Practice Essentials
Intestinal polyposis syndromes can be divided, on the basis of histology, into familial adenomatous polyposis (FAP), hamartomatous polyposis syndromes, and other rare polyposis syndromes, such as hereditary-mixed polyposis syndrome and serrated polyposis syndrome. Variants of FAP include Gardner syndrome, Turcot syndrome, and MYH-variant. Hamartomatous polyposis syndromes include Peutz-Jeghers syndrome, PTEN -associated hamartomatous syndromes (eg, Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome), familial juvenile polyposis, and Cronkhite-Canada syndrome. [1, 2, 3, 4, 5, 6]
A GI polyp is defined as a mass of the mucosal surface protruding into the lumen of the bowel (see the images below). Polyps can be neoplastic, nonneoplastic, or submucosal. GI polyposis is characterized by multiple polyps within the GI tract. The role of the radiologist in the diagnosis and evaluation of intestinal polyposis syndromes cannot be overemphasized, as missed polyps are potentially missed cancers. [7, 8] For polyps larger than 1 cm, the sensitivity of single- and double-contrast barium enema (DCBE) examination is 90-95%. DCBE is more sensitive in the detection of polyps smaller than 1 cm.
The prevalence of colorectal cancer in patients with SPS has been estimated to be 15-35%, although smaller studies have reported an incidence as high as 70%. This has led to stringent surveillance recommendations, with multiple guidelines recommending either annual or biennial colonoscopy. [9, 8, 10, 3]
Colorectal screening
According to the American Society of Colon and Rectal Surgeons, polyposis syndromes should typically be considered in patients with more than 20 lifetime adenomas, patients with a personal history of desmoid tumor or other extracolonic manifestations of familial adenomatous polyposis, or family members of individuals with known familial adenomatous polyposis, attenuated FAP, or MYH-associated polyposis. [3]
There are several colorectal screening options for average-risk individuals, including colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, double-contrast barium enema every 5 years, CT colonography every 5 years, and annual fecal occult blood testing. Colonoscopy is the gold standard for screening because it allows for both detection and excision of premalignant lesions. [6]
When combined with a sodium chloride enema technique, ultrasonography can be used to detect colonic polyps as small as 7 mm in 91% of patients. However, the technique is cumbersome and is not widely practiced. At present, this approach cannot replace a barium enema study or colonoscopy. Still, ultrasonography is an invaluable tool in the screening of patients with polyposis syndromes and in the screening of their families for associated cancers, such as those of the thyroid, breast, liver, ovaries, and uterus.
CT and magnetic resonance (MR) colonography (virtual colonoscopy) techniques are being developed for the imaging of colorectal polyps and cancer. [11, 12, 13, 14, 15, 16, 17, 5] The limited data available show that the sensitivity of both CT and MR colonography for polyps larger than 1 cm is 75-90%; however, the detection rate decreases precipitously for smaller polyps. Both CT and MR colonography allow an analysis in both the cross-sectional and virtual endoscopic formats. New developments, such as fecal tagging, are likely to increase the sensitivity of the techniques, and the noninvasive nature of the procedures increase patient acceptability. [18, 19, 20, 21, 22] CT and MR techniques have the added advantage of offering the capability of imaging extraintestinal disease associated with many of the colon polyposis syndromes. [23]
Capsule endoscopy (CE) was introduced over a decade ago and has since been used in the diagnosis of inflammatory disorders of the esophagus, small bowel, and colon; obscure gastrointestinal bleeding; complicated celiac disease; Crohn disease; and polyposis syndromes. Neumann and associates had reviewed the current applications and future prospects of imaging with CE. CE has proven its efficacy in multiple studies, which have shown that CE for these interventions is superior to radiologic interventions and push enteroscopy. With positive CE findings, balloon-assisted endoscopy offers the potential to apply targeted biopsies or therapy. [24]
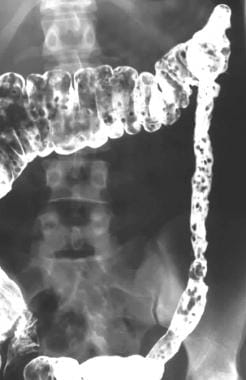 Colon, polyposis syndromes. Polyposis coli. Postevacuation image obtained after double-contrast barium enema study shows extensive polyposis of the colon.
Colon, polyposis syndromes. Polyposis coli. Postevacuation image obtained after double-contrast barium enema study shows extensive polyposis of the colon.
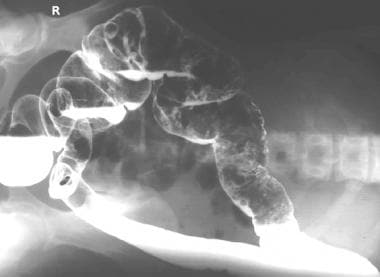 Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows multiple polyps in the transverse and descending colon.
Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows multiple polyps in the transverse and descending colon.
Nuclear medicine has no role in the diagnosis of colonic polyps. However, associated abnormalities in some of the polyposis syndromes lend themselves to radionuclide imaging. [25] One such disorder is Gardner syndrome, in which the osseous lesions, thyroid cancer, and small-bowel carcinoids can be imaged with radionuclides.
There are new endoscopic imaging technologies being studied that promise to generate images of the cellular structure of polyps. These are known as optical biopsy or virtual histology and include confocal laser endomicroscopy, optical coherence tomography, multiphoton microscopy, Raman spectroscopy, hyperspectral imaging, and molecular imaging. [5]
Inherited and nonfamilial polyposis syndromes
Polyposis syndromes may be classified as familial inherited (autosomal dominant) or nonfamilial. The inherited polyposis syndromes are further subdivided into 2 groups, depending on whether the polyps are adenomas or hamartomas. The adenomatous polyposis syndromes include the classic familial adenomatous polyposis (FAP), Gardner syndrome, and Turcot syndrome. Hamartomatous familial polyposis syndromes include Peutz-Jeghers syndrome, juvenile polyposis syndrome, Cowden disease, and Ruval-Caba-Myhre-Smith syndrome. The noninherited polyposis syndromes include Cronkhite-Canada syndrome and a variety of miscellaneous nonfamilial polyposis.
Serrated polyposis syndrome (SPS) is the most prevalent polyposis syndrome and characterized by numerous colonic serrated polyps. Although SPS is believed to be hereditary, it is not associated with a single gene mutation. SPS is diagnosed if at least one of the following World Health Organization (WHO) criteria is met [10] :
-
At least 5 serrated polyps proximal to the sigmoid, with 2 or more of these being ≥10 mm.
-
Any number of serrated polyps proximal to the sigmoid in an individual who has a first-degree relative with SPS.
-
20 or more serrated polyps of any size, distributed throughout the colon .
From a prognostic viewpoint, these syndromes must be recognized, because the adenomatous polyps are premalignant. These syndromes should be considered when an intestinal polyp is recognized in the young, when 2 or more polyps are seen in any patient, when colon carcinoma is discovered in patients younger than 40 years, and when extraintestinal manifestations associated with these syndromes are discovered.
GI polyps may be asymptomatic, but they may also occur with rectal bleeding and diarrhea. The urgency of case tracing and genetic counseling is related not so much to the symptoms of the disease but to the potential for the development of a colon carcinoma. It is probable that patients with familial polyposis, if untreated, will develop a colon carcinoma (see the images below). [26, 27, 28]
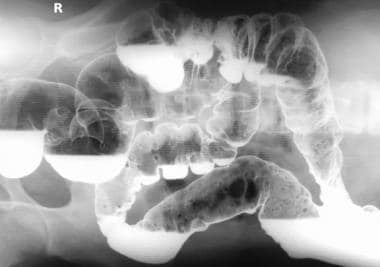 Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows numerous small polyps in the transverse and descending colon.
Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows numerous small polyps in the transverse and descending colon.
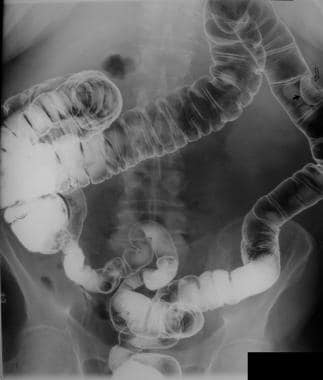 Colon, polyposis syndromes. Polyposis coli. Double-contrast enema study in a man with a family history of familial colonic polyposis shows a solitary polyp with malignant change.
Colon, polyposis syndromes. Polyposis coli. Double-contrast enema study in a man with a family history of familial colonic polyposis shows a solitary polyp with malignant change.
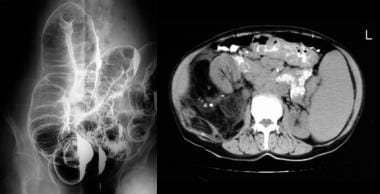 Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Left lateral decubitus image from a double-contrast barium enema study shows numerous polyps throughout the colon. Right, Contrast-enhanced CT scan through the upper abdomen shows massive splenomegaly, retroperitoneal lymphadenopathy, and multiple polypoid lesions in the transverse colon; these distort the colonic mucosa. The histologic diagnosis was a mantle lymphoma.
Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Left lateral decubitus image from a double-contrast barium enema study shows numerous polyps throughout the colon. Right, Contrast-enhanced CT scan through the upper abdomen shows massive splenomegaly, retroperitoneal lymphadenopathy, and multiple polypoid lesions in the transverse colon; these distort the colonic mucosa. The histologic diagnosis was a mantle lymphoma.
