Practice Essentials
Pancreatic cystic neoplasms represent a small yet increasingly detected entity of pancreatic abnormalities. Approximately 70% of pancreatic cystic neoplasms are discovered incidentally. The most frequently encountered neoplastic pancreatic cysts include intraductal papillary mucinous neoplasm (IPMN), serous cystadenoma (SCA), mucinous cystic neoplasm with ovarian stroma (MCN), and solid pseudopapillary epithelial neoplasm. IPMN is further subdivided into branch duct (BD), main duct, and combined forms. [1] Together, these constitute 90% of premalignant pancreatic, primarily cystic, tumors. [2] In contrast, pseudocyst, true epithelial cyst, lymphoepithelial cyst, and mucinous non-neoplastic cyst have no malignant potential. [3]
Malignancy occurs only in mucinous cysts. SCA is considered a nonmalignant lesion, and malignant serous tumors reported in the literature have not been found to meet the World Health Organization (WHO) criteria for SCA. [4] IPMN can progress from lower to higher grades of dysplasia and, ultimately, pancreatic ductal adenocarcinoma (PDAC). IPMNs involving the main duct have a higher malignancy rate than those in the branches, with the risk of malignancy estimated to be 62%; the risk for malignancy of MCNs is less than 15%. [5] Other, rarer cystic lesions, such as solid pseudopapillary epithelial neoplasm and cystic pancreatic neuroendocrine tumor (cPNET), tend to harbor features that suggest a specific diagnosis, usually leading to surgical removal. [3]
Mucinous cystic neoplasm with ovarian stroma (MCN)
MCNs are common in middle-aged women, are usually well defined, and are predominantly in the tail of the pancreas (>90%). Compared with serous cystic tumors, MCNs are larger (>20 mm in diameter) and less numerous (usually < 6). The biologic behavior of MCNs is variable, and different histologic patterns frequently coexist in the same tumor. The tumors may be entirely benign, as are mucinous cystadenomas or intraductal papillary mucinous adenomas. Some tumors are borderline, showing cellular dysplasia, whereas others are frankly malignant. Malignant cystadenocarcinoma and intramedullary mucinous carcinomas may be further subdivided into noninvasive and invasive types. Noninvasive MCN is associated with a 100% survival rate and zero percent chance of recurrence. The 5-year survival rate of invasive MCN is 20-60%. [6]
In a study of 47 cases of MCN by Ramia et al, 35 (74.5%) were found to be noninvasive. Mean preoperative size was 46 mm, and invasive tumors were larger (6 cm vs 4 cm). [6] Kim et al, in a study of 99 patients with MCN, found that the presence of symptoms, tumor size greater than 4 cm, and the radiologic finding of a solid component/mural nodule or duct dilatation were significantly associated with malignancy. [7]
(The radiologic characteristics of MCNs are demonstrated in the images below.)
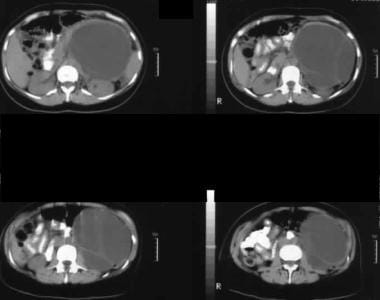 Nonenhanced axial CT scans. Image 1 shows a large septate mass in the left hypochondrium. Note the smooth external contour typical of a mucinous cystic neoplasm.
Nonenhanced axial CT scans. Image 1 shows a large septate mass in the left hypochondrium. Note the smooth external contour typical of a mucinous cystic neoplasm.
Intraductal papillary mucinous neoplasm (IPMN)
On the basis of involvement of the pancreatic duct, IPMNs are classified as either main duct IPMN, side-branch IPMN, or mixed variant IPMN involving both the main pancreatic duct and the side branches. Main duct IPMNs often have intestinal-type epithelium, and side-branch IPMNs usually have gastric-type epithelium. Although all morphologic variants of IPMN can progress to cancer, invasive adenocarcinoma originating in gastric-type IPMNs is associated with a significantly worse survival rate than that originating from other types of IPMNs. However, the imaging features are not specific for differentiating the various histologic variants of IPMNs.
Side-branch IPMNs are commonly detected in older men and are more frequently located in the proximal pancreas (head and uncinate process). An important differentiating feature between MCN and IPMN is visualization of pancreatic ductal communication. If a clear channel of communication with the pancreatic duct is visualized, the diagnosis of side-branch IPMN is almost certain because SCAs and MCNs do not communicate with the pancreatic ductal system.
Despite the low incidence of aggressiveness of mucinous cystic lesions 3 cm and smaller, the incidence is not low enough to dismiss the lesions entirely, and careful review of the imaging features is mandated. In addition, patients whose condition is found not to be suitable for surgical management often need frequent assessments for growth and change in imaging features.
(IPMNs are seen in the images below.)
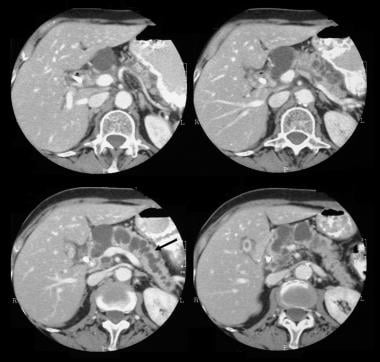 Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a 5.5-cm cystic tumor in the pancreatic head. Note the upstream, gross dilatation of the pancreatic duct. The accessory pancreatic duct is also dilated.
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a 5.5-cm cystic tumor in the pancreatic head. Note the upstream, gross dilatation of the pancreatic duct. The accessory pancreatic duct is also dilated.
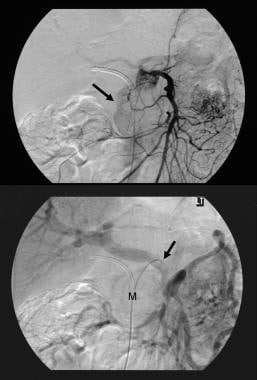 Pancreatic intraductal papillary mucinous tumor (IPMT). (Top) Superior mesenteric angiogram shows capillary vascularity in the mass in the pancreatic head during the arterial phase. (Bottom) The portal venous phase image shows displacement of the portal venous branches and encasement of the junction of the superior mesenteric vein and the portal vein. M denotes the pancreatic mass.
