Practice Essentials
Progressive multifocal leukoencephalopathy (PML) is a fatal subacute progressive demyelinating disease caused by the neurotrophic JC polyoma virus. Before the HIV epidemic, PML was rare and associated with other immunocompromised conditions, such as leukemia, lymphoma, systemic lupus erythematosus (SLE), organ transplantation, Wiskott-Aldrich syndrome, and severe combined immunodeficiency (SCID). During the first 2 decades of the HIV epidemic, PML was predominantly seen in patients with AIDS, but effective antiretroviral treatment and restitution of T cell function led to a decline in the occurrence of PML in this population. The advent of drugs that interfere with leukocyte-endothelial interaction, such as natalizumab for multiple sclerosis (MS) and efalizumab for psoriasis, has led to an increase in the number of cases of PML. Other immunosuppressive agents associated with PML include rituximab, brentuximab-vedotin (BV), alemtuzumab, eculizumab, infliximab, adalimumab, fumaric acid esters (FAE), dimethyl fumarate (DMF), fingolimod, and ibrutinib. [1]
PML-related immune reconstitution inflammatory syndrome (IRIS) was originally described in patients with AIDS who paradoxically deteriorated on starting highly active antiretroviral therapy. It is also a recognized complication following natalizumab cessation in patients with MS. [2, 3]
Imaging reveals characteristic multiple lesions in the subcortical hemispheric white matter or the cerebellar peduncles. PML lesions also occur in gray-matter areas such as the basal ganglia or thalamus. [1]
The characteristics of PML, as seen on magnetic resonance imaging (MRI) and computed tomography (CT) scans, are demonstrated below.
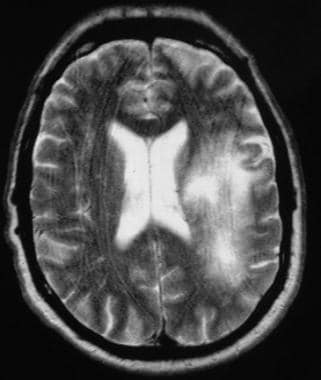 T2-weighted MRI in a patient infected with HIV demonstrates a hyperintense lesion in the left frontoparietal region in the subcortical and periventricular white matter. Biopsy confirmed progressive multifocal leukodystrophy.
T2-weighted MRI in a patient infected with HIV demonstrates a hyperintense lesion in the left frontoparietal region in the subcortical and periventricular white matter. Biopsy confirmed progressive multifocal leukodystrophy.
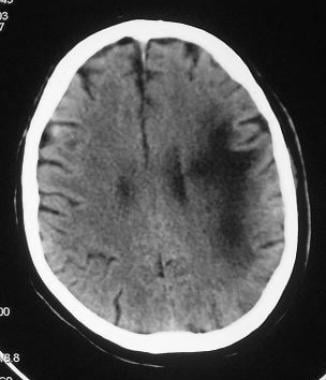 Nonenhanced CT of the head shows a hypoattenuating lesion in the subcortical white matter. Note the characteristic scalloped lateral margin.
Nonenhanced CT of the head shows a hypoattenuating lesion in the subcortical white matter. Note the characteristic scalloped lateral margin.
PML is characterized by 3 cardinal histopathologic features: demyelination, enlarged nuclei of oligodendrocytes, and bizarre astrocytes. [4] This unique cluster is not observed in other neurologic disorders. However, detection of JC virus DNA in cerebrospinal fluid (CSF) by polymerase chain reaction (PCR) and detection of specific lesions by brain MRI are both considered essential for the diagnosis of natalizumab-associated PML in patients with MS. In addition, strict pharmacovigilance by MRI can result in detection of small lesions and undetectable JCV DNA in CSF before a formal diagnosis of PML. [5]
Preferred examination
MRI is the preferred diagnostic imaging modality. It is sensitive to white-matter lesions and shows hyperintense lesions on T2-weighted (T2W) images in affected regions. Because of its superior contrast resolution, it can be used to detect subtle white-matter abnormalities, whereas CT depicts the lesions at an advanced stage. [6, 7, 8, 9] The regular use of MRI imaging in MS patients may be able to detect the onset of PML at a very early stage before patients become clinically symptomatic. [10]
Lesions are found at the gray matter–white matter interface and tend to involve the subcortical white matter. This predilection accounts for the scalloped margins of the lesions. Lesions are initially multiple and discrete, but they eventually may coalesce into large lesions. The lesions may occur anywhere, but they are most often seen in the parieto-occipital and frontal lobes.
Although MRI is more sensitive than CT scanning in the detection of the white-matter lesions of PML, it is contraindicated in patients with a cardiac pacemaker, in those with MRI-incompatible implants, and in those with intraocular metallic foreign bodies.