Limitations of techniques
The false-negative rate for the detection of polyps smaller than 1 cm is 7% with double-contrast barium enema. Small sessile polyps are easily missed. Interpretive problems may occur because of overlapping bowel loops, air bubbles, spasm, diverticula, a poorly prepared bowel, and barium flooding. Diverticula impacted with feces, lobulated diverticula seen en face, and edema of the diverticular neck caused by inflammatory changes can also mimic polyps and contribute to the false-positive rates.
The screening and surveillance of patients with many of the colonic polyposis syndromes include prolonged observation; barium enema studies are less acceptable for surveillance because of concerns about radiation exposure.
Ultrasonography remains operator dependent. Colonic and bowel gas and obesity degrade sonograms. The presence of feces and mucus also prevents the identification of colonic polyps. An assessment of the bowel thickness at the base of a polyp is important in identifying malignant transformation; however, this cannot always be evaluated by using the sodium chloride enema technique. Polyps smaller than 7 mm can be missed during ultrasonographic examination.
The main limiting factor for interpreting CT colonograms is time spent on reading the scans. For virtual colonoscopy to be a clinically feasible tool, the examination must be performed and the results interpreted in a time-efficient manner. For example, a large cohort of patients underwent both 2-dimensional (2D) and 3-dimensional (3D) imaging with antegrade and retrograde 3D navigation of the colon. Patients were in both the supine and prone positions. The median interpretation time for 2 radiologists was 31 minutes (2D imaging) and 27 minutes (3D imaging), respectively. The sensitivity of CT for polyps 10 mm or larger was over 90%. [29, 30, 31]
The time issue is especially a problem with multisection CT colonography, with which nearly 1000 images can be obtained per patient, depending on the section collimation and degree of overlap.
MR colonography shares several limitations with its CT counterpart. Inadequate colon distention, contrast opacification, and the presence of air bubbles and fecal masses may potentially cause problems in interpretation. [30, 32, 31, 33, 34, 35]
None of the above techniques are useful in differentiating hamartomatous polyps from adenomatous polyps.
Radiograph
With double-contrast barium enema (DCBE), the features common to all the colon polyposis syndromes is the finding of multiple (usually 10 or more) polyps in the colon. The appearances of polyps on a DCBE study are dependent on the angle at which they are viewed and their relationship to the barium pool. When viewed en face, wide-based or sessile polyps exhibit the meniscus sign. This sign is a clearly defined inner border, which represents the base of the polyp; this fades into a less clearly defined outer border, which represents normal mucosa. When viewed tangentially or obliquely, both sessile and intermediate polyps can look like a bowler hat because the meniscus at the base and barium covering the surface of the polyp are seen. The polyps exhibit features suggestive of a pedunculated form.
(See the images below.)
 Colon, polyposis syndromes. Polyposis coli. Postevacuation image obtained after double-contrast barium enema study shows extensive polyposis of the colon.
Colon, polyposis syndromes. Polyposis coli. Postevacuation image obtained after double-contrast barium enema study shows extensive polyposis of the colon.
 Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows multiple polyps in the transverse and descending colon.
Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows multiple polyps in the transverse and descending colon.
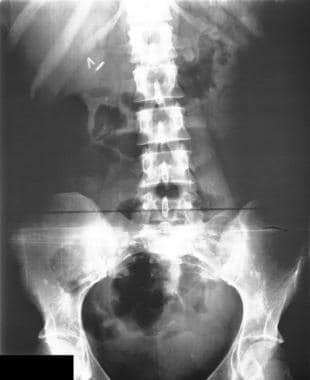 Colon, polyposis syndromes. Polyposis coli. Plain abdominal radiograph in a patient known to have Gardner syndrome shows multiple, small osteomas in the pelvis.
Colon, polyposis syndromes. Polyposis coli. Plain abdominal radiograph in a patient known to have Gardner syndrome shows multiple, small osteomas in the pelvis.
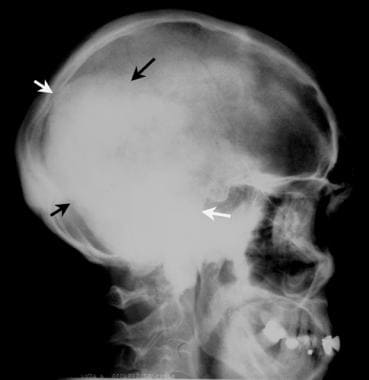 Colon, polyposis syndromes. Polyposis coli. Plain lateral skull radiograph in a patient with known Gardner syndrome shows a large osteoma in the occipital region (arrows).
Colon, polyposis syndromes. Polyposis coli. Plain lateral skull radiograph in a patient with known Gardner syndrome shows a large osteoma in the occipital region (arrows).