Pancreatic intraductal papillary mucinous tumor (IPMT). (Top) Superior mesenteric angiogram shows capillary vascularity in the mass in the pancreatic head during the arterial phase. (Bottom) The portal venous phase image shows displacement of the portal venous branches and encasement of the junction of the superior mesenteric vein and the portal vein. M denotes the pancreatic mass.
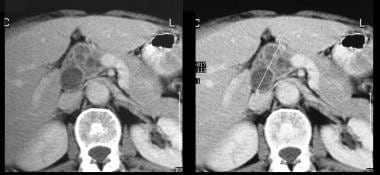 Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a multiseptate tumor in the head of the pancreas.
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a multiseptate tumor in the head of the pancreas.
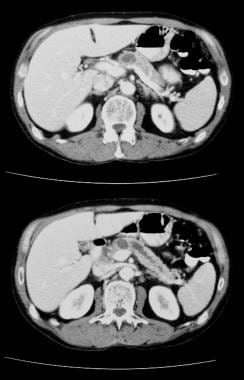 Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced CT scans through the pancreas show gross dilatation of the pancreatic duct. At surgery, IPMT was confirmed.
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced CT scans through the pancreas show gross dilatation of the pancreatic duct. At surgery, IPMT was confirmed.
Serous cystadenomas
Serous cystadenomas (microcystic adenomas) (see the images below) are the second most common cystic tumors of the pancreas. The clinical presentation of serous cystadenomas is similar to that of mucinous cystic pancreatic tumors.
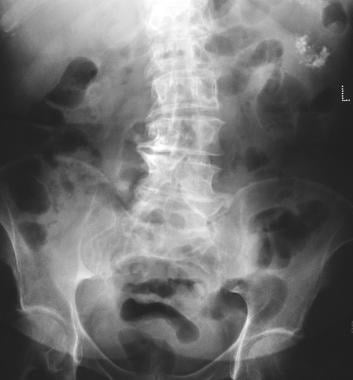 Pancreatic microcystic adenoma. Plain radiograph shows tumor calcification in a microcystic adenoma (left upper quadrant). Calcification in the microcystic adenoma presents as a central cluster arranged in a sunburst or stellate arrangement. Central calcification is better evaluated with CT than with radiography.
Pancreatic microcystic adenoma. Plain radiograph shows tumor calcification in a microcystic adenoma (left upper quadrant). Calcification in the microcystic adenoma presents as a central cluster arranged in a sunburst or stellate arrangement. Central calcification is better evaluated with CT than with radiography.
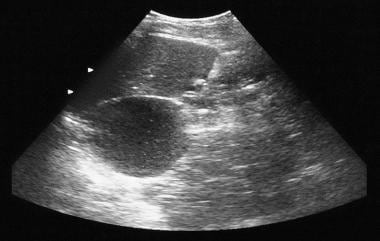 Pancreatic microcystic adenoma. Sonogram shows a cystic mass in the region of the tail of the pancreas.
Pancreatic microcystic adenoma. Sonogram shows a cystic mass in the region of the tail of the pancreas.
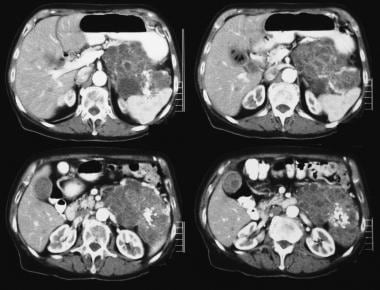 Pancreatic microcystic adenoma. Contrast-enhanced axial CT scans show a hypervascular tumor in the pancreatic tail with sunburst calcification. Note the Swiss-cheese enhancement.
Pancreatic microcystic adenoma. Contrast-enhanced axial CT scans show a hypervascular tumor in the pancreatic tail with sunburst calcification. Note the Swiss-cheese enhancement.
Because of increasing use of cross-sectional imaging, many of these tumors are detected as an incidental, asymptomatic finding.
On cross-sectional images, serous cystadenomas appear as numerous tiny cysts separated by delicate fibrous septa, which give them a honeycomb appearance. The cysts are filled with clear, watery fluid and are often arranged around a central stellate scar, which may be calcified. On CT scans, sunburst central calcification in a spongy mass is pathognomonic of this tumor, but this finding occurs in only 10% of patients.
Endoscopic ultrasonography (EUS) allows better resolution of the honeycomb structure than does CT. At times, the cysts may be large, a feature that makes it difficult to differentiate these cysts from MCNs.
Hypervascularity may be demonstrated on angiograms, and some tumors occur with intra-abdominal hemorrhage.
Differential diagnoses
Pancreatic pseudocyst or pancreatic fluid collections
Fluid collections occur in as many as 50% of cases of acute pancreatitis. Pseudocysts are usually seen as anechoic fluid spaces on sonograms, but they may show internal echoes if they contain necrotic tissue or clot.
Imaging findings that suggest a diagnosis of pseudocyst rather than of cystic neoplasm include the following: lack of septae, loculations, solid components, or cyst-wall calcifications on computed tomography (CT) scans; hypovascularity on angiograms; and communication between the cyst and pancreatic ducts on endoscopic retrograde cholangiopancreatography (ERCP). Most pseudocysts are extrapancreatic, whereas pancreatic cystic neoplasms are intrapancreatic.
Pancreatic abscess
Pancreatic abscess is usually secondary to infection of a pseudocyst, but in rare cases, it can occur as a result of direct spread from renal or colonic infection. Typically, a pancreatic abscess occurs 2-4 weeks after an episode of acute pancreatitis.