Prognosis
Natalizumab-associated PML has a mortality of 22% when identified at the time of symptoms, which is lower than that of the HIV-related form, although surviving patients can have significant morbidity. Older patients and those with poorer baseline function have a worse prognosis. MRI has been able to detect PML-related changes 3 to 4 months before development of symptoms. Because prompt detection and treatment of PML in the presymptomatic phase has been shown to improve outcomes, appropriate surveillance of patients taking natalizumab is essential. [2]
The currently known risk of developing PML during treatment with natalizumab is highest for patients with MS who have anti-JCV antibody-positive status, have received any prior immunosuppressant therapy of any duration at any time, and have been treated with natalizumab for 25 months or more. Patients who have all 3 of these risk factors should be closely monitored with MRI for potential signs of PML onset during natalizumab treatment and for 6 months after treatment. [6]
PML is an AIDS-defining illness. Patients whose MRI scans show enhancement, which is rare, and those with an increased CD4 count appear to have a better prognosis than do other patients; these findings probably represent their relatively good immune status.
Computed Tomography
CT scans usually show several bilateral, asymmetric hypoattenuating foci of various sizes without mass effect or enhancement. The lesions may involve the periventricular white matter, subcortical white matter, or both. Subcortical U-fiber involvement results in lesions having a lateral scalloped margin (as seen in the image below) that follows the gray matter–white matter junction. Although lesions may be seen on CT scanning, MRI offers superior sensitivity in the detection and characterization of the lesions. The diagnosis may be suspected with CT scanning, but MRI is needed for a more confident exclusion of other differential considerations. Artifacts, chronic infarcts, and chronic, ischemic white-matter changes may mimic true white-matter lesions of PML.
 Nonenhanced CT of the head shows a hypoattenuating lesion in the subcortical white matter. Note the characteristic scalloped lateral margin.
Nonenhanced CT of the head shows a hypoattenuating lesion in the subcortical white matter. Note the characteristic scalloped lateral margin.
Magnetic Resonance Imaging
MRI has far greater sensitivity than other studies in detecting the lesions of PML and in defining their extent of involvement. On diffusion-weighted images (DWI), lesions can show restricted diffusion, though this is uncommon. The extent of abnormal diffusion appears to be correlated with the speed of clinical progression. Areas with DWI abnormality may correspond to areas that are actively infected by the virus at the time of imaging, but this has been uncommonly reported. The lesions typically do not enhance and do not have mass effect; however, some reports describe lesions with faint peripheral enhancement or diffuse enhancement with mass effect, especially in the early stages. Enhancement could suggest a relatively good immune response and hence an improved prognosis.
On T2-weighted (T2W) images, lesions appear hyperintense and typically involve the periventricular and subcortical white matter, having a characteristic scalloped lateral margin when they involve the subcortical white matter. Lesions are more conspicuously visualized on fluid-attenuated inversion recovery (FLAIR) images, appearing hyperintense against a background of suppressed CSF signal intensity.
Lesions appear hypointense and well demarcated on T1-weighted (T1W) images, though they may be isointense in the initial phase of the disease. Although the disease may involve any part of the brain, lesions typically occur in the parieto-occipital lobes. Lesions appear to start in the subcortical white matter before extending to the periventricular white matter. Mass effect is infrequently described and usually minimal and correlated with shorter survival when seen on initial studies. T1W, T2W, and FLAIR MRI scans are presented below.
 T2-weighted MRI in a patient infected with HIV demonstrates a hyperintense lesion in the left frontoparietal region in the subcortical and periventricular white matter. Biopsy confirmed progressive multifocal leukodystrophy.
T2-weighted MRI in a patient infected with HIV demonstrates a hyperintense lesion in the left frontoparietal region in the subcortical and periventricular white matter. Biopsy confirmed progressive multifocal leukodystrophy.
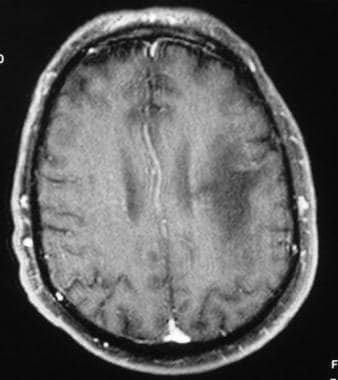 Contrast-enhanced T1-weighted MRI demonstrates a hypointense lesion predominantly in a subcortical, left frontoparietal location. Note the characteristic absence of enhancement and lack of mass effect.
Contrast-enhanced T1-weighted MRI demonstrates a hypointense lesion predominantly in a subcortical, left frontoparietal location. Note the characteristic absence of enhancement and lack of mass effect.