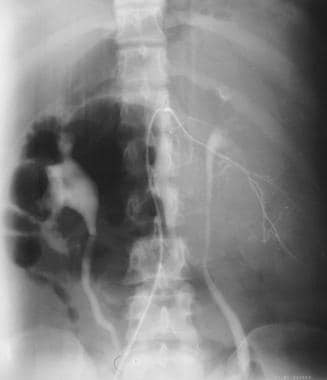 Colon, polyposis syndromes. Polyposis coli. Selective lumber arteriogram shows that the desmoid tumor derives its blood supply from the lumber artery.
Colon, polyposis syndromes. Polyposis coli. Selective lumber arteriogram shows that the desmoid tumor derives its blood supply from the lumber artery.
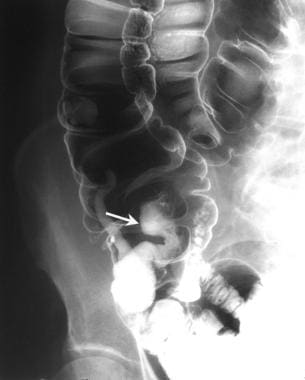 Colon, polyposis syndromes. Polyposis coli. Image shows a large polyp in the cecum on a stalk in the adult-type of juvenile polyposis. Histologically, the polyp was hamartomatous.
Colon, polyposis syndromes. Polyposis coli. Image shows a large polyp in the cecum on a stalk in the adult-type of juvenile polyposis. Histologically, the polyp was hamartomatous.
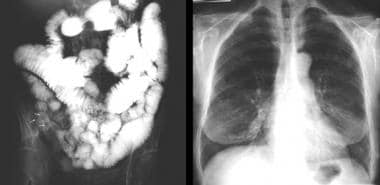 Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Chest radiograph of a woman known to have neurofibromatosis 1 (NF1) shows bilateral basal pulmonary fibrosis. Note the skin neurofibromas. Right, Barium follow-through study in the same patient shows multiple polyps within the cecum; these represent neurofibromas. The neurofibromas were confirmed during colonoscopy.
Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Chest radiograph of a woman known to have neurofibromatosis 1 (NF1) shows bilateral basal pulmonary fibrosis. Note the skin neurofibromas. Right, Barium follow-through study in the same patient shows multiple polyps within the cecum; these represent neurofibromas. The neurofibromas were confirmed during colonoscopy.
The target sign refers to the appearance of intermediate and pedunculated polyps seen en face. Adenomatous polyps can present as sessile, intermediate, or pedunculated forms. Hamartomatous polyps are commonly pedunculated; therefore, they can be large. Metaplastic polyps are often small and are most commonly sessile. Malignancy is suggested when polyps have an irregular, lobulated surface; when the base is wider than the height; and when the base of the polyp is retracted.
Patients with FAP coli who present early in the course of disease may have more than 100 sessile adenomas measuring 1-5 mm and occurring throughout the whole length of the colon. Later in the disease process, contrast-enhanced examination usually reveals sessile and pedunculated adenomas that are 0.5-1 cm or larger. Malignant transformation in larger adenomas may be seen.
The radiologic features in the colon are identical to those of Gardner syndrome, which is regarded as a variant of FAP with important extracolonic manifestations. These manifestations rarely include multiple osteomas of the skull and mandible, though these can be seen in any bone. In young persons, the symptoms of osteomas and other extracolonic manifestations often prompt an investigation of the large bowel.
In Turcot syndrome, the adenomatous polyps are fewer in number, lthough as many as 100 may be present, and they may be as large as 3 cm. The polyps of Peutz-Jeghers syndrome are hamartomatous, and they more commonly affect the small intestine and stomach than the colon. Their size varies from 0.1 to 5 cm in diameter. [36] As mentioned, the polyps may appear solitary, or they may be few in number, large, and pedunculated. This morphology commonly affects the colon; the lesions often become eroded, which may be seen on contrast-enhanced examination. More commonly, multiple, small, sessile polyps resembling those of FAP carpet the small intestine; this is demonstrated on barium follow-through study and small-bowel enteroclysis.
Degree of confidence
Because of the appearances of polyposis syndromes in the colon, there is often little doubt regarding the nature of the lesions, as demonstrated on optimal double-contrast examination in a well-prepared bowel. In young persons, who often have extracolonic manifestations of disease, multiplicity of polyps aids the diagnosis.
The radiographic detection of colonic polyps depends on the size of the lesion and the type of examination. For polyps measuring 1 cm or larger, the sensitivity of single-contrast barium enema and double-contrast barium enema (DCBE) studies is 90-95%. DCBE is more sensitive in the detection of polyps smaller than 1 cm. The false-negative rate for the detection of polyps smaller than 1 cm in size is 7% with DCBE study. These radiographic sensitivities for the detection of polyps larger than 1 cm are similar to those of colonoscopy.
