Practice Essentials
Cystic lesions of the pancreas are common, and 80-90% of these lesions are pseudocysts, or retention cysts. Cystic neoplasms of the pancreas are less common, accounting for about 10-15% of all cystic pancreatic lesions. True cysts of the pancreas are rare. The 2 most common cystic neoplasms of the pancreas are serous cystadenoma and mucinous cystic neoplasm. Serous cystadenomas are more common than mucinous cystic neoplasms, at a ratio of about 2:1. Intraductal papillary mucinous tumor (IPMT) is a more recently discovered cystic neoplasm that may be a variant of the mucinous cystic neoplasm. The important point to remember is that serous cystadenoma is benign, whereas the biologic behavior of the mucinous cystic neoplasm and the IPMT ranges from benign to malignant. Therefore, distinguishing these entities is of utmost importance. [1, 2, 3, 4, 5, 6]
Pancreatic serous cystadenomas are commonly found in women 60 years of age or older (approximately 75% of cases). In a study of 2622 patients with serous cystadenoma, 74% were women, with a mean age of 58 years. [7] These lesions are generally small (mean diameter, 31 mm), but giant pancreatic serous cystadenomas (≥10 cm) have been reported. [8] They can be categorized as microcystic, honeycomb, oligocystic, or solid, based on appearances on imaging. [5] Lesions occur more frequently in the body or tail of the pancreas. [9]
Serous cystadenomas are benign tumors that rarely transform into malignancies; surgery is generally indicated only for large serous cystadenomas. [6]
Imaging modalities
Ultrasonography can be used to detect, and sometimes characterize, features of this tumor, especially when the classic features are present. In comparison, computed tomography (CT) is superior in lesion depiction and characterization and is the preferred imaging modality in the initial workup of pancreatic lesions. However, the first test performed is usually ultrasonography because the typical presenting symptom is abdominal pain and/or nausea. [3, 10, 11, 12, 13]
Endoscopic ultrasonography allows high-resolution evaluation of cyst morphology. To avoid serious complications of pancreatic surgery, serous cystadenoma should be diagnosed accurately at the preoperative level. Preoperative diagnosis requires a multimodal diagnostic approach that includes imaging, cystic fluid biochemical analysis, and/or endoscopic ultrasound fine-needle aspiration (EUS-FNA). EUS-FNA can provide additional information, can be performed to diagnose malignancy, and can be used to monitor changes in pancreatic cysts. It can help identify lesions that will require resection. [12, 13, 14]
Magnetic resonance imaging (MRI) can be a useful problem-solving adjunct in select cases. For example, when evaluation of the ducts is needed, magnetic resonance cholangiopancreatography (MRCP) can be useful.
Findings from plain radiography and upper GI series are nondiagnostic, except the finding of a classic sunburst central calcification, which is suggestive of a serous cystadenoma.
(See the images below.)
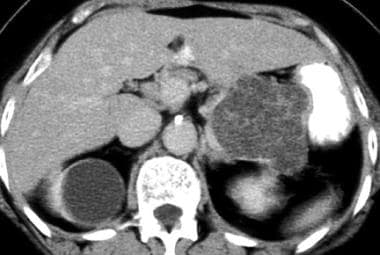 Serous cystadenoma on a contrast-enhanced CT scan. Note the Swiss cheese–like enhancement and the gentle external lobulation.
Serous cystadenoma on a contrast-enhanced CT scan. Note the Swiss cheese–like enhancement and the gentle external lobulation.
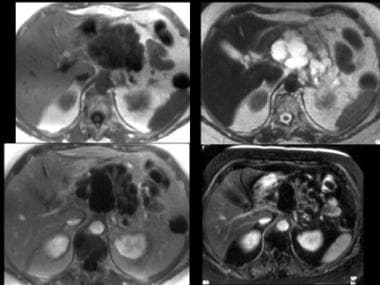 MRIs of serous cystadenoma. Top left, T1-weighted image; top right, T2-weighted image; bottom left, T1-weighted gadolinium-enhanced image; bottom right, fat-suppressed T1-weighted gadolinium-enhanced image. The mass is externally lobulated and hypointense on the T1-weighted image and is hyperintense on the T2-weighted image with septal enhancement and, atypically, some larger cysts. Image courtesy of Arnold C. Friedman, MD, FACR.
MRIs of serous cystadenoma. Top left, T1-weighted image; top right, T2-weighted image; bottom left, T1-weighted gadolinium-enhanced image; bottom right, fat-suppressed T1-weighted gadolinium-enhanced image. The mass is externally lobulated and hypointense on the T1-weighted image and is hyperintense on the T2-weighted image with septal enhancement and, atypically, some larger cysts. Image courtesy of Arnold C. Friedman, MD, FACR.
