Practice Essentials
In polycystic ovary syndrome (PCOS), enlarged ovaries with thickened sclerotic capsules and an abnormally high number of follicles are present. The follicles may concurrently exist in varying states of growth, maturation, or atresia. [1, 2, 3] PCOS is considered the most common endocrine disorder in the world in women of reproduction age. [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] In a study of follicular and endocrine characteristics of anovulatory and sporadic ovulatory cycles in women with PCOS, follicular excess, or increased follicle number, was observed on ultrasonography across anovulatory and sporadic ovulatory cycles in patients with PCOS. [1]
There are many associated morbidities with PCOS, including infertility, metabolic syndrome, obesity, impaired glucose tolerance, type 2 diabetes mellitus, cardiovascular risk, depression, obstructive sleep apnea, endometrial cancer, and nonalcoholic fatty liver disease/ nonalcoholic steatohepatitis. [15]
The American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society have published best practices for evaluating and treating PCOS. They state that the diagnosis of PCOS is based on the presence of at least 2 of the following 3 criteria: chronic anovulation, hyperandrogenism (clinical or biological), and polycystic ovaries. They note that ultrasonography now allows diagnosis of PCOM in patients with at least 25 small follicles (2-9 mm) in the whole ovary, and ovarian size of 10 ml is the threshold between normal and increased ovary size. [13, 14]
PCOS is usually diagnosed based on the Rotterdam criteria if 2 of 3 criteria are present: (1) oligo- and/or anovulation, (2) hyperandrogenism (HA) (clinical and/or biochemical), and (3) polycystic ovary morphology (PCOM) on ultrasonography (either 12 or more follicles measuring 2-9 mm in diameter and/or an increased ovarian volume >10 cm3). Irregular menstruation is defined as less than 21 days or more than 35 days or fewer than 8 cycles per year. Clinical HA includes hirsutism, acne, or alopecia. Biochemical HA typically refers to an elevated serum testosterone level. [16, 17, 18, 19, 20]
Although a multiplicity of clinical presentations exists for polycystic ovary disease, in 1935, Stein and Leventhal reported the classic symptomatology in a group of women who had amenorrhea, infertility, hirsutism, and enlarged polycystic ovaries. [21] The authors found that, after ovarian biopsy, the women began to menstruate regularly. As was discovered over time, women may have polycystic ovaries, yet their cases may not conform to all of the original criteria for this condition. Therefore, Stein-Leventhal syndrome became a subgroup of a more encompassing disease called polycystic ovary disease.
Evidence suggests that the underlying disorder of polycystic ovary disease is insulin resistance, with the elevated insulin levels stimulating excess ovarian androgen production. In such cases, there is a predispositon for type 2 diabetes mellitus and cardiovascular disease in later life. Metformin may be used in specific cases of polycystic ovary disease. [22, 23]
In adolescent girls, large, multicystic ovaries are a common finding. For adolescents in whom diagnosis of PCOS remains uncertain after clinical and laboratory evaluation, MRI may be considered as a diagnostic imaging modality. [13, 14, 24, 25] Many adults with PCOS present with pathognomonic symptoms as adolescents, making early diagnosis valuable because of the associated long-term metabolic and reproductive health sequalae. [26, 27]
(See the images of polycystic ovary disease below.)
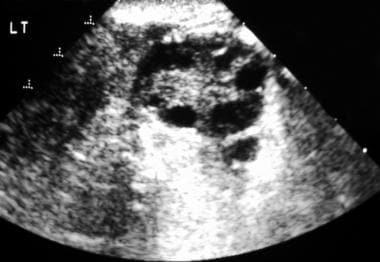 Transverse endovaginal sonogram of the left ovary. This image exhibits numerous peripheral follicles and hyperechoic stroma. Note that none of the follicles is larger than 1.2 cm.
Transverse endovaginal sonogram of the left ovary. This image exhibits numerous peripheral follicles and hyperechoic stroma. Note that none of the follicles is larger than 1.2 cm.
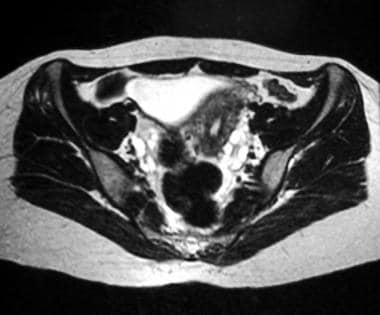 Axial T2-weighted magnetic resonance image of the pelvis. This image reveals multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the left side on this image.
Axial T2-weighted magnetic resonance image of the pelvis. This image reveals multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the left side on this image.