False positives/negatives
Polyps may not be detectable for many reasons. Small, sessile polyps smaller than 1 cm can be missed. Overlapping loops of bowel, air bubbles, spasm of the bowel, poor image quality, diverticula, poor bowel preparation (with feces or a wet colon), and excess barium can all compound the problem.
Diverticula can be particularly troublesome. Diverticula impacted with feces, lobulated diverticula seen en face, and edema of the diverticular neck caused by inflammatory changes can all mimic polyps. However, when multiple polyps are present, as in most of the polyposis syndromes, the diagnosis should rarely be missed. Double reporting of examination results aids in the diagnosis.
CT SCAN
Conventional CT
Conventional CT may show colonic polyps as intraluminal filling defects that cause deformation of the contrast material–filled lumen. Conventional abdominal CT is also a valuable tool in planning surgery for colon cancer, which can be a complication of colonic polyposis. On CT scans, colon cancer appears as a soft tissue–attenuating mass that narrows the colonic lumen. Colon cancer can also appear as focal bowel thickening and luminal narrowing. CT can also readily depict complications of colon cancer, such as obstruction, perforation and fistula formation, and liver, lymph node, and other distant metastases. Extraluminal spread is depicted as the loss of fat planes between the colon and adjacent organs (see the images below). [37, 38, 39, 40]
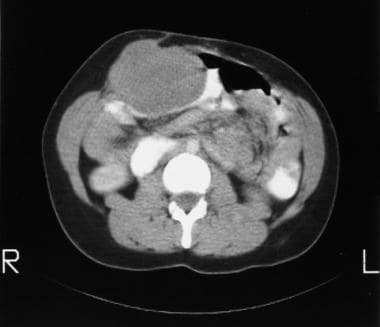 Colon, polyposis syndromes. Polyposis coli. Contrast-enhanced axial CT scans through the midabdomen in a 36-year old woman who had previously undergone total colectomy for Gardner syndrome. Image shows a large desmoid tumor, which is compressing a loop of small bowel. The tumor was resected.
Colon, polyposis syndromes. Polyposis coli. Contrast-enhanced axial CT scans through the midabdomen in a 36-year old woman who had previously undergone total colectomy for Gardner syndrome. Image shows a large desmoid tumor, which is compressing a loop of small bowel. The tumor was resected.
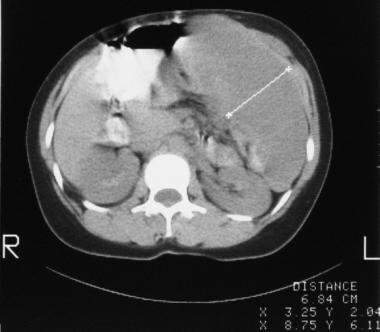 Colon, polyposis syndromes. Polyposis coli. Contrast-enhanced axial CT scans through the midabdomen in the same patient as in the previous images obtained 1 year later shows recurrence of the large, left-sided desmoid tumor.
Colon, polyposis syndromes. Polyposis coli. Contrast-enhanced axial CT scans through the midabdomen in the same patient as in the previous images obtained 1 year later shows recurrence of the large, left-sided desmoid tumor.
 Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Left lateral decubitus image from a double-contrast barium enema study shows numerous polyps throughout the colon. Right, Contrast-enhanced CT scan through the upper abdomen shows massive splenomegaly, retroperitoneal lymphadenopathy, and multiple polypoid lesions in the transverse colon; these distort the colonic mucosa. The histologic diagnosis was a mantle lymphoma.
Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Left lateral decubitus image from a double-contrast barium enema study shows numerous polyps throughout the colon. Right, Contrast-enhanced CT scan through the upper abdomen shows massive splenomegaly, retroperitoneal lymphadenopathy, and multiple polypoid lesions in the transverse colon; these distort the colonic mucosa. The histologic diagnosis was a mantle lymphoma.
CT colonography (virtual CT colonoscopy)
CT colonography typically refers to the evaluation of a cleansed and gas-distended colon to detect polyps and masses. A low-dose CT is performed in both the supine and prone positions. The images are interpreted using specialized software by viewing the axial, multiplanar reconstruction (MPR), and 3D endoluminal images.
A strategy of stool opacification with barium and fluid opacification with water-soluble contrast may also be used. The stool-opacified images may be viewed in axial and multiplanar reconstructed views. When stool and fluid opacification is used, an electronic subtraction program may be applied to permit a primary 3D or 2D interpretation of the subtracted images. Some investigators suggest using intravenous contrast in one view, particularly if there is a large amount of fluid that could hide polyps (even with the patient scanned both supine and prone) or when investigating a patient with a known obstructing carcinoma whose colonoscopy was incomplete.
