as 5 subpatterns in each. The sum of the 2 patterns is the Gleason score, which has prognostic significance. Patients with a low Gleason score do well clinically, whereas patients with a high score do poorly. A noninvasive prediction of tumor aggressiveness can be achieved using T2-weighted imaging, diffusion-weighted imaging, and spectroscopy.
Indications for prostate ultrasonography include the following [21] :
-
Guidance for biopsy in the presence of an abnormal digital rectal examination or elevated prostate specific antigen (PSA) or a suspicious prostatic lesion detected on MRI. This includes use of transrectal ultrasound (TRUS) biopsy as part of the TRUS/MRI fusion technique.
-
Assessment of prostate volume before medical, surgical, or radiation therapy and to calculate PSA density.
-
Real-time guidance for the placement of brachytherapy seeds.
-
Assessment of lower urinary tract symptoms.
-
Assessment of congenital anomalies.
-
Evaluation for suspected recurrence in the prostatectomy bed in patients who have undergone prostatectomy.
Staging
High-resolution T2-weighted imaging is helpful in local staging by noninvasive assessment of tumor spread outside the prostatic capsule or invasion of the seminal vesicles. Prostate cancer staging is performed using the TNM (tumor, node, metastasis) staging system that categorizes the primary tumor (T) as follows:
-
TX - Primary tumor is not assessable
-
T0 - No evidence of primary tumor
-
T1 - Clinically inapparent tumor, not palpable or visible by imaging; substages are as follows:
T1a - Incidental histologic finding in 5% or less of the tumor resected (tissue is obtained during transurethral resection for symptoms of outflow tract obstruction)
T1b - Incidental histologic finding in more than 5% of the tissue resected (tissue is obtained during transurethral resection for symptoms of outflow tract obstruction)
T1c - Tumor identified by needle biopsy (performed because of elevated PSA levels)
-
T2 - Tumor confined within the prostate; substages are as follows:
T2a - Tumor involving half of one prostate lobe or less
T2b - Tumor involving more than half of one prostate lobe
T2c – Tumor involving both lobes
-
T3 - Tumor extending through the prostatic capsule; substages are as follows:
T3a - Extracapsular extension (unilateral or bilateral)
T3b - Tumor invading the seminal vesicles
-
T4 - Tumor fixed or invading adjacent structures other than the seminal vesicles: external sphincter, rectum, levator muscles, and/or pelvic wall
Regional lymph nodes (N) are categorized as follows:
-
NX - Regional lymph nodes not assessable
-
N0 - No regional lymph node metastasis
-
N1 - Regional lymph node metastasis
Distant metastasis (M) is categorized as follows:
-
M0 - No distant metastasis
-
M1 - Distant metastasis; substages are as follows:
M1a - Nonregional lymph node metastasis
M1b - Bone metastasis
M1c - Metastasis at other sites
The usual sites of distant metastases from prostate cancer are the lymph nodes and bones. The spread of prostate cancer to the lymph nodes involves the obturator nodes and then the common iliac and para-aortic lymph nodes. Pelvic lymph nodes are involved initially; the inguinal nodes are not involved. Metastatic spread to bone is common in patients with advanced prostate cancer; this typically occurs as osteoblastic sclerotic metastases. Occasionally, lytic metastases are seen. Distant staging can be performed using bone scintigraphy (for bone metastases), complemented with targeted imaging with plain radiographs, CT scanning, or MRI of the abdomen (for lymph node metastases). Advances have led to the introduction of axial MRI and positron emission tomography (PET) scanning. [22, 16, 23]
Guidelines on staging and characterization of prostate cancer have been published by the National Comprehensive Cancer Network. [22]
Radiography
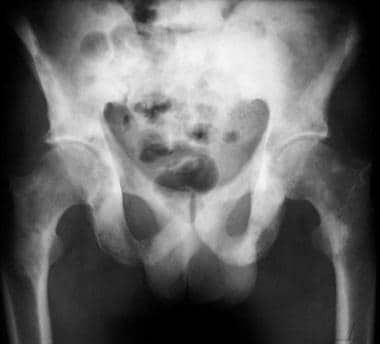
Plain radiographs of the pelvis cannot be used to demonstrate localized disease in the prostate, and they are generally only needed in the evaluation of metastatic disease. Most skeletal metastases from prostate cancer (about 85%) are osteoblastic and are visible as an area of abnormal tracer activity on a radionuclide bone scan. In case of doubt, targeted imaging with skeletal radiographs can help distinguish metastatic areas from degenerative disease. A chest radiograph may be used in the evaluation of a patient with known prostate cancer to assess chest symptoms, weight loss, localized bone pain, or constitutional symptoms.