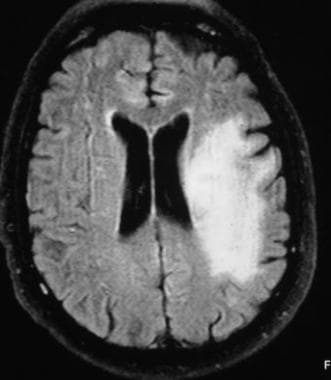 Fluid-attenuated inversion recovery (FLAIR) MRI shows a PML lesion with improved contrast after the suppression of cerebrospinal fluid signal intensity.
Fluid-attenuated inversion recovery (FLAIR) MRI shows a PML lesion with improved contrast after the suppression of cerebrospinal fluid signal intensity.
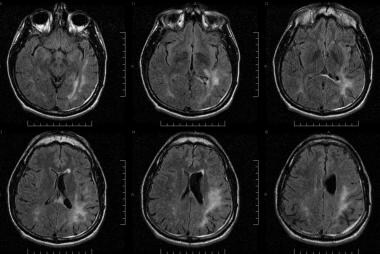 Fluid-attenuated inversion recovery (FLAIR) images in a patient with HIV infection presenting with visual defects, aphasia, and balance problems. Patchy, confluent, and hyperintense lesions are seen in the left occipitotemporoparietal lobes in the subcortical and periventricular white matter. The patient's clinical and radiologic features suggested progressive multifocal leukodystrophy, though cerebrospinal fluid results for the JC virus were negative.
Fluid-attenuated inversion recovery (FLAIR) images in a patient with HIV infection presenting with visual defects, aphasia, and balance problems. Patchy, confluent, and hyperintense lesions are seen in the left occipitotemporoparietal lobes in the subcortical and periventricular white matter. The patient's clinical and radiologic features suggested progressive multifocal leukodystrophy, though cerebrospinal fluid results for the JC virus were negative.
Distribution of lesions
A multifocal distribution pattern is seen. This pattern may be unilateral, but more often, it is bilateral and asymmetric. Lesions may start small, but they eventually enlarge and coalesce. Posterior-fossa involvement is common and is seen in up to one third of patients. Synchronous lesions are usually seen in the supratentorial compartment, though in 10% of patients, lesions are confined to the structures of the posterior fossa. PML may also appear to involve the basal ganglia and deep gray-matter nuclei because of white-matter fibers in these structures. When present, involvement of the gray matter is a secondary finding. In rare cases, lesions are single and can occur anywhere in the brain, including the brainstem.
PML in MS
Early, preferably asymptomatic, detection of PML may lead to more favorable outcomes with respect to survival and functional outcome. However, differentiating PML demyelinated plaques from MS plaques is a diagnostic challenge. Large, confluent, and granular hyperintense T2W lesions and deep gray-matter lesions are more common in patients with PML than in patients with MS. Crescent-shaped lesions in the cerebellar hemispheres suggest PML. Lesions larger than 3 cm are more likely to be associated with PML than MS. Periventricular white-matter lesions and Dawson fingers are common in MS and unexpected in PML. [10]
PML-related immune reconstitution inflammatory syndrome (PML-IRIS) is characterized by an increase in size of preexisting PML lesions. Edema, cerebral swelling, and mass effect may be seen, which are not typical of PML. [2, 10] The most prominent imaging finding is that of contrast enhancement, with variable, irregular, ill-defined contrast enhancement patterns. Punctate enhancement with a perivascular distribution, outside of the main PML lesion, is another sign of PML-IRIS. [2]
Prognostic utility of MRI in PML
Post et al studied the prognostic utility of MRI in PML. None of the MRI variables was predictive of patient survival, with the exception of mass effect, which was usually minimal, infrequent, and associated with decreased survival. Serial MRI studies showed progression of disease in 1 to 24 weeks, and a more rapid change was seen in many patients in just 9 weeks. [9] The authors suggested that findings of increasing atrophy, increasing confluence and extent of white-matter lesions, spread of disease across the corpus callosum, and increasing hypointensity of the lesions on follow-up T1W MRIs may be considered poor prognostic indicators or as failure of response to therapy. Stabilization or a decrease in the size of the lesion, clinical improvement, and loss JC viral detection on CSF PCR testing may indicate a response to therapy. However, improvements on MRI can lag clinical improvement by 2 to 6 months or may even indicate temporary worsening.
In PML patients undergoing highly active antiretroviral therapy (HAART), MRIs can show initial worsening in the first few months followed by stabilization and regression by 12 months. HAART consists of a combination of 3 or 4 anti-HIV drugs from the following classes: nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors.
