Practice Essentials
Viral pneumonia occurs due to aggression of the viral pathogens on the lung structures. Because of the clinical manifestations and the radiologic aspects, viral pneumonia was included in the broad category of atypical pneumonias. In this category, viral pneumonia and the other atypical bacterial pneumonias must be differentiated. This differentiation is sometimes difficult and should be based on clinical, radiologic, and microbiologic criteria. [1]
Clinical and radiologic data only suggest the diagnosis and can narrow the differential diagnosis. A precise etiologic diagnosis can be made only by performing virologic laboratory studies. Even in these conditions, some series yielded an unidentified causative microorganism in 50-80% of symptomatic patients. While highly sensitive nucleic acid detection methods and testing of multiple specimens improve sensitivity, multiple pathogens are often detected, and this adds complexity to the interpretation because the etiologic significance of results may be unclear (ie, the pneumonia may be caused by none, one, some, or all of the pathogens detected). [2]
COVID-19
Despite the high sensitivity that has been seen with chest CT for COVID-19, CT is considered to be too nonspecific to be recommended for common screening. Routine screening CT for diagnosis or exclusion of COVID-19 is currently not recommended by most professional organizations or the Centers for Disease Control and Prevention. However, compared to non-COVID-19 pneumonia, COVID-19 pneumonia has been more likely to have a peripheral distribution (80% vs. 57%), ground-glass opacity (91% vs. 68%), fine reticular opacity (56% vs. 22%), and vascular thickening (59% vs. 22%), but COVID-19 pneumonia has been less likely to have a central+peripheral distribution (14.% vs. 35%), pleural effusion (4.1 vs. 39%), and lymphadenopathy (2.7% vs. 10.2%). [3, 4, 5, 6, 7]
(See the image below.)
An accurate and early etiologic diagnosis is important because specific therapies are used against certain viruses.
Viral pneumonia can have different manifestations. Pneumonia in the otherwise healthy adult host (community-acquired pneumonia [CAP]) [8] differs from nosocomial pneumonia or pneumonia in an immunocompromised host. The spectrum of clinical severity can vary from mild and self-limited disease (usually in the immunocompetent adult host) to severe compromise (in immunocompromised patients and those at the extremes of age) to the need for mechanical ventilation (eg, for acute respiratory distress syndrome [ARDS]).
The Pneumonia Etiology Research for Child Health project (PERCH) reported that hypoxemia, tachypnea, crackles, and fever may predict a radiologic finding of pulmonary consolidation in children younger than 5 years with severe or very severe pneumonia. [9]
Viral etiologic agents
DNA and RNA viruses are involved in the etiology of viral pneumonia. Some are well-known lung pathogens that produce common clinical and radiologic manifestations. Others are rarely involved as lung pathogens.
Etiologic viruses include various families, as follows:
-
Adenoviridae (adenoviruses)
-
Coronaviridae (coronaviruses) - Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavius (SARS-CoV)
-
Bunyaviridae (arboviruses) -Hantavirus
-
Orthomyxoviridae (orthomyxoviruses) - Influenza virus
-
Papovaviridae (polyomavirus) – JC virus, BK virus
-
Paramyxoviridae (paramyxoviruses) -Parainfluenza virus (PIV), respiratory syncytial virus (RSV), human metapneumovirus (HMPV), measles virus
-
Picornaviridae (picornaviruses) – Enteroviruses, coxsackievirus, echovirus, enterovirus 71, rhinovirus
-
Reoviridae (rotavirus)
-
Retroviridae (retroviruses)- Human immunodeficiency virus (HIV), human lymphotropic virus type 1 (HTLV-1)
Most of the members of Herpesviridae family are documented lung pathogens in hosts with compromised cell immunity and include the following:
-
Herpes simplex virus 1 (HSV-1) and herpes simplex virus 2 (HSV-2), also called human herpesvirus 1 (HHV-1) and human herpesvirus 2 (HHV-2), respectively
-
Herpesvirus 6, herpesvirus 7, and herpesvirus 8
-
Varicella-zoster virus (VZV)
-
Cytomegalovirus (CMV)
-
Epstein-Barr virus (EBV)
Radiography
According to guidelines from the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA), posteroanterior (PA) chest radiographs should be obtained if pneumonia is suspected in adults. Lateral images should also be acquired if possible.