On images, these abscesses may appear similar to pseudocysts. Generally, the appearance depends on their age. In the acute phase, the changes may be subtle, with only loss of normal pancreatic contour associated with obliteration of the pancreatic outline and the peripancreatic vascular and other soft tissue spaces. These changes may be indistinguishable from those found in severe acute pancreatitis. In the subacute and chronic stages, when central necrosis occurs, an anechoic or complex cystic mass is usually seen. A debris level may be observed in the dependent portion of the abscess. In the subacute or chronic phase, through transmission is usually good except when gas is present within the abscess. In the presence of gas, the abscess may become echogenic and may shadow.
The walls of subacute and chronic pancreatic abscess have variable features. The walls may be thick, irregular, and well defined, or the abscess may have no definable wall at all. The sonographic findings are nonspecific, but in the appropriate clinical setting, a diagnosis of an abscess may be suggested and confirmed by means of percutaneous aspiration or CT.
Parasitic cysts
Echinococcus granulosis cysts and multilocularis cysts of the pancreas have been described, although pancreatic involvement is exceptionally rare. E. granulosis cysts may be unilocular, multilocular, or complex.
On imaging alone, differentiation of these and other cystic masses is difficult. Serologic tests may be useful in the appropriate clinical setting. E. multilocularis cysts show an echogenic infiltrative pattern. This diagnosis should be entertained in endemic regions when such a pattern is seen.
Solid and papillary epithelial pancreatic tumors
Solid and papillary epithelial neoplasms of the pancreas may be solid or cystic. These are rare tumors that are often mistaken for mucin-secreting tumors or nonfunctioning adenomas.
Solid and papillary epithelial pancreatic tumors are most often located in the pancreatic tail. They are large, well-encapsulated masses with areas of hemorrhage and necrosis. On sonograms, they appear as heterogeneous, round, solid masses with a cystic necrotic center and dystrophic calcification, which may shadow.
Dysontogenic cysts
Dysontogenic cysts are hamartomatous cysts that are often associated with renal cysts, cerebellar angiomas, and encephaloceles. Imaging reveals a large, thin-walled cyst with a mulberry configuration.
Pseudoaneurysms
Pseudoaneurysms are usually not truly intrapancreatic, and they may be confused with a pancreatic cyst. These aneurysms are a complication of pancreatitis in 3.5-10% of patients. Doppler sonography may show turbulent arterial flow within a pseudoaneurysm, whereas color flow Doppler sonography shows bidirectional flow and swirling within the anechoic mass. Doppler imaging may permit tentative identification of the artery feeding the pseudoaneurysm.
Retroperitoneal neurofibroma or schwannoma
These tumors may be hyperechoic or hypoechoic/cystic lesions with sporadic internal echoes. This is a common feature in larger tumors in which cystic degeneration and hemorrhage have occurred. The tumors are retroperitoneal but may mimic pancreatic masses.
Pancreatic sarcoma
Pancreatic sarcoma is a rare tumor of the mesenchymal supporting structures of the pancreas. It is a relatively sonolucent mass and may be mistaken for a fluid collection or pseudocyst. Sonographic results may be normal, or sonograms may demonstrate a retroperitoneal mass, which is relatively sonolucent compared to the surrounding tissues. Therefore, this lesion may be confused with a cystic pancreatic mass.
Pancreatic lymphoma
Primary pancreatic lymphoma is rare. The clinical presentation is not unlike that of pancreatic carcinoma. Sonography may reveal a homogeneous, sonolucent, or complex mass. These masses are usually echo-poor and may mimic cystic lesions. As the prognosis of a pancreatic lymphoma is favorable, its differentiation from a carcinoma is crucial. The correlation of sonographic, CT, and angiographic findings may result in a correct diagnosis. However, if doubt exists, sonography-guided biopsy may reveal the true nature of the mass.
Pancreatic acinar cell carcinoma
Pancreatic acinar cell carcinomas (PACCs) constitute approximately 1% of exocrine pancreatic tumors. PACC is an epithelial neoplasm with evidence of acinar differentiation. Patients with acinar cell carcinoma have a better prognosis than do patients with ductal-type adenocarcinomas but a worse prognosis than do patients with pancreatic endocrine tumors. On CT, PACC has been described in a variety of ways, including as a poorly defined dense mass; as a well-defined mass with central necrosis; and as a cystic mass surrounded by a thick hypervascular wall. [8]
Imaging modalities
MRI is the preferred technique for the diagnosis of cystic pancreatic tumors. MRIs are usually helpful in differentiating between pseudocysts and cystic neoplasms. [4, 9, 10, 11, 12] Magnetic resonance cholangiopancreatography (MRCP) can depict biliary and pancreatic duct anatomy noninvasively, and it helps in the diagnosis of intraductal tumors.
Ultrasonography is generally the first technique in a patient with epigastric symptoms. This is an excellent modality for the diagnosis of cystic pancreatic masses. Sonography also provides an opportunity for guided intervention, such as aspiration and biopsy. Doppler sonography provides an added benefit in the evaluation of hypervascular tumors and vascular thrombosis/occlusion associated with pancreatic tumors. Echo-enhanced power Doppler sonography has high sensitivity and specificity in the differential diagnosis of pancreatic tumors. [13, 14]
In equivocal cases or in cases in which malignancy is highly suspected, EUS-FNA (endoscopic ultrasound fine-needle aspiration) gives the best diagnostic yield, as it permits the acquisition of cytologic samples and cystic fluid for the analysis of various tumor markers. [15]
Occasionally, despite complete evaluation of a cystic mass, the type of cyst may remain indeterminate. Although expensive and invasive, laparoscopic sonography, biopsy of the cyst wall, and analysis of the cystic aspirate significantly contribute to the differential diagnosis of pancreatic cystic lesions.
CT shows tumor calcification and is an excellent modality for the detection of local and distant metastases. Although CT and MRI cannot be used to differentiate mucin content from pancreatic juice, communication between the cystic lesion and the dilated main pancreatic duct (MPD) and a bulging papilla with a patulous orifice are characteristics of intraductal papillary mucinous tumor (IPMT). The internal architecture of mucinous tumors is displayed at least as well on MRI scans as it is on CT scans, with the exception of calcification within the lesion (which MRI has only a limited ability to reveal).