(The image below depicts prostate cancer metastases on radiography.)
Computed Tomography
CT scanning has little value in demonstrating intraprostatic pathology and in local staging. However, it may be helpful in detecting metastatic disease, such as lymph node involvement or bone metastases.
Nodal staging is indicated in patients with a prostate-specific antigen (PSA) value of 20 ng/mL or higher, a clinical stage T2b or higher, and a Gleason score of 7 or higher. [24] CT or MRI scans depict lymph node enlargement and have similar accuracy for the evaluation of lymph node metastases. [16] However, nodal staging relies on assessment of lymph node size, and neither CT scan nor MRI can demonstrate cancer within lymph nodes that are not enlarged.
In case of doubtful radionuclide tracer activity, targeted imaging with CT scanning can be helpful in diagnosing osteoblastic and osteolytic skeletal metastases. CT scanning may also be used to depict soft-tissue metastases elsewhere in the body. Common sites of distant metastases include the liver, lung, mediastinum, pleura, brain, and adrenal glands. [23]
(The CT scans below depict metastatic prostate cancer.)
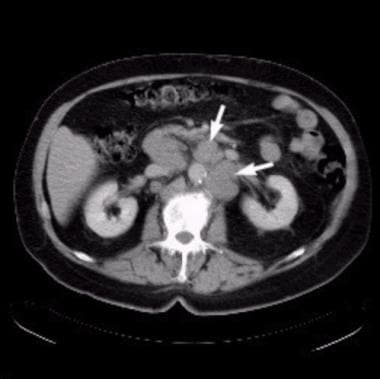 Axial computed tomography (CT) scan at the level of the kidneys shows extensive para-aortic lymphadenopathy (arrows), which results from advanced prostate cancer
Axial computed tomography (CT) scan at the level of the kidneys shows extensive para-aortic lymphadenopathy (arrows), which results from advanced prostate cancer
Magnetic Resonance Imaging
MRI evaluation for prostate cancer includes morphologic imaging (T1- and T2-weighted), complemented with one or more functional techniques (diffusion-weighted imaging, dynamic contrast-enhanced MRI, and/or spectroscopy). The technique is therefore called multiparametric MRI (mpMRI). [17] Potential roles of MRI are in guiding prostate biopsy, local staging of biopsy-proven cancers, treatment planning, and posttreatment surveillance. [18]
The National Comprehensive Cancer Network (NCCN) advises that although standard MRI techniques can be considered for initial evaluation of high-risk patients, mpMRI can be used in the staging and characterization of prostate cancer. In addition, the NCCN guidelines recommend considering mpMRI in patients who are undergoing active surveillance if anterior and/or aggressive cancer is suspected when PSA increases and systematic prostate biopsies are negative. [22]
Morphologic MRI (T1- and T2-weighted imaging)
On T1-weighted images, the prostate appears homogeneous with medium signal intensity; neither the zonal anatomy nor intraprostatic pathology is displayed, but if the MRI is performed after biopsy, postbiopsy hemorrhage can be identified as areas of high T1-signal intensity.
T2-weighted sequences exquisitely depict the prostatic zonal anatomy. The central gland usually consists of nodular areas of varying signal intensity, depending on the relative amount of hypointense stromal and hyperintense glandular elements. [3, 4] The normal peripheral zone has high signal intensity (as it is mainly composed of numerous ductal and acinar elements with hyperintense secretions). [3]
Most prostate cancers can be visualized as low-signal-intensity areas within the high-signal-intensity normal tissue background of the peripheral zone. Because about 70% of all prostate cancers occur within the peripheral zone, [25] morphologic T2-weighed imaging can thus depict the majority of all prostate cancers. On the other hand, low-signal-intensity tumors in the central gland are usually indistinguishable from far more common hypointense stromal hyperplasia. [26, 19] Therefore, central gland tumors are more difficult to detect than peripheral zone cancers.
T2-weighted imaging can be performed on a 1.5-Tesla unit, preferably with use of an endorectal coil, or on a 3-Tesla unit. Reported sensitivities (22-85%) and specificities (50-99%) vary widely, [27] the latter illustrating the fact that low-signal-intensity areas are by no means specific for prostate cancer, since benign conditions such as prostatitis, hemorrhage, hyperplastic nodules, or posttreatment (hormonal or irradiation) changes may equally show low signal intensity (see the images below).