Thorough colonic cleansing is required to eliminate false-positive scans caused by fecal residue. Dry preparation by using sodium chloride cathartics is preferred to a colonic lavage, to minimize the retention of fluid that might hide polyps. After colonic cleansing, the patient is placed in the left lateral decubitus position on the CT table. An endorectal tube is passed, and the large bowel is insufflated with air or carbon dioxide using either manual insufflation or a mechanical pump.
To minimize respiratory motion, a fast scanner is needed. Multidetector-row CT is the preferred imaging modality, but electron-beam CT also can be used. Even single-section helical CT can be used, but it may require multiple breath holds to scan the abdomen and pelvis. A CT scanogram is first obtained to allow the optimal areas to be covered and also to ensure that optimal distention of the colon has been achieved.
With multisection imaging, the typical CT parameters include a 4 × 1-mm section detector configuration, 120 kV, 0.5-second gantry rotation, and effective 50 mAs. A variable pitch between 6 and 7 is used so that the entire abdomen and pelvis can be covered within a 30-second breath-hold. This imaging results in 12 and 14 mm of coverage per second. If helical CT is used, satisfactory images can be obtained by using 5-mm beam collimation, a reformatting index of 2 mm, an acquisition time of 1 second per revolution, a pitch of 1.25, 110 kVp, 200 mAs, and the smallest field of view to fit a 512 × 512 matrix. Most investigators do not recommend a beam collimation above 3 mm.
CT images are reconstructed as 1.25-mm-thick sections with a 1-mm reconstruction interval. Scans are usually obtained with the patient in both the supine and prone positions to obtain optimal views of all colonic segments by moving fluid, stool, and gas to facilitate the differentiation of feces and polyps. The images are networked to a workstation, where data interpretation can proceed. A wide variety of surface and volume-rendering packages are now commercially available. These can be used to obtain the virtual colonoscopic fly-through on a workstation.
The optimal evaluation of CT colonograms requires access to supine and prone images and 2D, 3D, and MPR images. Several workstations allow axial supine and prone images to be displayed adjacent to each other. The ability to quickly change window and/or level settings from wide to narrow facilitates data interpretation.
One major drawback of reading the 2D, MPR, and 3D reconstructed images is the time required to interpret them. However, as experience with 2D imaging as a primary interpretation technique has increased, the time to evaluate colonographic data has decreased. The reason for this is that, with experience, confidence in differentiating bulbous folds and fecal material from polyps increases; therefore, the utility of MPR and 3D is confined to problem solving.
The interpretation of primary 2D images requires an evaluation of the entire colon. The process is facilitated by access to a workstation that allows a rapid cine function so that scrolling through the colon is easy. Because the colon is a tortuous and rather redundant organ, scrolling should be performed so that the entire colon is evaluated.
A 12% incidence of incidental extracolonic findings is reported, even when low dose CT is used and intravenous contrast is not used. [41, 42, 43, 44, 45, 46, 47, 48, 49]
Degree of confidence
Three-dimensional virtual colonoscopic techniques have the appeal of truly simulating conventional colonoscopy. Several commercial workstations are incorporating an automated center line that allows the computer to automatically generate images and make cine images of the colon traversing this center line. With improvements in the software, one can then navigate through the colon and evaluate suspicious abnormalities.
Several improvements in CT colonographic technique have increased its diagnostic performance. These improvements include the abilities to maximize colonic distention, to obtain data in the supine and prone positions, and to perform CT colonography in patients with well-prepared clean colons.
Virtual colonoscopy does not yet compete with colonoscopy in the demonstration of small colonic polyps, although one trial by Pickhardt et al using stool/fluid opacification and electronic subtraction with a primary 3D read achieved results comparable to optical colonoscopy for 1-cm polyps. [50] CT is not the modality of choice for examining the colon in polyposis syndromes. With good oral preparation and gaseous distention, CT colonography with scanning in the prone and supine positions reliably depicts larger polyps.
Polyps smaller than 1 cm may not be detected, even with prone and supine scanning. CT has good sensitivity for the detection of clinically important polyps. CT may be helpful in less mobile and/or older persons, as well as those in whom malignant transformation is suspected; therefore, it is a useful adjunct to a contrast-enhanced examination.
Although CT colonography is a relatively noninvasive imaging procedure, some aspects of the procedure may produce some anxiety and potential discomfort for patients; examples include bowel preparation and colonic insufflation. Most studies have shown a small to significant patient preference for CT colonography over optical colonoscopy, particularly when patients are asked how frequently they would be willing to be reevaluated at various intervals.
Performing both supine and prone imaging doubles the patient radiation dose. However, this is essential to optimize bowel distention; redistribution of residual fluid; and differentiation of fecal material from polyps, since visualization of mobility of a filling defect implies residual fecal material. With high contrast at the air-mucosa interface, the milliampere-second setting can be sufficiently reduced to limit the patient radiation dose to levels comparable to that of a barium enema study or less. Ongoing research continues on the use of very low doses (20-50 mAs).