Spiral and/or multisection CT are excellent techniques for imaging the pancreas, providing superb spatial resolution and anatomic detail. With thin collimation and arterial and venous phases and multiplanar and/or 3-dimensional (3D) reconstructions, excellent detail of the vascular anatomy is depicted; most centers now seldom use angiography to assess pancreatic tumors.
Plain radiographs are often obtained to look for pancreatic calcification. Upper GI barium studies may be performed in the context of epigastric pain. With pancreatic tumors, barium studies may depict extrinsic displacement of the stomach and duodenum.
Limitations of techniques
MRI is not universally available, is expensive, and poses a problem for patients with claustrophobia. The American College of Gastroenterology (ACG) guidelines recommend caution when using imaging to diagnose cyst type or concomitant malignancy; the accuracy of MRI or MRCP in diagnosing cyst type is 40-50% and is 55–76% in determining benign vs. malignant tumors. [9]
Visceral gas, patient habitus, and operator dependency limit the value of sonography. Laparoscopic ultrasonography is invasive. EUS imaging cannot reliably distinguish benign from malignant IPMNs, and it is unclear whether imaging features of mucinous lesions with increased malignant potential are sufficiently predictive to influence clinical management. When surgical histology is used as a reference standard, the diagnostic accuracy of EUS imaging ranges from 40 to 96%. A single prospective study demonstrated that the sensitivity (56%) and specificity (45%) of EUS morphology alone for differentiating mucinous cysts (mucinous cystic neoplasms and IPMNs) from nonmucinous cysts were low, resulting in poor overall accuracy (51%). [16]
In a study by Huynh of accuracy of cyst size by CT, MRI, and EUS, cyst size measured by CT and MRI overestimated pathologic size by 0.33 cm and 0.27 cm, respectively, whereas EUS underestimated pathologic size by 0.05 cm. For pathologically confirmed cysts less than 3 cm, MRI overestimated pathologic size by 0.30 cm. For cysts 3 cm or greater, EUS underestimated pathologic size by 0.35 cm, and MRI and CT overestimated pathologic size by 0.23 cm and 0.51 cm, respectively. [17]
Plain radiographs and upper GI barium studies are nonspecific, and similar findings may be encountered in a variety of pathologies. CT carries a significant ionizing radiation burden and uses iodinated contrast material with a risk of anaphylaxis and nephrotoxicity.
Cross-sectional studies, including ultrasonography, CT, and MRI, cannot be used to distinguish between mucinous cystadenoma and cystadenocarcinoma unless the tumor has metastasized or invaded neighboring organs. Angiography is nonspecific and invasive. It also requires iodinated contrast medium, with the risk of anaphylaxis and nephrotoxicity.
Guidelines
ACG guidelines recommend cyst surveillance be offered to surgically fit candidates with asymptomatic cysts that are presumed to be IPMNs or MCNs. All surgically resected IPMNs require postoperative surveillance, but resected MCNs without pancreatic cancer do not. MRCP is the preferred modality for surveillance; EUS may also be the primary surveillance tool when MRI scans are contraindicated. In the absence of concerning features that warrant increased surveillance or referral for further evaluation, cyst size guides surveillance intervals for presumed IPMNs and MCNs. [9]
Guidelines for the diagnosis and management of pancreatic cysts have been published by the following organizations:
-
American College of Gastroenterology (ACG)
-
European Study Group on Cystic Tumours of the Pancreas
-
American College of Radiology (ACR)
The ACG guidelines recommend magnetic resonance imaging (MRI) or magnetic resonance cholangiopancreatography (MRCP) as the preferred diagnostic modality because of their noninvasiveness, lack of radiation, and greater accuracy in assessing communication between the main pancreatic duct and the cyst (which is a characteristic of side-branch IPMNs). Pancreatic protocol computed tomography (CT) or endoscopic ultrasound (EUS) were deemed "excellent alternatives" if MRI is contraindicated. EUS fine-needle aspiration (FNA) and cyst fluid analysis should be considered in cysts in which the diagnosis is unclear and where the results are likely to alter management. [9]
Analysis of cyst fluid carcinoembryonic antigen (CEA) may be considered to differentiate IPMNs and MCNs from other cyst types, but it cannot be used to identify IPMNs and MCNs with high-grade dysplasia or pancreatic cancer. IPMNs or MCNs with any of the following features should undergo EUS with or without FNA and/or be referred to a multidisciplinary group for further evaluation [9] :
-
Any of the following symptoms or signs: jaundice secondary to the cyst, acute pancreatitis secondary to the cyst, significantly elevated serum CA 19-9.
-
Any of the following imaging findings: the presence of a mural nodule or solid component either within the cyst or in the pancreatic parenchyma, dilation of the main pancreatic duct of >5 mm, a focal dilation of the pancreatic duct that is concerning for main duct IPMN or an obstructing lesion, or mucin-producing cysts measuring ≥3 cm in diameter
-
The presence of high-grade dysplasia or pancreatic cancer on cytology
The European Study Group guidelines also recommend MRI as the preferred method for the diagnosis of pancreatic cystic neoplasms (PCNs). Multimodality imaging should be considered in cases where the identification of calcification is important, for tumor staging, or for diagnosing postoperative recurrent disease. However, the accuracy remains relatively low for identifying the specific type of PCN, for differentiating small PCN from nonneoplastic or nonepithelial cysts, or for connection to the ductal system. [4] CT should be considered in the following clinical situations [4] :
-
For the detection of parenchymal, mural, or central calcification, and especially when differentiating pseudocysts associated with chronic pancreatitis from pancreatic cystic neoplasms.
-
When there is suspicion of malignacy or concomitant pancreatic cancer and when assessment of vascular involvement, peritoneal disease, or metastatic disease is required.
-
When there is suspicion of postoperative recurrence of pancreatic cancer.