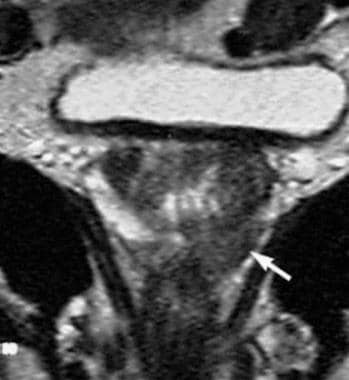 Coronal, T2-weighted magnetic resonance imaging (MRI) study of the prostate gland obtained by using an external coil. Low signal intensity (arrow) is seen on the left side of the prostate at the site of a biopsy-proven prostate cancer.
Coronal, T2-weighted magnetic resonance imaging (MRI) study of the prostate gland obtained by using an external coil. Low signal intensity (arrow) is seen on the left side of the prostate at the site of a biopsy-proven prostate cancer.
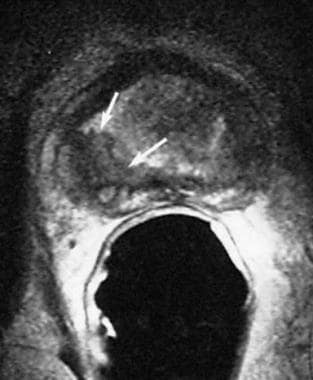 Endorectal, axial, T2-weighted magnetic resonance imaging (MRI) scan in a patient with a prostate-specific antigen level of 8 ng/mL and right-sided prostate cancer. Low signal intensity is demonstrated in the right peripheral zone (arrow).
Endorectal, axial, T2-weighted magnetic resonance imaging (MRI) scan in a patient with a prostate-specific antigen level of 8 ng/mL and right-sided prostate cancer. Low signal intensity is demonstrated in the right peripheral zone (arrow).
An important role of morphologic T2-weighted MRI is the assessment of local extracapsular extension and invasion of the seminal vesicle in a patient with no documented distant metastases. Signs of extracapsular spread include (1) irregular bulging of the prostatic outline (see the image below), (2) breach of the capsule with infiltration of the periprostatic fat, (3) asymmetry of the neurovascular bundles, and (4) loss of the rectoprostatic angle. [28, 29] Seminal vesicle invasion may be suspected in the presence of an abnormally low signal intensity within the lumen of the seminal vesicle or by focal thickening of the seminal vesicle walls. [30]
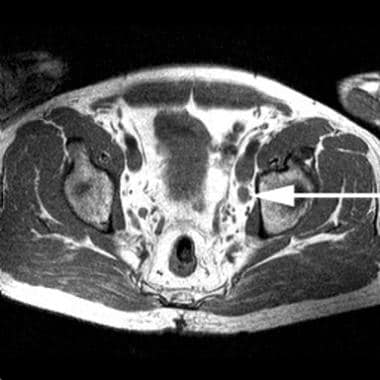 Patient with biopsy-proven prostate cancer. Axial, T1-weighted magnetic resonance imaging (MRI) scan of the pelvis shows an enlarged left obturator node (arrow).
Patient with biopsy-proven prostate cancer. Axial, T1-weighted magnetic resonance imaging (MRI) scan of the pelvis shows an enlarged left obturator node (arrow).
For local staging, the reported sensitivities range from 14 to 100% and specificities range from 67 to 100%. [27] Because MRI cannot detect microscopic invasion, low sensitivity values are not unexpected. The main indication for local MRI staging, however, is the assessment of capsular and vesicular integrity in a patient clinically staged as T1c or T2c. Such patients obviously should not be inappropriately upstaged by MRI, and therefore a conservative approach is adopted in which only unequivocal capsular or vesicular extensions are assigned a T3 status. This implies high specificity reading (no false positives) at the expense of a lower sensitivity.
Functional MRI
To increase both the sensitivity and specificity of MRI in the detection of prostate cancer, several functional techniques can be added. These take advantage of various tumor phenotypes, such as cellular density (diffusion-weighted imaging), angiogenesis (dynamic contrast-enhanced MRI), and tumor metabolism (magnetic resonance spectroscopy).