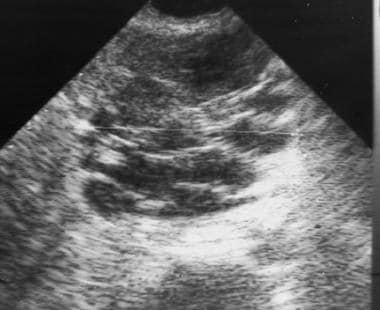 Sonogram of serous cystadenoma. The large mass in the head of the pancreas is externally lobulated, with some cystic-appearing regions, some solid-appearing regions, and increased through transmission. Image courtesy of Arnold C. Friedman, MD, FACR.
Sonogram of serous cystadenoma. The large mass in the head of the pancreas is externally lobulated, with some cystic-appearing regions, some solid-appearing regions, and increased through transmission. Image courtesy of Arnold C. Friedman, MD, FACR.
Because of significant overlap in the imaging findings of mucinous and serous pancreatic tumors, these tumors should be followed up with surveillance CT scanning to assess interval growth if aspiration is not performed.
A serous cystadenoma should be diagnosed with caution unless the lesion has all of the typical findings. If a mucinous cystic lesion is incorrectly diagnosed as a serous cystadenoma, and if surveillance CT or MRI scanning is not performed, there may be grave consequences. [15]
When stellate calcification or the classic CT scan features of central sunburst are present in a multilocular cystic mass in an older woman, some institutions accept this as a clearly benign finding. However, in one series, CT scanning was useful in depicting the lesions but was not useful in differentiating benign and malignant tumors, serous and mucinous tumors, or pseudocysts and neoplasms.
No radiographic abnormalities are associated with serous cystadenoma except those related to a mass that is large enough to displace or obstruct the bowel and those related to prominent central calcification. The main mimics of this tumor are pseudocysts and mucinous cystic tumors.
Serous cystadenomas typically demonstrate intense peripheral hypervascularity at angiography, although this finding is nonspecific.
Diagnosis
The exact etiology of serous cystadenomas is not well understood. Genetic mutations may contribute to formation of serous cystadenomas. Many patients present without any specific signs or symptoms. Often, pancreatic serous cystadenomas are detected incidentally by abdominal ultrasonography or cross-sectional imaging studies performed for another condition. [9]
The main differential diagnoses include mucinous cystic neoplasm, IPMT, pseudocyst, focal pancreatitis, and adenocarcinoma.
When patients are symptomatic, the most common presentation consists of abdominal pain and/or a palpable mass in the upper abdomen. Less commonly, patients with advanced cystic lesions present similarly to those with pancreatic ductal carcinoma, with abdominal pain, weight loss, early satiety secondary to compression of the stomach wall by the adjacent pancreatic mass (gastric outlet obstruction), obstructive jaundice due to obstruction of the biliary tree (may lead to acute cholangitis), and pancreatic duct obstruction causing pancreatic exocrine insufficiency and recurrent pancreatitis. [9]
Diagnosis of pancreatitis and pseudocyst is generally straightforward, especially in a clinical setting of chronic alcoholism with a history of pancreatitis. If the tumor is large enough, symptoms related to mass effects may predominate. Smaller tumors may be discovered incidentally on abdominal ultrasonogram or on CT scan.
Imaging findings are usually confirmatory in difficult cases. Adenocarcinomas are most commonly solid and therefore are infrequently confused with cystic neoplasms. Other entities can have imaging features that are highly suggestive of one entity rather than another. Communication with the pancreatic duct strongly suggests mucinous cystic neoplasm or IPMT, rather than serous cystadenoma.
A central stellate scar with calcification and a grapelike cluster of cysts and external lobulation strongly suggest serous cystadenoma. However, imaging features of these entities can overlap considerably; therefore, analysis of percutaneous cells and cystic fluid is often required for diagnosis. In fact, approximately 10% of all serous cystadenomas have cystic components larger than 2 cm that cannot be distinguished from mucinous cystic neoplasms.
Overall, serous cystadenomas are considered benign with small malignant potential (< 3%). The prognosis for patients with serous cystadenoma is excellent. Reports describe long-term survival after resection. Studies have concluded that any serous cystadenoma has the potential to grow over time regardless of tumor subtype, size, or location; therefore, all serous cystic neoplasms should be followed up regularly. [9]
Computed Tomography
Classically, these lesions have a mean diameter of 5 to 8 cm (range, 4-20 cm) and a lobulated external contour. They are composed of a grapelike cluster or a honeycomb pattern of 6 or more uniformly sized cysts that measure 2 cm or smaller. They tend to occur in the head or neck of the gland, although biliary obstruction is present in only about 15% of cases. [5, 6, 8, 16]
In about 30% of cases, a central, stellate, late-enhancing scar is evident with calcification. Small septa and internal debris may be seen in individual cysts. Because the capsule of these tumors is poorly developed, they are often poorly differentiated from the surrounding pancreatic parenchyma. No communication with the pancreatic duct occurs, except in rare cases.