Radiologic findings of adult viral pneumonia are variable and overlapping. The correlation between pathologic and radiologic findings is good. Because the viruses are intracellular pathogens, most pathologic changes in the setting of viral pneumonia occur in the epithelium and adjacent interstitial tissue. According to the virulence and the rate of the development of infection, 2 types of pathologic reactions and radiologic aspects can be observed: (1) usual, long-standing, or insidious course of pneumonia; and (2) rapidly progressive or virulent pneumonia. [10, 11]
The usual form (long-standing or insidious course of pneumonia) is characterized by lymphatic infiltrates in the alveolar septa. These sometimes extend to the lung adjacent to the terminal and respiratory bronchioles or even throughout the lobule in rare cases. On radiologic studies, these findings appear as 4- to 10-mm, poorly defined nodules and patchy areas of peribronchial ground-glass opacity and airspace consolidation, with variable hyperinflation. [12]
The rapidly progressive or virulent pneumonia with diffuse alveolar hemorrhage extends to the interstitium and the air space (with interstitial infiltrate, airspace hemorrhage, edema, fibrin, type 2 pneumocytes hyperplasia, hyaline membrane formation). The chest radiograph shows the rapid confluence of patchy, unilateral, or bilateral consolidations and ground-glass opacity or poorly defined centrilobular nodules.
Adenovirus pneumonia
Pathologic findings of adenoviral pneumonia are represented by patchy areas of hemorrhagic consolidation evolving to necrosis and diffuse alveolar hemorrhage, necrotizing bronchiolitis with overinflation, and atelectasis. [13] The usual radiographic findings are diffuse bilateral bronchopneumonia and severe overinflation. Lobar collapse and atelectasis is a frequent complication; right upper-lobe atelectasis is most common in infants, and left lower-lobe collapse is common in older children. Radiologic changes resolve in 2 weeks in uncomplicated cases.
About 53% of children with acute adenoviral pneumonia develop a form of chronic disease: bronchiectasis, obliterative bronchiolitis, interstitial fibrosis, or unilateral hyperlucent lung syndrome. Approximately 64% of complicated cases are described in children younger than 2 years.
In lung transplant recipients, adenoviral pneumonia is most severe, with the highest rate of mortality compared with those of patients with other respiratory viruses. The radiologic findings have been found to be more severe, typically, than those found in RSV or PIV pneumonia. Changes consist of progressive homogeneous consolidations developing over days or weeks. Pleural effusions are seen in 20% of patients.
CMV pneumonia
The pathologic findings in CMV pneumonia differ according to the degree of the host's immunosuppression. In moderately immunocompromised transplant patients, the interstitial pneumonia, inflammatory or hemorrhagic nodules, organizing pneumonia, and severe necrotizing pneumonia are due to T-cell–mediated immune mechanism.
Patients with increased immunosuppression, such as those with AIDS, have a high density of CMV inclusion bodies. [14] These are directly responsible for severe pneumonitis or diffuse alveolar damage. In recipients of solid-organ transplants, CMV pneumonia often appears normal or minimally abnormal on chest radiographs. In a series of lung transplant recipients with proven CMV pneumonitis, only one third of patients had abnormal radiographs. No deaths were recorded in the group with normal radiographs, as compared with the 18% mortality in the group with radiographic abnormalities.
When abnormal, chest radiographs reveal an interstitial pattern of disease, which is usually diffuse and which involves the bases. The interstitial pattern consists of accentuation of Kerley A and Kerley B lines or of diffuse, hazy, ground-glass opacities.
Relatively few reports note CMV pneumonia in immunocompetent hosts. Interstitial infiltrates were described in a few patients from a series of 34 immunocompetent patients.
Coxsackievirus pneumonia
In the few reported cases of coxsackievirus pneumonia, the radiographic pattern consists of fine perihilar infiltration. In the cases with pleurodynia, parenchymal consolidation in the lung bases may be observed.