Diffusion-weighted imaging
Diffusion-weighted imaging provides information about the amount of random Brownian movements of water molecules. Protons are very mobile in normal water-rich glandular tissue, but they are restricted in their movement in densely packed water-poor tissue, such as tumor areas, which contain many hydrophobic cell membranes. As a result, prostate cancer in both the peripheral zone and the transition zone displays significantly lower diffusion than it does in benign prostatic tissue. [31, 20]
Diffusion-weighted imaging of the prostate is fast and easy. It produces poor spatial resolution as compared to T2-weighted images, but it is useful as a supplementary technique in drawing attention to areas of suspicion at 1.5 Tesla and 3 Tesla. A correlation seems to exist between the degree of diffusion restriction and tumor aggressiveness (Gleason score). [32]
In one meta-analysis, of 21 studies, [33] the pooled sensitivity and specificity of diffusion-weighted imaging was found to be 62% and 90%, respectively. In a second meta-analysis, of 10 studies, [34] pooled sensitivity and specificity of T2-weighted imaging combined with diffusion was 76% and 82%, respectively, and was superior to T2-weighted imaging alone.
Dynamic contrast-enhanced imaging
After an intravenous bolus injection of 0.1 mmol/kg of a gadolinium contrast agent, the prostate is serially imaged with a T1-weighted sequence every 2-5 seconds. [35] Most prostate carcinomas show earlier and more pronounced contrast enhancement, although some overlap still is apparent in the enhancement patterns between tumors and benign conditions such as prostatitis, postbiopsy hemorrhage, and benign prostatic hyperplasia. Accuracies of 70-90% have been reported for dynamic contrast-enhanced MRI in the primary diagnosis of prostate carcinoma in the peripheral zone. [35, 36] The role of contrast-enhanced MRI is primarily to improve specificity, because T2-weighted MRI is more sensitive. [18]
(See the image below.)
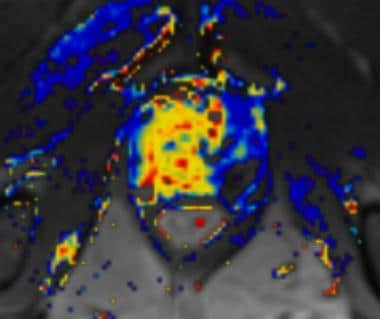 Dynamic contrast-enhanced MRI scan in a patient with an extensively enhancing prostate carcinoma in the right prostate halve.
Dynamic contrast-enhanced MRI scan in a patient with an extensively enhancing prostate carcinoma in the right prostate halve.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy provides information about the relative concentration of cellular metabolites in the prostate, such as citrate and choline. Citrate is a marker of normal prostatic tissue, while an increased concentration of choline is suggestive of a tumor lesion. [37] The complementary changes of both metabolites are used to predict the presence or absence of prostate cancer.
When used in combination with T2-weighted images, sensitivities and specificities ranging from 59-94% and 80-95%, respectively, have been reported. [38] A useful correlation between the choline-to-citrate ratio and tumor aggressiveness (Gleason score) has also been demonstrated, [39] and a particularly high negative predictive value was found in ruling out high-grade prostate cancer (ie, Gleason 4+3 or higher grade) in men presenting with an increased prostate-specific antigen (PSA) value. [40]
In a systematic review, magnetic resonance spectroscopy had the highest sensitivity (92%) of the MRI techniques, as well as a higher specificity than T2-weighted MRI. [41]
(See the image below.)
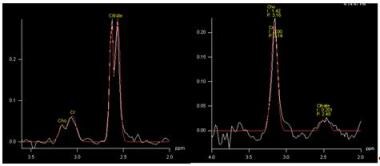 Proton magnetic resonance spectrum in a normal voxel (left image: high citrate peak and low choline peak) and a cancer voxel (right image: low citrate and high choline peak).
Proton magnetic resonance spectrum in a normal voxel (left image: high citrate peak and low choline peak) and a cancer voxel (right image: low citrate and high choline peak).
Nodal staging
Nodal staging relies on assessment of lymph node size, and neither CT scanning nor MRI can demonstrate cancer within lymph nodes that are not enlarged. A technique to detect clinically occult lymph node metastases using MR lymphography with a highly lymphotropic MR contrast agent was reported. Intravenous lymphotropic paramagnetic nanoparticles of iron oxide were administered, and patients were examined using MRI 24 hours after contrast administration. Small lymph node metastases were identified with higher sensitivity than with conventional MRI. [42]
Ultrasonography
Transrectal ultrasonography (TRUS)
TRUS is widely available, well tolerated by patients, and relatively inexpensive. It is optimally performed with high-frequency TRUS probes, and the entire prostate is imaged in the transverse and sagittal plane. The prostate volume can be approximated by multiplying the height, depth, and width of the prostate by 0.52 (prolate ellipsoid formula). [21]
With TRUS, the prostate is shown to be divided into an isoechoic peripheral zone and a more heterogeneous central gland, comprising the transition zone. Calcifications (corpora amylacea) are common at the boundary between the peripheral zone and the central gland. The seminal vesicles can be visualized as convoluted hypoechoic cystic structures.