The European Study Group guidelines find EUS helpful for identifying PCN with features that should be considered for surgical resection. Similar to MRI and CT, EUS has low accuracy for identifying the exact type of PCN. EUS-FNA improves diagnostic accuracy for differentiating mucinous versus nonmucinous PCN, as well as malignant versus benign PCN, in cases where CT or MRI findings are unclear. Additional recommendations for the use of EUS-FNA include the following:
-
EUS-FNA should only be performed when the results are expected to change clinical management.
-
EUS-FNA should not be performed if the diagnosis is already established by cross-sectional imaging or where there is a clear indication for surgery.
-
Relative contraindications for EUS-FNA in PCN is a distance of >10 mm between the cyst and the transducer, the presence of a high-risk of bleeding due to bleeding disorder, or the use of dual antiplatelet drugs.
Radiography
Plain radiographs may show tumor calcification in 10-15% of cases of microcystic adenomas. Calcification in microcystic adenoma presents as a central cluster arranged in a sunburst or stellate arrangement (as seen in the image below). In mucinous-type tumors, calcification tends to occur at the periphery of the tumor or in the walls of the cysts and to appear curvilinear. CT is more sensitive for detecting calcification than plain radiography.
 Pancreatic microcystic adenoma. Plain radiograph shows tumor calcification in a microcystic adenoma (left upper quadrant). Calcification in the microcystic adenoma presents as a central cluster arranged in a sunburst or stellate arrangement. Central calcification is better evaluated with CT than with radiography.
Pancreatic microcystic adenoma. Plain radiograph shows tumor calcification in a microcystic adenoma (left upper quadrant). Calcification in the microcystic adenoma presents as a central cluster arranged in a sunburst or stellate arrangement. Central calcification is better evaluated with CT than with radiography.
Upper GI barium studies are nonspecific and usually show extrinsic displacement of the stomach or duodenum. As most symptomatic cystic neoplasms are large, these findings are not infrequent.
Endoscopic retrograde cholangiopancreatography (ERCP) and/or pancreatoscopy are rarely indicated for the evaluation of pancreatic cystic lesions. In main-duct IPMN, duodenoscopy may reveal the highly specific finding of mucus extruding from a patulous pancreatic orifice. This pathognomonic finding is seen in 20-55% of patients with main-duct IPMN and has been seen more frequently in malignant disease in some, but not all, studies. A pancreaticoduodenal fistula extruding mucus is seen in up to 2% of IPMN cases and suggests malignant invasion. [16]
ERCP in MCN rarely shows cyst communication with pancreatic ducts, but it frequently shows duct displacement by mass effect or ductal obstruction.
(See the image below.)
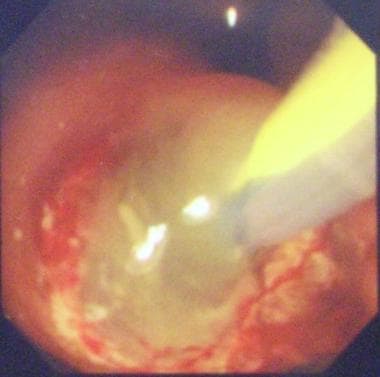 ERCP typically shows a patulous ampulla of Vater with discharging mucus, which is often diagnostic for IPMT.
ERCP typically shows a patulous ampulla of Vater with discharging mucus, which is often diagnostic for IPMT.
ERCP has a higher risk of adverse events and a lower sensitivity and specificity for identifying the type of PCN than conventional radiology does, and EUS should not be used for this indication. [4]
The accuracy rate of pancreatoscopy is higher in main-duct (MD) IPMN (88%) than in branch-duct (BD) IPMN (67%). Intraoperative main pancreatic duct (MPD) pancreatoscopy combined with frozen section of intraductal biopsies may be helpful in establishing the extent of IPMN involvement of the MPD and in assisting surgical decision-making regarding the extent of resection required. [4]
There are several causes of calcification in the pancreatic bed; these include chronic pancreatitis, pancreatic hemorrhage, abscess, infarction, hyperparathyroidism, cystic fibrosis, and kwashiorkor. Many pancreatic tumors can become calcified, including adenocarcinoma (rare), islet cell tumor, microcystic adenoma–sunburst calcification, MCN (macrocystic cystadenoma), cavernous lymphangioma, hemangioma, and colonic carcinoma metastases.
Abnormalities on upper GI barium series are also nonspecific and can be caused by many neoplastic and nonneoplastic masses near the stomach and duodenum. Pancreatic duct calculi and chronic pancreatitis may mimic IPMT, and vice versa.
Computed Tomography
Mucinous cystic neoplasms
Nonenhanced CT scans of mucinous cystic neoplasms show a well-defined, unilocular or multilocular, externally smooth, round-to-ovoid mass with fluid attenuation.
(See the image below.)
 Nonenhanced axial CT scans. Image 1 shows a large septate mass in the left hypochondrium. Note the smooth external contour typical of a mucinous cystic neoplasm.
Nonenhanced axial CT scans. Image 1 shows a large septate mass in the left hypochondrium. Note the smooth external contour typical of a mucinous cystic neoplasm.
The attenuation values of the multilocular cysts vary according to the degree of hemorrhage or protein in the mucoid cysts. Larger cysts may demonstrate small daughter cysts along their internal surface. Typically, they show a well-defined, multilocular cystic mass with thick internal septa separating the different cystic cavities of varying sizes. The cysts are 2-26 cm. [18, 19]
(See the images below.)
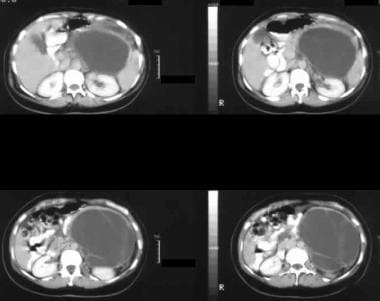 Enhanced axial CT scans show a large septate mass in the left hypochondrium with rim enhancement and enhancement of the septa. At surgery, a mucinous adenoma was confirmed. Note the smooth external contour typical of a mucinous cystic neoplasm.