EBV pneumonia
EBV lung involvement is characterized by mononuclear infiltrates in bronchovascular bundles and interlobular septa and also in alveolar exudates. Chest radiographic analysis in 59 cases of infectious mononucleosis revealed splenomegaly as the most common finding (47%), followed by hilar lymph node enlargement (13%), a diffuse reticular pattern indicating interstitial disease (5%), and bilateral or unilateral pleural effusions.
Pulmonary consolidation in infectious mononucleosis associated with interstitial pulmonary infiltrates is rare.
Echovirus pneumonia
Echovirus pneumonia has a pattern of increased bronchovascular markings and bilateral hilar lymph node enlargement.
Rhinovirus pneumonia
Alveolar and/or interstitial pulmonary infiltrates, consolidation, and complicated bronchiolitis are the most common findings for rhinovirus pneumonia. Pleural effusions are less common. [15]
Hantavirus pneumonia
As with other viral etiologies, interstitial and airspace edema, interstitial lymphocyte infiltrates, epithelial necrosis, and vascular thrombosis are seen in hantavirus pneumonia. Particular aspects of hantaviral lesions are extensive cellular debris with destruction of type I cells and a predominance of type II pneumocytes, neutrophil infiltrates, and fibrosing alveolitis.
Chest radiographs show interstitial edema with or without progression to airspace disease, with a central or bibasilar distribution and common pleural effusions. Pulmonary capillary leak syndrome of hantaviral infection may be secondary to the associated renal failure.
HIV pneumonia
Fine reticular or reticulonodular infiltrates in the pulmonary interstitium and coarse reticulonodular infiltrates or opacities with superimposed patchy alveolar infiltrates have been described in patients with AIDS or AIDS-related complex (ARC) and biopsy-proven lymphocytic interstitial pneumonia. This disease is considered a benign reaction of bronchial-associated lymphatic tissue to HIV. Radiographic findings are stable throughout the course of the disease in 75% of patients.
HSV pneumonia
HSV pneumonia is usually characterized by alveolar necrosis and proteinaceous exudates, with or without necrotizing bronchopneumonia. The focal infiltrates are thought to be the expression of aspirated secretions, and the diffuse bilateral infiltrates reflect hematogenous spread.
A study of 23 patients with HSV-1 pneumonia showed patchy segmental or subsegmental airspace opacities in 100% and a lobar distribution and ground-glass opacities in 48%. About 30% of patients had additional reticular opacities. The pattern was diffuse and multifocal in all, scattered in 82%, peripheral in 9%, and central in 4%. Pleural effusions were detected in 52%. The drawback of this study was that some of the bilateral consolidations might have been secondary to ARDS and not HSV infection. [16]
Another study of 17 patients with HSV pneumonia did not reveal a high correlation between ARDS and HSV pneumonia. The most consistent findings were bilateral opacities with an air space (3 of 14) or a mixed airspace and interstitial pattern (8 of 14). The pattern of opacities was bilateral and diffuse (12 of 14), and the extent was lobar (6 of 14), or it involved almost the entire lung (6 of 14). Pleural effusions (mostly moderate) were described in 8 patients, and atelectasis was found in 5. The presence of 2 normal chest radiographs in this series can be explained by the contamination of BAL samples obtained from the upper airways.
In neonates with HSV-2 infection contracted during delivery, progressive development from a normal chest radiograph to interstitial changes, airspace consolidation, and diffuse consolidation of both lungs is described. [17] Interstitial disease is diffuse, usually bilateral, with granularity and linear and opaque strands in hilar and peribronchial areas. Diffuse alveolar infiltrates increase lung opacification without volume loss. Diffuse consolidation is the expression of pulmonary hemorrhage with necrotizing pneumonitis. Pleural effusions may be seen.
In a series of 42 patients with HSV pneumonia, all radiographs showed abnormalities: pulmonary infiltrates (93%), pleural effusions (29%), and atelectasis (12%). In a series of 7 patients with HSV pneumonia after heart transplantation, 5 had diffuse bilateral changes, either mixed interstitial with an air space or interstitial and micronodular.