(See the image below.)
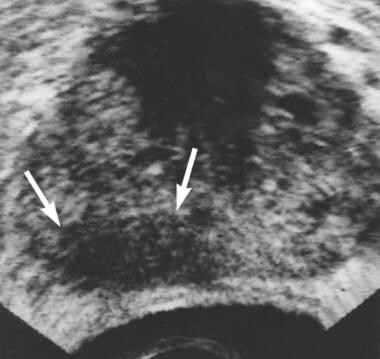 Axial transrectal ultrasonographic scan shows extensive hypoechoic area (arrows) in the right peripheral zone. Biopsy revealed prostatic adenocarcinoma.
Axial transrectal ultrasonographic scan shows extensive hypoechoic area (arrows) in the right peripheral zone. Biopsy revealed prostatic adenocarcinoma.
TRUS in diagnosis
Prostate cancers can be visualized as hypoechoic nodules within the isoechoic normal peripheral zone, but they may be isoechoic, hyperechoic, or multifocal as well, so TRUS has major limitations regarding fully demonstrating prostate cancers. Furthermore, TRUS has a low specificity because many nonmalignant conditions (eg, prostatitis, prostatic atrophy, infarction, granulomatous prostatitis) may appear as similarly hypoechoic areas in the peripheral zone of the prostate. For this reason, the sensitivity and specificity of TRUS are far too low for sonographic prostate cancer screening, and therefore, the main roles of TRUS are measuring the prostate volume (for estimation of the prostate-specific antigen [PSA] density) and providing guidance for biopsy of the prostate. [43]
(See the images below.)
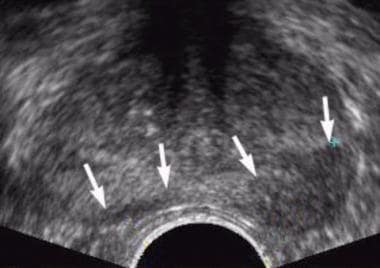 Axial transrectal ultrasonographic scan shows a hypoechoic area in left peripheral zone and a small hypoechoic area in right peripheral zone (arrows). Biopsy revealed an adenocarcinoma (Gleason grade 6).
Axial transrectal ultrasonographic scan shows a hypoechoic area in left peripheral zone and a small hypoechoic area in right peripheral zone (arrows). Biopsy revealed an adenocarcinoma (Gleason grade 6).
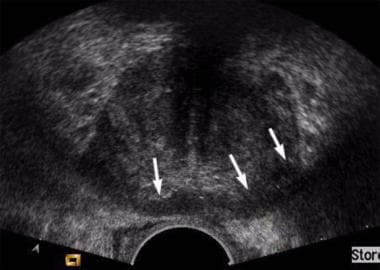 Axial transrectal sonogram in a patient with normal results during digital rectal examination and a prostate-specific antigen (PSA) level of 9 ng/mL. Image shows extensive bilateral, but predominantly left-sided, hypoechoic areas in the peripheral zone (arrows). Biopsy confirmed a Gleason grade 8 prostate cancer. Minor capsular irregularity is present on the left; this is consistent with a T3 tumor.
Axial transrectal sonogram in a patient with normal results during digital rectal examination and a prostate-specific antigen (PSA) level of 9 ng/mL. Image shows extensive bilateral, but predominantly left-sided, hypoechoic areas in the peripheral zone (arrows). Biopsy confirmed a Gleason grade 8 prostate cancer. Minor capsular irregularity is present on the left; this is consistent with a T3 tumor.
Ancillary sonographic tools may improve, to some extent, the diagnostic performance of TRUS.
Color or power Doppler ultrasonography may show increased vascularity in a cancer area, but a wide range of diagnostic accuracies have been reported. [43] No cancer-specific flow pattern has been identified, and some cancers do not show any focal hypervascularity.
(See the image below.)