Enhanced axial CT scans show a large septate mass in the left hypochondrium with rim enhancement and enhancement of the septa. At surgery, a mucinous adenoma was confirmed. Note the smooth external contour typical of a mucinous cystic neoplasm.
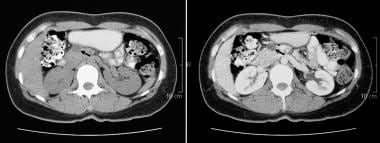 Nonenhanced (left) and contrast-enhanced axial CT scans through the pancreas. These scans confirm the presence of a cystic mass in the anterior part of the head of the pancreas. The contrast-enhanced image shows a septum within the mass. At surgery, a mucinous adenoma was confirmed.
Nonenhanced (left) and contrast-enhanced axial CT scans through the pancreas. These scans confirm the presence of a cystic mass in the anterior part of the head of the pancreas. The contrast-enhanced image shows a septum within the mass. At surgery, a mucinous adenoma was confirmed.
Visualization of nodular or papillary excrescences with irregular borders of the septa is possible. If present, calcification is curvilinear or punctate and confined to the cyst wall or septa. Contrast-enhanced CT scans show enhancement of the cyst wall, internal septations, mural nodules, and other intracavitary projections. CT may also allow the identification of solid components associated with cystic elements, which are features of borderline or malignant tumors but not benign variants. CT more clearly demonstrates enhancement of cystic walls and septa than do other studies.
Intraductal papillary mucinous neoplasms
Main-duct IPMNs may be focal or diffuse, findings that are reflected on imaging. However, main-duct tumors may be difficult to differentiate from chronic pancreatitis, as the imaging features may be similar. [20]
(See the images of IPMNs below.)
 Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a 5.5-cm cystic tumor in the pancreatic head. Note the upstream, gross dilatation of the pancreatic duct. The accessory pancreatic duct is also dilated.
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a 5.5-cm cystic tumor in the pancreatic head. Note the upstream, gross dilatation of the pancreatic duct. The accessory pancreatic duct is also dilated.
 Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a multiseptate tumor in the head of the pancreas.
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a multiseptate tumor in the head of the pancreas.
 Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced CT scans through the pancreas show gross dilatation of the pancreatic duct. At surgery, IPMT was confirmed.
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced CT scans through the pancreas show gross dilatation of the pancreatic duct. At surgery, IPMT was confirmed.
In the early stages of focal or segmental involvement by IPMN, the features may be difficult to differentiate from focal chronic obstructive pancreatitis on cross-sectional imaging. In these cases, the findings on ERCP may be diagnostic. Rarely, segmental pancreatic duct dilatation may acquire a cystic appearance, whereas the MPD and the rest of the pancreas appear normal. Cases with a cystic appearance may mimic a peripheral MCN. However, with a peripheral MCN, the pancreatic duct is almost always normal in appearance.
When the tumor involves the head of the pancreas, imaging reveals upstream dilatation of the pancreatic duct.
IPMN can be more confidently diagnosed when imaging reveals a filling defect in the main duct or a branch pancreatic duct. The filling defects are hyperechoic on sonograms, high attenuating on CT scans, and hypointense on T2-weighted MRIs relative to the surrounding fluid.
When there is diffuse involvement of the MPD, dilatation is present along the whole length of the pancreatic duct. This dilatation is often associated with diffuse and generally uniform pancreatic atrophy. These features may be difficult to differentiate from those of chronic pancreatitis. However, dilatation of a branch duct is a frequent finding in IPMN, and the presence of mural nodules and mucin blobs is another finding that may be a clue to the diagnosis.
In late cases with advanced disease, mass effect from the tumor may cause common bile duct (CBD) compression and dilatation of the biliary tree and compression or displacement of the stomach and duodenum. Pancreatobiliary fistula may be a late complication. Advanced stages of the disease may be complicated by pseudomyxoma peritonei as a result of dissemination of the disease to the peritoneum and retroperitoneum. Peritoneal seedlings can be identified as small foci that appear hyperechoic on sonography and hyperattenuating on CT.
Branch-duct IPMNs may be easier to identify than main-duct IPMNs, as the former generally appear as mass lesions on imaging. When these lesions are small, they are commonly an incidental finding in patients undergoing imaging for unrelated conditions. Branch IPMN is most frequently encountered in the region of the uncinate process. Branch IPMN may be microcystic or macrocystic.
The microcystic variety may mimic serous cystadenomas/cystadenocarcinomas on imaging, but communication with the MPD (which is frequently dilated) may be a clue to the diagnosis. The macrocystic variety must be differentiated from other cystic masses.
The thickness of the cyst wall and septa is variable with benign tumors; they tend to be thin and regular. In malignant tumors, the walls and septa appear irregular and thick, with solid nodules.
Degree of confidence
CT findings can be highly specific and are usually sufficient for a confident diagnosis. Bulging of the papilla into the duodenal lumen is virtually diagnostic of IPMT and is well demonstrated with CT and MRI.
CT is not useful for differentiating mucinous cystadenoma from cystadenocarcinomas, with the exception that papillary excrescences suggest malignancy and metastases prove malignancy. Mural nodules in IPMN can be difficult to differentiate from mucin blobs, as a clear attachment of the mural nodules to the pancreatic duct wall must be demonstrated; this may be difficult to define. However, mural nodules tend to enhance after the intravenous administration of contrast material, whereas mucin blobs do not.
When IPMN involves the entire MPD, differentiation from chronic pancreatitis may not be possible on imaging.