Influenza-virus pneumonia
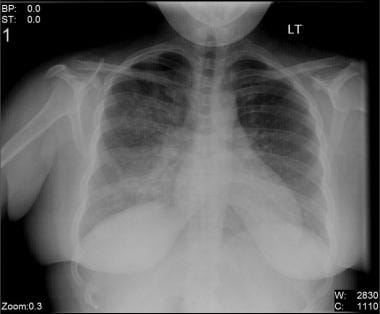
Chest radiographic changes in influenza pneumonia (seen in the image below) range from mild interstitial prominence to poorly defined, 1- to 2-cm patchy areas of consolidation, to extensive airspace disease due to hemorrhagic pulmonary edema. Alveolar hemorrhage can be seen as small centrilobular nodules. Pleural effusion is rare and usually represents bacterial infection. Cavity formation suggests bacterial superinfection with Staphylococcus organisms. [18]
Radiographic findings in lung transplant recipients with influenza pneumonia are nonspecific, ranging from subtle heterogeneous and linear opacities to homogeneous lobar consolidation involving the lower lobes more than the upper lobes. Chest radiograph infiltrates are seen in 36% of infected patients. Patients with chest radiographic changes appear to have outcomes more severe than those of other patients.
In a single-site retrospective review, airspace consolidations (89%) and peribronchial ground-glass opacities (89%) predominantly affecting mid and lower lung zones were the most common imaging findings in patients with H1N1 pneumonia admitted to the ICU. Findings were bilaterial in 94% of the patients. [19]
Measles-virus pneumonia
Primary measles pneumonia results in mixed reticular opacities and airspace consolidation. Lymph node enlargement in the hilum can be seen in children. The pathologic basis for these findings is epithelial hyperplasia in bronchioles and peribronchial alveoli, multinucleated giant cells in the alveoli, and diffuse alveolar damage. [20]
Atypical measles pneumonia appears with spherical or segmental consolidation that clears rapidly. Hilar lymph node enlargement and pleural effusions are frequently associated.
Pneumonia due to bacterial superinfection is segmental in distribution, it affects 1 or both lower lobes, and it is frequently associated with atelectasis. The presence of a dense opacity is more suggestive of a bacterial etiology (88%) than a viral etiology (36%).
PIV pneumonia
Radiographic changes in PIV pneumonia are relatively nonspecific and consist of diffuse or focal accentuation of lung markings caused by peribronchial or peribronchiolar infiltration in the lower lobes.
The giant-cell pneumonia produced by PIV-3 may be complicated by alveolar proteinosis; 1 case is reported in a recipient of transplanted umbilical-cord blood. The radiologic aspect was nonspecific and consisted of bilateral patchy infiltrates.
RSV pneumonia
The radiologic pattern of RSV pneumonia is the expression of mucosal necrosis and interstitial inflammation associated with bronchial narrowing and occlusion and bronchial wall thickening. The typical radiologic appearance of RSV lower respiratory infection is not well defined yet. The typical findings are still considered nonspecific. [21]
A 1974 study of 126 children with acute RSV lower respiratory infection showed typical features of collapse or airtrapping in small areas of consolidation. Airtrapping and peribronchitis was most common in infants younger than 6 months, whereas consolidation was most often seen after the age of 6 months. Atelectasis was a rare finding and was not correlated with age. [22]
Other authors showed that the variability of lung infiltration is correlated with the severity of infection. Atelectasis is more common in children with positive bacterial swabs than in others. (Lung infiltration is demonstrated in the image below.)
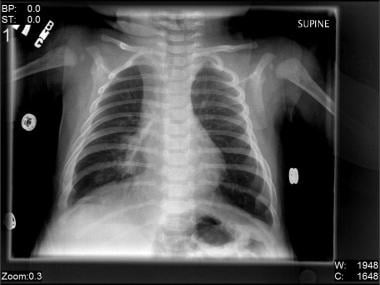 Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).
Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).
Lobar emphysema may be associated with RSV pneumonia.
A study of 128 chest radiographs of children with lower respiratory infection showed mainly lobar pneumonia, bronchopneumonia, or normal findings in infants younger than 6 months. Children older than this had mainly peribronchitis or interstitial pneumonia, as depicted on the chest images.