False positives/negatives
Adequate bowel preparation is absolutely vital for confident detection of significant lesions because residual fecal material may be indistinguishable from polyps or neoplasms, or it may obscure polyps, making their detection impossible.
Other problems in detection of polyps with CT colonoscopy arise with small, sessile polyps (< 1 cm). Distinguishing polyps from haustral folds can also be difficult; thus, a clean, well-distended colon visualized prone and supine is important. Flat polyps, flat cancers, mobile pedunculated polyps, and mobile loops of colon can cause false-negative results. A review of the images in a soft tissue window/level setting helps detect foci of wall thickening.
With workstations incorporating a center path line, problems are encountered when segments of the colon are not well distended and the center line cannot be generated. Also, in overdistended bowel segments, the center line may jump to an adjacent distended loop. Problems with the workstation may also occur with a 3D-viewing facility, which does not always ensure that the entire colonic surface is evaluated.
Further limitations of both primary 2D and primary 3D techniques are that they cannot be used as the sole technique for image interpretation. Use of the 3D technique alone may lead to many false-positive results. Just as 3D imaging and MPR are used for problem solving when 2D imaging is the primary interpretation technique, 2D imaging must be used as a problem-solving method for 3D imaging. This is to aid in the evaluation of the attenuation characteristics of a lesion and to determine whether a filling defect is a mural abnormality or an extrinsic defect.
Often, lesions are detected by using 3D CT colonographic techniques that demonstrate morphology consistent with a polyp or neoplasm. When these same areas are investigated with 2D CT, a variety of normal structures may be found, including fecal material and extrinsic defects.
Finally, the amount of time for data evaluation with these 3D techniques may limit its usefulness in a clinical setting. Primary 3D imaging techniques need to become quicker and more automated, with easier navigation, before they can become a primary viewing technique. However, given these limitations, polyps smaller than 5 mm can be routinely detected by using 3D endoluminal imaging in both antegrade and retrograde fashion.
One study involved the use of axial images, as well as complete 3D endoluminal navigation in antegrade and retrograde directions in both the supine and prone position. The detection of polyps smaller than 5 mm was 59%. This rate compares favorably to a report in which only 2D imaging was used as the primary data interpretation technique. Another study showed that 68% of polyps 5 mm or smaller that were missed with a primary 2D technique were either hyperplastic or normal colon, as determined at pathology.
MRI
GI polyps in patients with polyposis syndrome may be visualized on MRIs by using breath-hold T1-weighted and breathing-independent snapshot T2-weighted techniques.
Semelka and Marcos described MR findings in a small series of patients with GI polyposis syndromes. [51] The study included 6 patients: 3 with FAP, 1 with Peutz-Jeghers syndrome, 1 with Gardner syndrome, and 1 with neurofibromatosis. The investigators used breath-hold T1-weighted sequences, both standard and with fat suppression, before and after the administration of gadolinium-based contrast agent. They also performed breath-independent single-shot half-Fourier rapid acquisition with relaxation enhancement (RARE) T2-weighted sequences. In all patients, polypoid lesions exhibited signal intensity comparable to that of bowel on nonenhanced images, and they showed enhancement similar to that of bowel on early and late enhanced images. Polyp enhancement on gadolinium-enhanced images is an important finding that should distinguish polyps from bowel contents.
Polyps larger than 2 cm were observed in 1 patient with FAP and in the patient with Gardner syndrome. Both these polyps showed mild heterogeneity on late gadolinium-enhanced fat-suppressed images. Multiple colonic polyps ranging in size from 5 mm to 3 cm were observed in 1 patient with FAP. A solitary polyp associated with enteroenteric intussusception was observed in the patient with Peutz-Jeghers syndrome. Gastric polyps measuring 5 mm to 6 cm were observed in Gardner syndrome; 1- to 2-cm polyps were found within the duodenum and jejunum in the patient with neurofibromatosis. [51]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Virtual MR colonoscopy
Studies have shown that MR-based endoluminal assessment of the colon is possible. Patient preparation is similar to that with CT colonoscopy. After thorough cleansing of the colon, the patient is positioned prone on the imaging table, and the colon is filled with 1500-2000 ml of water and 15-20 ml of 0.5 M gadopentetate dimeglumine via an endorectal tube. Colonic filling is monitored by means of a 2D spoiled gradient-echo sequence with the acquisition of 1 image every 2 seconds. When the entire colon is filled with contrast agent, a 3D Fourier transform gradient-echo sequence is used to image the colon. As with CT colonoscopy, the images are transferred to a workstation and interpreted in both multiplanar reformatted and virtual colonoscopic views.