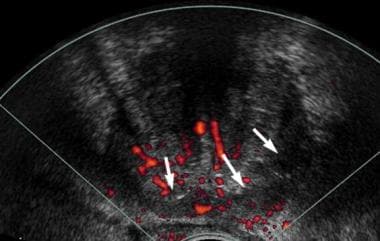 Axial transrectal ultrasonographic power Doppler scan in the same patient as in the previous image. The patient had normal results with digital rectal examination and a prostate-specific antigen (PSA) level of 9 ng/mL. A generalized increase in vascularity was noted in the posterior aspect of the prostate (arrows). However, this finding is not specific to the hypoechoic area in the left peripheral zone, illustrating the difficulty of using Doppler techniques in the assessment of prostate cancer.
Axial transrectal ultrasonographic power Doppler scan in the same patient as in the previous image. The patient had normal results with digital rectal examination and a prostate-specific antigen (PSA) level of 9 ng/mL. A generalized increase in vascularity was noted in the posterior aspect of the prostate (arrows). However, this finding is not specific to the hypoechoic area in the left peripheral zone, illustrating the difficulty of using Doppler techniques in the assessment of prostate cancer.
In elastography, prostate cancers appear as areas of decreased elasticity (increased stiffness), and both qualitative and quantitative methods have been developed to assess the tissue elasticity. Sensitivities and specificities range from 71-82% and 60-95%, respectively. [44] Elastography-guided transrectal biopsies have also been shown to double the cancer detection rate when compared to the standard systematic biopsy strategy. [45]
(See the image below.)
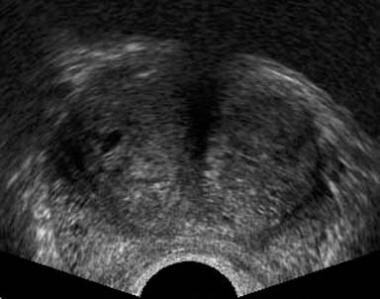 Axial transrectal ultrasonographic scan in a patient with clinical benign prostatic hyperplasia (BPH) and a serum prostate-specific antigen (PSA) level of 11 ng/mL. Enlargement of the transition zone is present, but no focal abnormality is observed in the peripheral zone. Systematic, 6-core biopsy revealed adenocarcinoma from both lobes of the prostate (ie, this is an isoechoic tumor in the peripheral zone of both prostatic lobes).
Axial transrectal ultrasonographic scan in a patient with clinical benign prostatic hyperplasia (BPH) and a serum prostate-specific antigen (PSA) level of 11 ng/mL. Enlargement of the transition zone is present, but no focal abnormality is observed in the peripheral zone. Systematic, 6-core biopsy revealed adenocarcinoma from both lobes of the prostate (ie, this is an isoechoic tumor in the peripheral zone of both prostatic lobes).
HistoScanning
Prostate HistoScanning (PHS) is a tissue characterization system used to enhance prostate cancer detection via transrectal ultrasound imaging. HistoScanning integrates specific acoustic signatures from different tissue types (eg, irregular morphology, increased vascularization, modifications in stiffness) into a characterization algorithm to detect and localize prostate cancer and enable transrectal biopsy targeting. [46]
TRUS in staging
TRUS can demonstrate bulges of the prostate capsular outline or overt extracapsular extension. Peripheral zone tumors longer than 2.3 cm that contact the fibromuscular rim surrounding the prostate may be associated with extracapsular invasion. Nevertheless, TRUS findings generally have been found to be inaccurate in the staging of localized prostate cancer.
TRUS-guided biopsy
Samples should include cores obtained during systematic biopsy, but they can be supplemented with cores directed to an abnormal focus detected on TRUS or MRI.
The original systematic approach to biopsy included the acquisition of 6 cores, with 1 core taken bilaterally at the base, mid gland, and apex in a parasagittal plane (ie, a "sextant" biopsy). Current practice is to obtain 10-12 cores, including cores from the peripheral zone as well as from the central gland. Some authors describe a saturation biopsy approach in which as many as 40 cores are obtained under general anesthesia or sedation. The precise biopsy approach must be individually tailored on the basis of the patient's clinical features.
Nuclear Imaging
Radionuclide bone scanning after the injection of a technetium-99m (99mTc) tracer is the current standard for assessing potential bone metastases from prostate cancer in patients with a prostate-specific antigen (PSA) value above 20 ng/mL, with a Gleason sum of 4+3 or higher, or with symptoms that might be attributable to potential bone metastasis. [24] Bone scans have a high sensitivity but low specificity for metastatic prostate cancer. When in doubt (eg, degenerative vs metastatic disease), targeted imaging with plain films, CT scanning, or MRI may be necessary. With diffuse bone metastases, a "superscan" may be seen; this superscan demonstrates high uptake throughout the skeleton, with poor or absent renal excretion of the tracer.