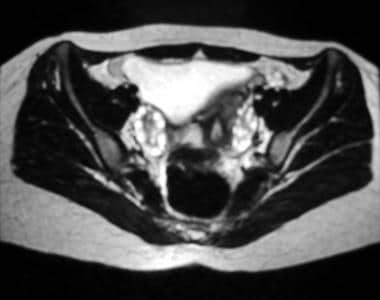 Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the right side on this image.
Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the right side on this image.
As more information regarding the nature of the condition has come to light, other terms have been applied, including polycystic ovarian/ovaries syndrome and polyfollicular ovarian disease. In actuality, polycystic ovaries are not the primary cause of amenorrhea or hirsutism in this condition. Rather, they are simply one sign of an underlying endocrinologic disorder that ultimately results in anovulation.
Shah et al proposed that premenarcheal girls presenting with ovarian torsion without obvious ovarian pathology be screened for ultrasound and biochemical evidence of polycystic ovary syndrome. In a retrospective observational case series, the authors studied PCOS in 6 premenarcheal adolescents and 6 adults with unexplained ovarian torsion. The authors suggested that, in those with evidence of PCOS, treatment with oral contraceptives be considered, taking into account age and pubertal development, to decrease ovarian volume. [28]
According to the Rotterdam consensus and Androgen Excess & PCOS Society, ultrasound criteria consist of the presence of 12 or more follicles within the ovary, with a diameter of 2-9 mm and/or ovarian volume 10 cm3 or greater. [29]
Imaging modalities
Polycystic ovaries are most often diagnosed by means of laboratory studies. The initial screening tests may include determinations of the blood serum levels of thyroid-stimulating hormone (TSH), FSH, LH, and prolactin (PL). The ratio of the FSH level to the LH level is useful in the diagnosis. TSH or PL levels may be useful in identifying an etiology, such as hyperthyroidism or a prolactinoma. In some patients, testosterone and dihydroepiandrosterone sulfate (DHEAS) levels or a progesterone challenge is useful. [6, 30]
Typically, a radiologic evaluation for polycystic ovaries is reserved for patients who have equivocal laboratory findings. However, radiologists make a significant number of incidental diagnoses. Should the radiologist's assistance be requested in the diagnosis of polycystic ovary syndrome, the imaging method of choice is transabdominal and/or transvaginal ultrasonography. Magnetic resonance imaging (MRI) is useful as an adjunct; however, although MRI is more sensitive than ultrasonography, its findings are less specific. The benefits of cross-sectional imaging and functional neuroimaging have been studied. [31, 32]
In a case-control study, by Fondin et a,l of 110 adolescent girls (age range, 13-17 yr) who underwent pelvic MR imaging, the most accurate diagnostic criteria on MRI for PCOS were ovarian volume, follicles per ovary measuring 9 mm or less, and peripheral distribution of follicles. [33]
Assessment of anti-Mullerian hormone levels may offer promise as a method of dertermining the presence of PCOS. [34]
Polycystic ovary syndrome is not a primary disease process. When polycystic ovaries are discovered at radiologic examination, further diagnostic tests are needed to determine the etiology.
Limitations of techniques
When the laboratory values are interpreted together with a thorough history as well as physical examination findings, they are useful in the diagnosis of polycystic ovaries. In some patients, such information may lead to a specific cause of the ovarian dysfunction (eg, hyperprolactinemia). When hormone levels do not provide adequate information, ultrasonography may prove useful; however, in the absence of correlative information, the significance of the radiologic findings is difficult to determine. The primary limitation of ultrasonography is that a radiologic diagnosis of polycystic ovaries does not reveal the underlying pathology, if it exists. Further studies are usually necessary to determine the cause of the radiologic finding.
Although polycystic ovaries are occasionally identified in patients without PCOS, this possibility does not relieve the radiologist of the responsibility to report the finding and recommend further clinical and biochemical evaluations.
Magnetic Resonance Imaging
Ovaries can be identified on MRIs in more than 95% of premenopausal women (see the images below). On T1-weighted images, the ovaries have homogeneously low signal intensity, and they are easily distinguished from the surrounding pelvic fat. T2-weighted images reveal high signal intensity within the fluid-filled follicles of the ovarian cortex. The ovarian stroma remains dark on these images. [35, 36, 37, 38, 39]
 Axial T2-weighted magnetic resonance image of the pelvis. This image reveals multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the left side on this image.
Axial T2-weighted magnetic resonance image of the pelvis. This image reveals multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the left side on this image.
 Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the right side on this image.
Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the right side on this image.