Kluger et al described a potential false-positive CT finding for a mucinous cystic neoplasm in the head of the pancreas. The authors presented a case of a 49-year-old woman with 2 years of intermittent epigastric pain found to have an 8.5-cm head-of-the-pancreas mass on CT. CT findings were suggestive of a mucinous cystic neoplasm for which she underwent pancreaticoduodenectomy. Histology revealed a bronchogenic cyst of the head of the pancreas. [21]
Bronchogenic cysts of ventral foregut origin can migrate into the abdomen before fusion of the diaphragm. These cysts can be easily misinterpreted as benign and malignant retroperitoneal lesions. Both mucinous cystic neoplasms and bronchogenic cysts can undergo malignant transformation. They can also become infected and hemorrhagic. With increasing use of high-resolution cross-sectional imaging, it is likely that more of these lesions may be recognized. The final diagnosis is offered by the histologic presence of ciliated respiratory epithelium and cartilage on pathology.
Magnetic Resonance Imaging
MRI is an excellent modality for the depiction of cystic pancreatic masses, showing their internal architecture to advantage. As with other cross-sectional imaging techniques, several mimics of MCN can be depicted on MRIs. These include benign and malignant neoplastic cysts and cysts of inflammatory origin.
The radiologic findings on cross-sectional imaging correlate well with the macroscopic features of mucinous cystadenoma and cystadenocarcinoma. Cystic lesions in MCN are hypointense or hyperintense on T1-weighted MRIs depending on protein content. Breathing-independent T2-weighted images, such as half-Fourier acquired single-shot turbo spin-echo (HASTE) images, show multiple hyperintense cysts separated by multiple hypointense septa. Intracystic excrescences and mural nodules also have low signal intensity, but they enhance significantly with gadolinium-based contrast agents.
(See the images below.)
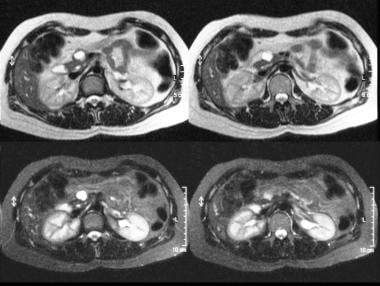 T2-weighted (top) and short-tau inversion recovery (STIR) (bottom) MRIs through the pancreas show a hyperintense lesion in the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
T2-weighted (top) and short-tau inversion recovery (STIR) (bottom) MRIs through the pancreas show a hyperintense lesion in the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
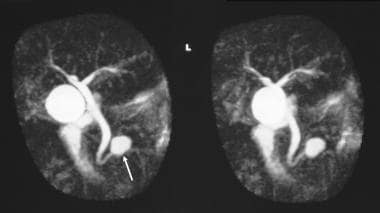 Magnetic resonance cholangiopancreatogram (MRCP) shows a cystic mass in the region of the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
Magnetic resonance cholangiopancreatogram (MRCP) shows a cystic mass in the region of the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
MRCP allows depiction of pancreatic duct or CBD changes that may be associated with the tumors.
Differentiation between serous and mucinous neoplasms may be difficult on MRIs, as there is variability in the MRI appearance of serous cystadenomas and overlap with mucinous neoplasms. CT criteria can be applied to MRI, except that calcification is often missed on MRI. Most serous tumors require histologic confirmation.
Magnetic resonance cholangiopancreatography (MRCP) is useful in differentiating between benign and malignant mucinous tumors, including IPMT of the pancreas. [20] The presence of mural nodules is suggestive of malignancy; however, the absence of mural nodules does not indicate that the tumor is benign.
A maximum MPD diameter greater than 15 mm and diffuse dilatation of the MPD are suggestive of malignancy in main-duct tumors. Among branch-duct tumors, malignant tumors tend to be larger than benign tumors; however, this finding is variable. The presence of MPD dilatation may be helpful in determining malignancy of branch-duct tumors. [22]
To study the frequency of incidental pancreatic cysts in asymptomatic individuals, Kromrey performed an MRCP examination in 1077 participants enrolled in a population-based cohort study. Of the original group, 676 people underwent a repeat examination 5 years later. At the time of the initial exam, 49% had at least one cyst of 2 mm or greater in diameter. The incidence of new cysts during the follow-up period was 2.6% per year. Cyst presence was strongly related to age; by 75 years of age, 75% of participants had one or more cysts. The discrimination between benign and premalignant or malignant pancreatic cysts is of high importance so as to avoid unnecessary procedures, such as radiologic follow-up examinations or surgical resection. [2]
According to a study of 63 MCN cases by Vullierme et al, the combination of signal heterogeneity on T2-weighted imaging, wall thickness 5 mm or greater, the presence of mural nodules 9 mm or greater, and enhancing septa had an area under the ROC (receiver-operating characteristic) curve of 0.97 for the diagnosis of malignant MCN. [10]
Hong et al found that enhancing mural nodules 5 mm or more and main pancreatic duct (MPD) diameter 10 mm or more on MRI were significant predictors of malignant IPMN. [11]
Ultrasonography
At presentation, mucinous cystic pancreatic neoplasms are usually larger than 5 cm. The walls of mucinous cysts are composed of thick, fibrous stroma that sometimes contain dystrophic calcification. Sonography reveals a large, cystic mass (as seen in the images below) that sometimes contains numerous septa, tumor excrescences, and debris. The tumors may be 2-23 cm, and they usually have sharply marginated walls and smooth borders. The cystic portions show a good through transmission. [13, 15]
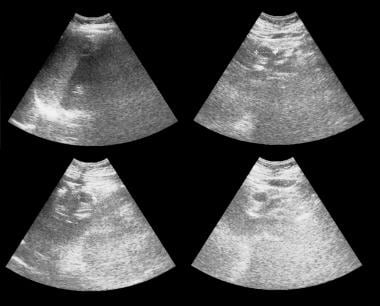 Axial and sagittal sonograms through the pancreas show a 1.93-cm cystic mass in the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
Axial and sagittal sonograms through the pancreas show a 1.93-cm cystic mass in the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
Microcystic adenomas (serous cystadenoma), an example of which is seen below, are mostly complex, with numerous internal echoes resulting in the appearance of an externally lobulated solid mass, sometimes with good through transmission. The central scar and calcification may be demonstrated on CT scans but are not well depicted on sonograms.