A study performed in Germany demonstrated 3 major radiologic findings in 108 cases of confirmed RSV lower respiratory infection: normal chest radiographic results (30%), central pneumonia (32%), and peribronchitis (26%). Other findings were emphysema (11%), pleural effusion (6%), bronchopneumonia (6%), atelectasis (5%), and pneumothorax (0.9%). Age-specific differences were not confirmed. Sensitive laboratory testing to confirm RSV infection and to rule out bacterial superinfection may explain the differences between this study and previous ones.
In adults, the radiographic aspect is frequently complicated by bacterial infection. In a study in Ohio, 40% patients had evidence of pneumonia or consolidation; in 35%, a lobar distribution was observed. Pleural effusions are seen in 5% of cases.
In lung transplant recipients, RSV and PIV pneumonias tend to be less symptomatic and without radiographic findings. Authors have described diffuse homogeneous consolidations in similar patients.
SARS-virus pneumonia
Extensive reports have been published about radiologic findings in SARS since the onset of the initial outbreaks in Asia and later in Canada in 2003. Pathologic changes of SARS consist of diffuse alveolar damage with a small amount of interstitial lymphocytic infiltrate. The early phase is characterized by pulmonary edema with hyaline membrane formation, and the organizing phase is characterized by cellular fibromyxoid organizing airspace exudates. These findings explain why most images of patients admitted with SARS infection are nonspecific and indistinguishable from those of other viral or bacterial bronchopneumonias.
The disease cannot be ruled out in patients with negative radiologic findings. Radiographic reexamination, dynamic observation, and digital radiography can be used to increase test sensitivity. Most authors emphasize the need for serial chest radiographs.
A study of 13 Canadian healthcare workers with probable SARS revealed 3 distinctive radiographic patterns. [23, 24] The most common pattern (seen in 76.9% of cases) was focal peripheral airspace disease at presentation with gradual resolution. Some patients had normal radiographs initially: 15.4% later developed focal airspace disease, and 7.7% had round pneumonia, a rare finding confirmed with other studies. Bilateral disease was seen in 53.8% of patients, and unilateral involvement was seen in 46.2%. All patients had mid- and lower-lung airspace disease, and 46.2% had additional upper-lung infiltrates. No evidence of pleural thickening, effusion, lymphadenopathy, cavities, or clinically significant airway changes was found.
A retrospective study of 62 children with SARS from Canada, Singapore, and Hong Kong found normal chest radiographs in 35.5%. [25] Prominent radiologic findings in the remaining children were areas of consolidation (ground-glass opacities or focal, lobar, or multifocal opacities; 45.2%), which were often peripheral and in the lower lobes. Peribronchial thickening was noted in 14.5%. Radiographic evidence of adenopathy was not seen. According to the authors, radiography has 2 major roles in SARS. The first is to depict pulmonary involvement in the suspected cases of SARS, and the second is to show radiologic changes characteristic of other bacterial or granulomatous diseases. Extensive pleural effusions, pneumothorax, pneumatocele, lung abscess, cavitation, and adenopathy are uncommon radiologic findings in SARS. [26]
VZV pneumonia
VZV invasion of the lungs causes swelling, proliferation of type II cells, endothelial damage in the small blood vessels, and desquamation of alveolar septal cells with alveolar septae mononuclear infiltration. Fibrinous exudate organizing in hyaline membranes and focal hemorrhagic necrosis are common.
After the patient recovers from initial disease, spherical nodules are seen. They consist of an outer, fibrous, lamellate capsule enclosing areas of hyalinized collagen or necrotic tissue, with variable degrees of calcification.
The radiographic pattern is scattered, ill-defined, 5- to 15-mm nodular opacities (acinar nodular pattern). These are confluent and fleeting and identical in immunocompetent and immunocompromised hosts. The nodules are seen in the lung periphery (bases), coalescing near the hila; these probably reflect contiguous spread from tracheobronchitis. Reticular markings, pleural effusions, and hilar adenopathy are rarely seen.