Gallium-68 prostate-specific membrane antigen (PSMA) PET/CT has been shown in studies to more accurately stage cancer recurrences and facilitate personalized treatment. [8, 9, 10] Imaging agents targeting PSMA, such as gallium-68-labeled PSMA-targeted compounds and 18F-labeled radiotracers, have been shown to provide superior diagnostic performance, with a positive predictive value of exceeding 95%. [11, 12, 13, 14]
Bone scans have a high sensitivity but low specificity for metastatic prostate cancer. Isotopic bone scans revealing metastatic prostate cancer are shown below.
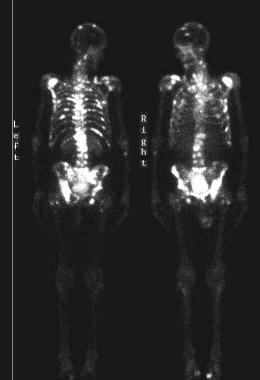 Isotopic bone scans show multiple areas of increased tracer activity from metastatic prostate cancer.
Isotopic bone scans show multiple areas of increased tracer activity from metastatic prostate cancer.
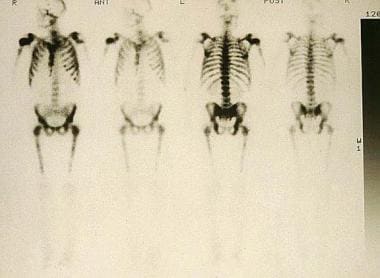 Isotopic bone scans. Diffuse metastases demonstrate a superscan appearance. Note that no renal excretion of radioactive tracer is demonstrated.
Isotopic bone scans. Diffuse metastases demonstrate a superscan appearance. Note that no renal excretion of radioactive tracer is demonstrated.
Choline C-11 seems to be the best tracer for the detection of lymph node metastases and is also promising for identifying bone metastases. Unfortunately, the half-life of this radionuclide is very short, so it can only be used in centers with an on-site cyclotron. An alternative that might solve this inconvenience is 18F-fluorocholine. Other potentially useful tracers are 11C-acetate and 18F-fluoroacetate for lymph node metastases and 11C-acetate, 11C-methionine, and 18F-fluoride for bone metastases. [47]
Flotufolastat F18 is indicated for PET imaging of PSMA-positive lesions in men who have prostate cancer with suspected metastasis and are candidates for initial definitive therapy, and for those with suspected recurrence on the basis of an elevated PSA. Approval was based on the SPOTLIGHT and LIGHTHOUSE studies, which detected recurrence and metastatic lesions in newly diagnosed men and those with undetectable metastasis at baseline with conventional imaging. [48, 49]
Approval of the radiotracer fluciclovine F18 for PET imaging in men with suspected prostate cancer recurrence was based on a comparative trial with 11C-choline. Sensitivity rates for 11C-choline and fluciclovine F18 were 32% and 37%; specificity, 40% and 67%; accuracy, 32% and 38%; and positive predictive value (PPV), 90% and 97%. [50] A second trial observed that the diagnostic performance of fluciclovine PET/CT imaging for recurrent prostate cancer was superior to that of routine clinical CT, and fluciclovine PET/CT provided better delineation between prostatic recurrence and extraprostatic recurrence. [51]
NCCN guidelines include scanning using 18F-NaF as the tracer for subsequent PET scanning in men with prostate cancer who undergo a bone scan to search for metastatic disease. The guidelines also note that choline C-11 PET/CT or PET/MRI or fluciclovine F-18 PET/CT or PET/MRI can be considered for further soft tissue evaluation. [52, 22]
-
Axial transrectal ultrasonographic scan shows extensive hypoechoic area (arrows) in the right peripheral zone. Biopsy revealed prostatic adenocarcinoma.
-
Axial transrectal ultrasonographic scan shows a hypoechoic area in left peripheral zone and a small hypoechoic area in right peripheral zone (arrows). Biopsy revealed an adenocarcinoma (Gleason grade 6).
-
Axial transrectal sonogram in a patient with normal results during digital rectal examination and a prostate-specific antigen (PSA) level of 9 ng/mL. Image shows extensive bilateral, but predominantly left-sided, hypoechoic areas in the peripheral zone (arrows). Biopsy confirmed a Gleason grade 8 prostate cancer. Minor capsular irregularity is present on the left; this is consistent with a T3 tumor.