 Pancreatic microcystic adenoma. Sonogram shows a cystic mass in the region of the tail of the pancreas.
Pancreatic microcystic adenoma. Sonogram shows a cystic mass in the region of the tail of the pancreas.
Although histologic studies remain the criterion standard for the differential diagnosis of pancreatic tumors, Rickes et al concluded in a report that echo-enhanced power Doppler sonography has a high sensitivity and specificity for such diagnosis. [23] In their study, the authors selected 137 patients with a mean age of 60 years and clinically suspected pancreatic tumors. The patients were assessed with conventional sonography and nonenhanced and echo-enhanced power Doppler sonography. The sensitivity of echo-enhanced power Doppler sonography with respect to diagnosing pancreatic carcinoma was 87%, and its specificity was 94%. The corresponding values for chronic pancreatitis were 85% and 99%, respectively.
EUS appears to be reliable in distinguishing between most benign lesions and neoplastic cystic lesions. In equivocal cases or in cases in which malignancy is highly suspected, EUS-guided FNA gives the best diagnostic yield, as it permits the acquisition of cytologic samples and cystic fluid for the analysis of various tumor markers. When the lesion is differentiated from other cystic masses and when the tumors are large and symptomatic, the best course of action is surgical resection. When small cystic lesions are encountered in asymptomatic patients, follow-up EUS may suffice. [15]
There is a wide differential diagnosis of cystic lesions in the pancreas, and therefore, the potential for false-positive results is significant. For smaller cystic lesions, a false-negative finding is possible in obese patients or in patients with gaseous distention.
Nuclear Imaging
Sperti et al concluded in a study that fluorodeoxyglucose (FDG) positron emission tomography (PET) is more accurate than CT scanning in identifying malignant pancreatic cystic lesions and should be used, in combination with CT and tumor marker assay, in the preoperative evaluation of patients with pancreatic cystic lesions. [24] In 56 patients with a suspected cystic tumor of the pancreas, values for sensitivity, specificity, and positive and negative predictive values for FDG-PET in detecting malignant tumors were 94%, 97%, 94%, and 97%, respectively. For CT scanning, these values were 65%, 87%, 69%, and 85%.
A study by Sendler and associates, however, indicated that PET does not allow the precise exclusion of malignant tumors and that, therefore, the use of invasive diagnostic procedures may not be reduced by employing this modality. In their study, FDG-PET was performed in 46 patients admitted to the hospital for pancreatic tumor surgery, [25] and the resulting values for sensitivity and specificity were 86% and 67%, respectively.
-
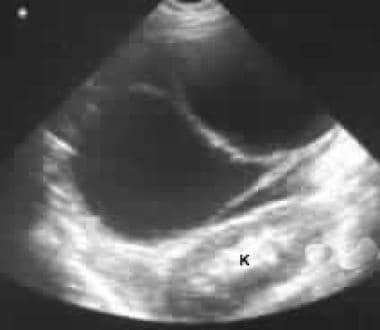
Sonogram through the left hypochondrium shows a large septate mass anterior to the kidney (K).
-
Nonenhanced axial CT scans. Image 1 shows a large septate mass in the left hypochondrium. Note the smooth external contour typical of a mucinous cystic neoplasm.
-
Enhanced axial CT scans show a large septate mass in the left hypochondrium with rim enhancement and enhancement of the septa. At surgery, a mucinous adenoma was confirmed. Note the smooth external contour typical of a mucinous cystic neoplasm.
-
Contrast-enhanced axial CT scans through the tail of the pancreas show a large, enhancing tumor occupying the left hypochondrium, with cystic and solid components. At surgery, a mucinous carcinoma of the pancreatic tail was confirmed.
-
Superior mesenteric angiograms show a hypervascular tumor. The tumor also derives its blood supply from the celiac axis (not shown). At surgery, a mucinous carcinoma of the pancreatic tail was confirmed.
-
Axial and sagittal sonograms through the pancreas show a 1.93-cm cystic mass in the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
-
Nonenhanced (left) and contrast-enhanced axial CT scans through the pancreas. These scans confirm the presence of a cystic mass in the anterior part of the head of the pancreas. The contrast-enhanced image shows a septum within the mass. At surgery, a mucinous adenoma was confirmed.
-
T2-weighted (top) and short-tau inversion recovery (STIR) (bottom) MRIs through the pancreas show a hyperintense lesion in the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
-
Magnetic resonance cholangiopancreatogram (MRCP) shows a cystic mass in the region of the head of the pancreas. At surgery, a mucinous adenoma was confirmed.
-
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a 5.5-cm cystic tumor in the pancreatic head. Note the upstream, gross dilatation of the pancreatic duct. The accessory pancreatic duct is also dilated.
-
Pancreatic intraductal papillary mucinous tumor (IPMT). (Top) Superior mesenteric angiogram shows capillary vascularity in the mass in the pancreatic head during the arterial phase. (Bottom) The portal venous phase image shows displacement of the portal venous branches and encasement of the junction of the superior mesenteric vein and the portal vein. M denotes the pancreatic mass.
-
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced axial CT scans through the pancreas show a multiseptate tumor in the head of the pancreas.
-
Pancreatic intraductal papillary mucinous tumor (IPMT). Contrast-enhanced CT scans through the pancreas show gross dilatation of the pancreatic duct. At surgery, IPMT was confirmed.
-
Pancreatic microcystic adenoma. Plain radiograph shows tumor calcification in a microcystic adenoma (left upper quadrant). Calcification in the microcystic adenoma presents as a central cluster arranged in a sunburst or stellate arrangement. Central calcification is better evaluated with CT than with radiography.
-
Pancreatic microcystic adenoma. Sonogram shows a cystic mass in the region of the tail of the pancreas.
-
Pancreatic microcystic adenoma. Contrast-enhanced axial CT scans show a hypervascular tumor in the pancreatic tail with sunburst calcification. Note the Swiss-cheese enhancement.
-
ERCP typically shows a patulous ampulla of Vater with discharging mucus, which is often diagnostic for IPMT.