The radiographic manifestations usually appear 2-5 days after the rash does. They tend to clear in 3-5 days in mild disease and take up to several weeks or months to clear in widespread disease.
An apparently unique complication of acute VZV pneumonia consists of the late appearance (years after onset of pneumonia) of 2- to 3-mm dense calcifications, which are well defined, scattered, and predominant in the lower half of the lungs. The frequency of these calcifications is 1.7-2.0% in adults with previous VZV pneumonia.
Degree of confidence
Several reports have suggested that the chest radiography cannot be used to differentiate nonbacterial pneumonia from bacterial pneumonia. The limited number of patients and microbiologic techniques used and the wide variation in descriptive radiologic terms limited the results, and no general conclusions can be drawn.
Achieving standardization of radiology results reporting is important to provide confidence in the interpretation of pneumonia etiology. The PERCH study reaffirmed findings that observer agreement is best for consolidation and poorest for findings of other infiltrates. [27]
One group from Finland enrolled 215 children with CAP. Their results showed that 71% of children with alveolar (especially lobar) infiltrates, as shown on chest radiographs, had evidence of bacterial infection. One half of the children with interstitial infiltrates as the sole radiographic finding had bacterial infection. Therefore, interstitial infiltrates were not a reliable indication of viral pneumonia. [28]
Specific organism diagnosis of a viral pneumonia cannot be made on the basis of imaging features alone. The radiographic manifestations depend on the patient's immunologic status and preexisting or coexisting lung diseases. Many pathogens can have overlapping radiographic features, and not all physicians agree on the meaning of some descriptive terms. The imaging aspects should be integrated with clinical and epidemiologic data and confirmed by means of virology testing. Recognition of the radiologic findings helps in narrowing the differential diagnosis and in assessing the evolution of disease and complications.
Computed Tomography
Computed tomography (CT) scanning remains a useful adjunct to radiologic investigation of viral pneumonia. Some authors consider CT useful in differentiating diffuse interstitial lung disease from infectious conditions in ICU patients. [29]
In an immunocompetent host with pneumonia, CT is indicated only in complicated cases and in association with invasive procedures. [30] CT is frequently appropriate for cases with normal, equivocal, or nonspecific radiographic findings.
High-resolution CT scanning
High-resolution CT (HRCT) can be used to guide diagnostic procedures such as bronchoalveolar lavage (BAL) or transbronchial biopsy, and it is helpful in differentiating infectious disease from noninfectious acute parenchymal lung disease. However, HRCT has limited value in making a specific diagnosis.
In ICU patients, HRCT has not yet been evaluated as a diagnostic test for opportunistic infections in the ventilated patient with diffuse interstitial lung disease. In this setting, invasive procedures (eg, open lung biopsy) or semi-invasive procedures (eg, transbronchial lung biopsy or BAL) are required for establishing an etiologic diagnosis.
General findings on CT scans
CT findings in viral pneumonia are nonspecific and overlapping and consist of centrilobular nodules, ground-glass attenuations with a lobular distribution, segmental consolidation, and/or diffuse ground-glass attenuation with thickened interlobular septa. Similar to the radiographic findings, CT findings follow the appearance of pathologic lesions.
The centrilobular nodules (airspace nodules or acinar nodules) are 6-10 mm. They are best appreciated in early disease and best seen at the edge of the pathologic process, where consolidation is incomplete.
Some authors consider these centrilobular nodules the main CT feature that differentiates bacterial pneumonia from atypical pneumonia. These nodules are found in 64% of patients with atypical pneumonia and 77% with viral pneumonia, as opposed to 11-17% of those with bacterial pneumonia. Predominant nodular patterns were reported in M pneumoniae pneumonia (89%) and fungal pneumonia (65%).
In viral pneumonia, the centrilobular nodules are typically associated with a background of diffuse ground-glass attenuation and/or reticulation. The ground-glass attenuations are nonspecific findings, defined as a localized increase in lung attenuation that allows visualization of vascular structures through the affected region. Viral pneumonia is commonly associated with nodules and focal or diffuse areas of airspace consolidation as well.