-
Axial transrectal ultrasonographic power Doppler scan in the same patient as in the previous image. The patient had normal results with digital rectal examination and a prostate-specific antigen (PSA) level of 9 ng/mL. A generalized increase in vascularity was noted in the posterior aspect of the prostate (arrows). However, this finding is not specific to the hypoechoic area in the left peripheral zone, illustrating the difficulty of using Doppler techniques in the assessment of prostate cancer.
-
Axial transrectal ultrasonographic scan in a patient with clinical benign prostatic hyperplasia (BPH) and a serum prostate-specific antigen (PSA) level of 11 ng/mL. Enlargement of the transition zone is present, but no focal abnormality is observed in the peripheral zone. Systematic, 6-core biopsy revealed adenocarcinoma from both lobes of the prostate (ie, this is an isoechoic tumor in the peripheral zone of both prostatic lobes).
-
Sonogram shows an extensive, hypoechoic T3 tumor (arrowheads). Capsular irregularity is present, particularly on the right and posteriorly, with a suggestion of infiltration into the rectal wall (arrow).
-
Coronal, T2-weighted magnetic resonance imaging (MRI) study of the prostate gland obtained by using an external coil. Low signal intensity (arrow) is seen on the left side of the prostate at the site of a biopsy-proven prostate cancer.
-
Endorectal magnetic resonance imaging (MRI) scan in a patient with extensive prostate carcinoma. Image shows a bulge in the capsular outline on the right side (arrow). This is a stage T3 tumor.
-
Endorectal, axial, T2-weighted magnetic resonance imaging (MRI) scan in a patient with a prostate-specific antigen level of 8 ng/mL and right-sided prostate cancer. Low signal intensity is demonstrated in the right peripheral zone (arrow).
-
Patient with biopsy-proven prostate cancer. Axial, T1-weighted magnetic resonance imaging (MRI) scan of the pelvis shows an enlarged left obturator node (arrow).
-
Isotopic bone scans show multiple areas of increased tracer activity from metastatic prostate cancer.
-
Isotopic bone scans. Diffuse metastases demonstrate a superscan appearance. Note that no renal excretion of radioactive tracer is demonstrated.
-
Pelvic radiograph shows widespread, osteoblastic, sclerotic metastases from prostate cancer.
-
Axial computed tomography (CT) scan at the level of the kidneys shows extensive para-aortic lymphadenopathy (arrows), which results from advanced prostate cancer
-
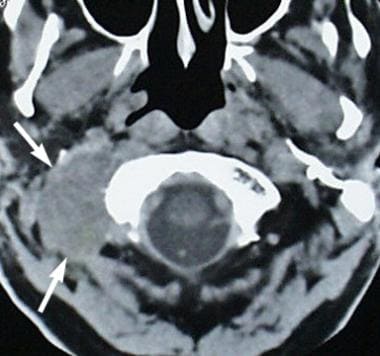
Metastatic prostate cancer (arrows) involves the soft tissues at the right side of the skull base. The patient presented with right-sided cranial nerve XII palsy.
-
Lytic bone metastasis involving the left iliac wing. Note surrounding soft tissue mass.
-
Transverse, T2-weighted MRI study of the prostate gland obtained by using an endorectal coil on a 1.5-Tesla scanner. Low signal intensity is seen on the left side of the prostate at the site of a biopsy-proven prostate cancer.
-
Axial, T2-weighted MRI scan in a patient with an anteriorly located prostate cancer. Low signal intensity is demonstrated in the right anterior portion of the peripheral zone. Scan obtained on a 3-Tesla MR-scanner without use of an endorectal coil.
-
Endorectal MRI scan in a patient with extensive prostate carcinoma. Image shows a bulge in the capsular outline on the left side. This is a stage T3a tumor.
-
Dynamic contrast-enhanced MRI scan in a patient with an extensively enhancing prostate carcinoma in the right prostate halve.
-
Proton magnetic resonance spectrum in a normal voxel (left image: high citrate peak and low choline peak) and a cancer voxel (right image: low citrate and high choline peak).
-
Axial transrectal ultrasonographic scan depicting the peripheral zone (PZ) and central gland (CG) of the prostate.
-
Axial transrectal sonogram in a patient with a prostate-specific antigen (PSA) level of 11.2 ng/mL. Image shows a nodular hypoechoic area in the left peripheral zone. Biopsy confirmed a Gleason grade 8 prostate cancer.
-
Axial transrectal elastography scan showing an area of increased stiffness in the left peripheral zone, corresponding to a Gleason 7 prostate cancer.