Thurnher et al reported 2 patients with long-term responses to HAART, apparent increased mass effect and enhancement before eventual improvement, and atrophy of the involved brain parenchyma. [11] T1W MRIs but not FLAIR MRIs showed hypointensity. Two nonresponders initially had extensive changes, without enhancement or mass effect after the start of therapy. Their T1W MRIs also showed hypointense areas that were hyperintense on FLAIR images. This increasing hypointensity on T1W MRIs might have been due to increasing demyelination, which appears hyperintense on FLAIR images. This finding might indicate a worsened prognosis. Increased T1W hypointensity in responders was due to gliosis and necrosis seen in burned-out lesions; this finding is hypointense on FLAIR images.
Berger et al found enhancement in 8.9% of short-term survivors, in contrast to 50% in long-term survivors. Enhancement suggests an improved immune response and hence an improved prognosis. [12]
In a study by Collazos et al, contrast enhancement was seen on images in patients with PML treated with HAART only when their CD4+ count increased. In these patients, the PML lesions changed from enhancing to nonenhancing on follow-up MRI after 3 to 6 months of therapy. [13]
Magnetic resonance spectroscopy
Evaluation of the PML lesions with MR spectroscopy reveals reduced N -acetylaspartate (NAA) and creatine levels, increased choline levels, and an excess of lipids and sometimes of myo-inositol. In some cases, lactate is present. Cell membrane and myelin breakdown is the presumed cause of elevated choline levels; neuronal loss leads to decreased NAA concentrations; and glial-cell proliferation elevates myo-inositol values. The mild elevation of lactate and lipid levels may be due to the activity of macrophages and the breakdown products of myelin.
The elevation of choline and myo-inositol values is seen in the early phase of the disease. In the later phase, all the metabolites are decreased. These metabolic abnormalities are not specific for PML and may be similar in other lesions complicating HIV disease.
Magnetization transfer imaging
PML lesions appear to have strongly reduced magnetization transfer ratios. According to Ernst et al, these features may help in distinguishing lesions from white-matter lesions of HIV leukoencephalopathy. Large prospective studies are needed to assess the utility of newer MR techniques, such as MR spectroscopy and magnetization transfer imaging, in the diagnosis and follow-up of PML. [14]
Degree of confidence
MRI has far greater sensitivity than that of other studies in depicting the lesions of PML and in defining their extent of involvement. Although MRI results may suggest the diagnosis of PML in the appropriate clinical setting, typical features of PML are often nonspecific, and other differential considerations, such as ischemic changes, HIV leukoencephalopathy, and gliotic changes from previous trauma, must be considered. When the lesions of PML are atypically enhancing or when they have mass effect, other lesions, such as those due to toxoplasmosis, lymphoma, or other intracranial masses, should be considered.
The presence of multiple pathologies in immunocompromised conditions adds to the complexity of image interpretation. Infection coexisting with other opportunistic infection may be responsible for some atypical manifestations of the disease and for an apparent response to some form of therapy in some patients.
It is unusual for a normal variant to mimic PML lesions on MRI.
PML must be distinguished from HIV leukoencephalopathy. PML tends to be multifocal, with bilateral, asymmetric, and predominantly subcortical involvement. In comparison, HIV leukoencephalopathy tends to produce lesions that are usually diffuse, bilateral, and symmetric; these predominantly affect the periventricular white matter.
Diffuse cortical atrophy and ventricular dilatation are not predominant findings of PML and may be helpful in distinguishing PML from HIV leukoencephalopathy, which is usually associated with substantial atrophy. Lesions appear well defined and hypointense on T1W images in PML, whereas they appear isointense and poorly defined in HIV leukoencephalopathy. Clinical correlation may be helpful, as patients with PML have progressive focal motor and sensory neurologic deficits, whereas those with HIV leukoencephalopathy present with global cognitive changes and dementia.
-
T2-weighted MRI in a patient infected with HIV demonstrates a hyperintense lesion in the left frontoparietal region in the subcortical and periventricular white matter. Biopsy confirmed progressive multifocal leukodystrophy.
-
Contrast-enhanced T1-weighted MRI demonstrates a hypointense lesion predominantly in a subcortical, left frontoparietal location. Note the characteristic absence of enhancement and lack of mass effect.
-
Fluid-attenuated inversion recovery (FLAIR) MRI shows a PML lesion with improved contrast after the suppression of cerebrospinal fluid signal intensity.
-
Nonenhanced CT of the head shows a hypoattenuating lesion in the subcortical white matter. Note the characteristic scalloped lateral margin.
-
Fluid-attenuated inversion recovery (FLAIR) images in a patient with HIV infection presenting with visual defects, aphasia, and balance problems. Patchy, confluent, and hyperintense lesions are seen in the left occipitotemporoparietal lobes in the subcortical and periventricular white matter. The patient's clinical and radiologic features suggested progressive multifocal leukodystrophy, though cerebrospinal fluid results for the JC virus were negative.