Degree of confidence
As with CT, virtual MR colonoscopy does not yet compete with colonoscopy for the demonstration of small colonic polyps. The overall experience with the technique is limited. However, the lack of adverse effects and the lack of ionizing radiation warrant further consideration of MR colonography as a tool for GI polyp screening. The limited experience so far suggests that polyps as small as 6 mm in size can be assessed, as can the inner wall itself
Inadequate colonic distention, contrast opacification, and air bubbles and fecal masses may present potential problems in the interpretation of the images.
Ultrasound
Ultrasonography is insensitive in the diagnosis of colonic polyps. However, with malignant transformation when the bowel wall is infiltrated, bowel wall thickening may be seen. If the bowel wall is thickened, compressed, or obliterated, a carcinoma should be suspected.
A target or an atypical target sign is generally seen with asymmetric, hypoechoic thickening of the bowel wall. These occur in association with a central echogenic area caused by the presence of intraluminal air and mucus.
The colon may be sonographically examined for polyps or colon carcinoma by means of the retrograde instillation of fluid (generally warm sodium chloride solution) into the colon. A warm isotonic sodium chloride enema may improve visualization of the rectosigmoid. Real-time examination of the colon can be performed with this technique.
Colonic polyps appear as hyperechoic structures projecting into the lumen of the colon. Endorectal ultrasonography can be used in the assessment of rectal carcinoma. [52, 53, 54, 55]
Degree of confidence
With the sodium chloride enema technique, colonic polyps larger than 7 mm can be identified in 91% of patients. However, at present, ultrasonography cannot replace a barium enema study or colonoscopy for the detection of colonic polyps. Still, sonography is an invaluable tool in the screening of patients with polyposis syndromes and in the screening of their families for associated cancers, such as those of the thyroid, breast, liver, ovaries, and uterus.
False positives/negatives
Colonic polyps may be difficult to identify on sonograms obtained with the patient in the supine position because air normally collects anteriorly, causing distal acoustic shadowing that obscures the field. Moreover, the presence of feces and mucus also prevents the identification of colonic polyps. Adequate assessment of the bowel thickness at the base of the colonic polyp may not be possible by using the saline enema technique. Also, polyps smaller than 7 mm usually cannot be identified by using the sodium chloride enema method.
-
Colon, polyposis syndromes. Polyposis coli. Postevacuation image obtained after double-contrast barium enema study shows extensive polyposis of the colon.
-
Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows multiple polyps in the transverse and descending colon.
-
Colon, polyposis syndromes. Polyposis coli. Left lateral decubitus image obtained as part of a barium enema study shows numerous small polyps in the transverse and descending colon.
-
Colon, polyposis syndromes. Polyposis coli. Plain abdominal radiograph in a patient known to have Gardner syndrome shows multiple, small osteomas in the pelvis.
-
Colon, polyposis syndromes. Polyposis coli. Plain lateral skull radiograph in a patient with known Gardner syndrome shows a large osteoma in the occipital region (arrows).
-
Colon, polyposis syndromes. Polyposis coli. Contrast-enhanced axial CT scans through the midabdomen in a 36-year old woman who had previously undergone total colectomy for Gardner syndrome. Image shows a large desmoid tumor, which is compressing a loop of small bowel. The tumor was resected.
-
Colon, polyposis syndromes. Polyposis coli. Contrast-enhanced axial CT scans through the midabdomen in the same patient as in the previous images obtained 1 year later shows recurrence of the large, left-sided desmoid tumor.
-
Colon, polyposis syndromes. Polyposis coli. Selective lumber arteriogram shows that the desmoid tumor derives its blood supply from the lumber artery.
-
Colon, polyposis syndromes. Polyposis coli. Image shows a large polyp in the cecum on a stalk in the adult-type of juvenile polyposis. Histologically, the polyp was hamartomatous.
-
Colon, polyposis syndromes. Polyposis coli. Double-contrast enema study in a man with a family history of familial colonic polyposis shows a solitary polyp with malignant change.
-
Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Chest radiograph of a woman known to have neurofibromatosis 1 (NF1) shows bilateral basal pulmonary fibrosis. Note the skin neurofibromas. Right, Barium follow-through study in the same patient shows multiple polyps within the cecum; these represent neurofibromas. The neurofibromas were confirmed during colonoscopy.
-
Colon, polyposis syndromes. Polyposis coli, differential diagnosis. Left, Left lateral decubitus image from a double-contrast barium enema study shows numerous polyps throughout the colon. Right, Contrast-enhanced CT scan through the upper abdomen shows massive splenomegaly, retroperitoneal lymphadenopathy, and multiple polypoid lesions in the transverse colon; these distort the colonic mucosa. The histologic diagnosis was a mantle lymphoma.