Adenoviral pneumonia
Most reports of adenoviral pneumonia describe the late changes that appear after the initial pneumonia resolves. CT scans show pulmonary hyperinflation with nonhomogeneous air-trapping and variable degrees of bronchiectasis after the pneumonia resolves. [31] Late pathologic changes consist of obliterating bronchiolitis induced by necrotizing bronchiolitis and bronchiectatic changes, absorption atelectasis, and follicular bronchiolitis.
CMV pneumonia
CT findings of CMV pneumonia are diverse and have been described in various patients. Findings in patients without AIDS include a mixed pattern of nodules, ground-glass attenuations, and consolidation [32, 33] ; these seem to differ from findings in patients with AIDS, who have masslike lesions. [34] One case report notes a cavitary lesion in an immunocompetent patient proven to have CMV pneumonia by means of BAL and lung biopsy. [35, 36]
EBV pneumonia
The most common CT findings in EBV-associated lymphoproliferative disease of the lung are multiple nodules with peribronchial distribution. [37, 38]
HIV and HTLV pneumonia
In HIV-related lymphocytic interstitial pneumonia, CT scans may show areas of ground-glass attenuation, ill-defined nodules, and multiple cystic lesions.
Lymphocytic interstitial pneumonia is one of the lung complications of HTLV 1–associated bronchopneumopathy.
CT findings of multiple cystic lesions, small nodules, thickening of the interlobular septa, and peribronchial or peribronchiolar thickening have been described. These lesions are correlated with pathologic findings of infiltrates of mononuclear cells in peribronchial and alveolar interstitial tissue and with infiltrates of peribronchiolar T cells.
Other CT findings may include ground-glass attenuation, focal areas of consolidation, small nodules, and thin-walled cystic nodules. Multiple cysts are secondary to partial bronchiolar obstruction and produced by the lymphocytic infiltrate; these cysts are the most characteristic lesions of lymphocytic interstitial pneumonia.
HSV pneumonia
Approximately one third of patients with HSV-1 pneumonia have coexisting bacterial pneumonia. Therefore, the delineation of typical CT findings due to HSV-1 is problematic. [39]
Influenza-virus pneumonia
CT findings in influenza pneumonia include airspace consolidation or ground-glass attenuation with a lobular distribution. These aspects are considered to be the expression of hyaline membrane formation in the peribronchiolar alveoli. [40, 41]
Bilateral multifocal peribronchovascular or subpleural consolidations are reported in immunocompetent patients.
Measles-virus pneumonia
CT findings in measles virus pneumonia include diffuse alveolar damage that produces airspace and interstitial disease. The CT appearance is that of ground-glass attenuation, airspace consolidation, and small centrilobular nodules.
An analysis of CT scans in 4 patients with measles pneumonia revealed bronchial wall thickening, centrilobular nodules in ground-glass attenuations, interstitial lesions (interlobular septal thickening, fissure thickening), pleural effusion, and lymphadenopathy. The marked centrilobular nodules in the ground-glass attenuations and interlobular septal thickening might be specific findings in measles virus pneumonia.
SARS-virus pneumonia
Even with chest radiography as the first imaging study, the early diagnosis of SARS is limited because of the increased likelihood of missing small, patchy, ground-glass attenuations or because of false-negative result. CT scanning has not yet been adopted as a screening tool.
HRCT is more sensitive than chest radiography, but its use is limited, especially during outbreaks, because of the highly infectious nature of the disease and because of cumbersome isolation and infection control measures. HRCT does seem to be useful for early diagnosis, especially for patients in whom SARS is highly suspected, such as close contacts or those with typical symptoms, and when radiographic findings are normal, subtle, or equivocal. HRCT may also be helpful in assessing complications. [42, 43]
An analysis of 27 confirmed cases from Hong Kong provided the best description of CT findings in SARS. [44] Defined HRCT patterns, though nonspecific, were observed in different phases of SARS. During the first week, ground-glass attenuations (33.3%) occurred in clusters of round or wedge-shaped patterns, a crazy-paving pattern (37%), or a combination of both. A sharply defined line separated the diseased areas from the normal areas. Focal areas of subpleural sparing were noted. In this phase, 20-30% of patients had changes on HRCT, with normal chest radiographs.