Polycystic ovaries are characterized by numerous, small (< 1 cm), peripheral cysts that are located throughout the cortex. The ovaries may be slightly larger than normal; however, the ovarian stroma is hypertrophic. Often, the fibrous capsule surrounding the ovary is prominent.
In a case-control study, by Fondin et a,l of 110 adolescent girls (age range, 13-17 yr) who underwent pelvic MR imaging, the most accurate diagnostic criteria on MRI for PCOS were ovarian volume, follicles per ovary measuring 9 mm or less, and peripheral distribution of follicles. [33]
Although MRI is sensitive to the presence of follicular cysts, this modality is not specific enough to permit the diagnosis of polycystic ovary disease without corroborating laboratory values and features from the patient's history.
Greater experience is necessary before sufficient criteria can be determined for the diagnosis of PCOS. Changes seen in polycystic ovaries have also been noted in patients without polycystic ovary syndrome, in patients with oligomenorrhea without a diagnosis of polycystic ovaries, and in patients taking exogenous steroids or clomiphene. The diagnosis remains a clinical one.
Ultrasonography
Polycystic ovaries typically exhibit 3 characteristics on ultrasonographic examination: bilateral enlarged ovaries, multiple small follicles, and increased stromal echogenicity (see the images below). [40, 41, 42, 43, 44, 45, 46, 47]
 Transverse endovaginal sonogram of the left ovary. This image exhibits numerous peripheral follicles and hyperechoic stroma. Note that none of the follicles is larger than 1.2 cm.
Transverse endovaginal sonogram of the left ovary. This image exhibits numerous peripheral follicles and hyperechoic stroma. Note that none of the follicles is larger than 1.2 cm.
Usually, the ovaries are enlarged symmetrically, and the shapes change from ovoid to spherical. Ovarian volume can increase by as much as 6 mL; however, almost 30% of patients with a biochemical and pathologic diagnosis of polycystic ovaries have no increase in ovarian volume.
The typical polycystic ovary contains numerous follicles at any given time. The follicles are small (0.5-0.8 cm), and no dominant follicle is present. Characteristically, the follicles are peripherally located in the cortex; however, they can occur anywhere in the ovarian parenchyma. The diagnosis of polycystic ovaries should be reserved for patients with at least 5 of these follicles in each ovary.
Typically, the ovaries are hypoechoic in relation to the surrounding pelvic fat and myometrium. Polycystic ovaries often display increased echogenicity; however, as many as one third may remain isoechoic or hypoechoic relative to the myometrium.
In a study of follicular and endocrine characteristics of anovulatory and sporadic ovulatory cycles in women with PCOS, follicular excess, or increased follicle number, was observed on ultrasonography across anovulatory and sporadic ovulatory cycles in patients with PCOS. [1]
Degree of confidence
Ultrasonography has a largely corroborative role in the diagnosis of polycystic ovary syndrome. In a patient with a biochemical diagnosis of polycystic ovaries, ultrasonographic findings may confirm the clinical diagnosis, but they cannot exclude it. Alternatively, the incidental discovery of polycystic ovaries during ultrasonography is not a reliable indicator of PCOS.
Almost 30% of patients with endocrinologic findings of polycystic ovaries may have normal-sized ovaries on sonograms. Less than 50% of patients with biochemical features of polycystic ovaries and increased ovarian volume have the classic finding of multiple, small, peripheral follicles. Ultimately, the diagnosis should be made on clinical and biochemical grounds. However, normal ultrasonographic findings should not exclude the diagnosis.
Alternatively, when polycystic ovaries are an incidental radiologic finding, approximately 25% of the patients have no clinical abnormality. Whether these patients have polycystic ovary syndrome remains a matter of debate. Again, correlation with biochemical and clinical findings is necessary before a definitive diagnosis is made.
-
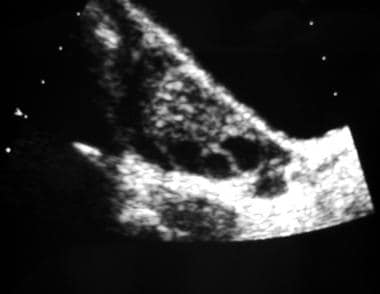
Longitudinal transabdominal sonogram of an ovary. This image reveals multiple peripheral follicles.
-
Transverse endovaginal sonogram of the left ovary. This image exhibits numerous peripheral follicles and hyperechoic stroma. Note that none of the follicles is larger than 1.2 cm.
-
Axial T2-weighted magnetic resonance image of the pelvis. This image reveals multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the left side on this image.
-
Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates multiple subcapsular follicles in both ovaries; the follicles are more conspicuous on the right side on this image.