In the subacute phase, thin or thick reticular lines developed in attenuations, producing a lattice effect. A marble aspect was seen in 18.5% of patients, and pleural effusions were seen in 25.9%.
If the disease progresses further, ground-glass attenuations tend to develop into consolidation (48%) and atelectasis (masslike organizing density). During this phase, the disease may be complicated by pneumomediastinum (25.9%) with coexisting subcutaneous emphysema and localized pneumothoraces. Changes in this phase are associated with architectural distortion, pleural thickness, and traction bronchiectasis.
During the recovery phase, most of the changes improve. Masslike shadows may persist and transform into fibrosis and scarring, with associated blebs and traction bronchiectasis. Chronic irreversible changes, such as honeycombing, are observed in 25.9% of patients. A bilateral scattered distribution is noted in 89%, with all-lobe involvement in 63%. No lymphadenopathy or cavitations are described.
Other retrospective studies from Hong Kong showed abnormal HRCT findings in all patients with symptoms and a high clinical suspicion of SARS, with or without initially normal radiographs. HRCT findings were similar to those previously described: ground-glass attenuations (68.4% of involved segments), pure consolidation (16.8%), or a combination of both (14.8%). Other findings were thickening of intralobular interstitium (32.3%), and interlobular septae (24.2%).
Almost one half of the patients had multifocal and/or bilateral involvement. The crazy-paving pattern was confirmed in only some florid cases. The lesions were smaller and the number of lobes involved were fewer in patients with normal chest radiographs than in others. Affected segments were predominantly in the lower lobes. CT scans confirmed that the lesions were mainly peripheral in the group with normal radiographs, as opposed to the mixed central and peripheral location in the group with abnormal chest radiographs.
A review of pediatric patients' findings in these series confirmed the observation of mild lesions, with the predominance of ground-glass attenuation and consolidation reported by other authors. The absence of cavitation, calcification, and lymphadenopathy in both adult and pediatric patients was consistent with other reports. Pleural effusions were not reported in all studies.
Even if CT is not routinely used to monitor the evolution of the disease and the response to treatment, some emphasize the usefulness of HRCT at 6 months. Late HRCT findings combined with clinical features are accurate in defining lung damage. About 60% of patients with dyspnea and reduced effort tolerance after discharge have evidence of fibrosis (parenchymal bands, traction bronchiectasis). These patients are relatively old and have worsened disease and changes and elevated peak lactate dehydrogenase levels. In this setting, HRCT can help differentiate between reversible ground-glass attenuation and irreversible fibrosis.
VZV pneumonia
A study in immunocompetent patients with VZV pneumonia revealed 5- to 10-mm nodules with or without surrounding ground-glass attenuation, patchy ground-glass attenuation, and coalescence of lesions. Patchy ground-glass attenuation and coalescing of ill-defined nodules were correlated with the consolidation shown on chest radiographs. Nodules depicted on CT resolved concomitantly with the skin lesions.
CT is indicated in immunocompetent patients with VZV pneumonia and equivocal radiographic findings or in patients requiring an evaluation of other combined or underlying pulmonary disease.
COVID-19
Despite the high sensitivity that has been seen with chest CT for COVID-19, CT is considered to be too nonspecific to be recommended for common screening. Routine screening CT for diagnosis or exclusion of COVID-19 is currently not recommended by most professional organizations or the Centers for Disease Control and Prevention. However, compared to non-COVID-19 pneumonia, COVID-19 pneumonia has been more likely to have a peripheral distribution (80% vs. 57%), ground-glass opacity (91% vs. 68%), fine reticular opacity (56% vs. 22%), and vascular thickening (59% vs. 22%), but COVID-19 pneumonia has been less likely to have a central+peripheral distribution (14.% vs. 35%), pleural effusion (4.1 vs. 39%), and lymphadenopathy (2.7% vs. 10.2%). [3, 4, 5, 6, 7]
-
Bilateral interstitial infiltrates in a 31-year-old patient with influenza pneumonia.
-
Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).