(See the images below.)
 Serous cystadenoma on a contrast-enhanced CT scan. Note the Swiss cheese–like enhancement and the gentle external lobulation.
Serous cystadenoma on a contrast-enhanced CT scan. Note the Swiss cheese–like enhancement and the gentle external lobulation.
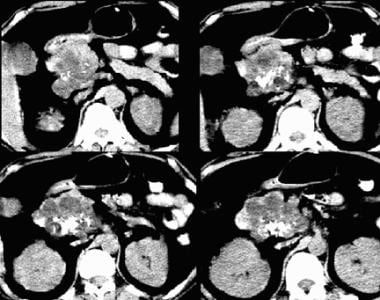 Serous cystadenoma on a nonenhanced CT scan. Note central calcification, attenuation similar to that of water, and external lobulation.
Serous cystadenoma on a nonenhanced CT scan. Note central calcification, attenuation similar to that of water, and external lobulation.
A serous cystadenoma generally has attenuation similar to that of water on nonenhanced scans, and it typically enhances with contrast in a Swiss cheese–like pattern. Some tumors have a few cysts larger than 2 cm.
In a retrospective review, Yang and colleagues examined the utility of textural features in combination with morphologic characteristics in the differential diagnosis of pancreatic serous and mucinous cystadenomas. They concluded that the combination of morphologic characteristics and textural features can significantly improve the diagnostic performance of CT, which may provide a reliable method for selecting patients with surgical intervention indications in consideration of the different treatment principles of these 2 diseases. [17]
Magnetic Resonance Imaging
Serous cystadenomas are usually hyperintense on T2-weighted images and hypointense on T1-weighted images (see the image below). Wang and coworkers investigated the difference in tumor textures presented on T1-weighted images (T1WI) and T2-weighted images (T2WI) and determined that texture analysis of T1WI and T2WI may be a simple and effective tool in the differential diagnosis between pancreatic serous cystadenoma and mucinous cystadenoma. [18] Occasionally, debris (especially hemorrhage) in the cysts alters this signal intensity pattern. Septa are well depicted on T2-weighted images, but the central scar is not seen. [19]
 MRIs of serous cystadenoma. Top left, T1-weighted image; top right, T2-weighted image; bottom left, T1-weighted gadolinium-enhanced image; bottom right, fat-suppressed T1-weighted gadolinium-enhanced image. The mass is externally lobulated and hypointense on the T1-weighted image and is hyperintense on the T2-weighted image with septal enhancement and, atypically, some larger cysts. Image courtesy of Arnold C. Friedman, MD, FACR.
MRIs of serous cystadenoma. Top left, T1-weighted image; top right, T2-weighted image; bottom left, T1-weighted gadolinium-enhanced image; bottom right, fat-suppressed T1-weighted gadolinium-enhanced image. The mass is externally lobulated and hypointense on the T1-weighted image and is hyperintense on the T2-weighted image with septal enhancement and, atypically, some larger cysts. Image courtesy of Arnold C. Friedman, MD, FACR.
Use of a gradient-echo pulse sequence with a long echo time (TE) may bring out susceptibility effects from the calcified scar. MRCP is helpful in demonstrating the relationship of the mass to the main pancreatic duct. The main duct is almost never obstructed, but the duct and its branches may be gently splayed and draped.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). This disease has occurred in patients with moderate to end-stage renal disease after they were given a gadolinium-based contrast agent to enhance magnetic resonance imaging or magnetic resonance angiography scans.
Rarely, a fluid-filled diverticulum of the transverse duodenum may mimic a cystic pancreatic mass.
Ultrasonography
The cluster-of-grapes pattern and external lobulation may be seen. However, when cysts are small, the mass can be echogenic (because of the large number of acoustic interfaces) and can appear solid (see the image below). This finding may suggest the presence of an adenocarcinoma. The presence of increased through transmission, even if the mass is fairly echogenic, should suggest the diagnosis. [12, 20]
In some studies, contrast-enhanced ultrasound has been shown to be superior to standard ultrasound in diagnosing cystic pancreatic lesions and in characterizing different pancreatic pathologies. Serous cystadenomas show features of a head-neck location, a lobulated shape, a honeycomb pattern, and a thin wall (< 3 mm). [21]
Nuclear Imaging
Octreotide (OctreoScan) can be used to detect pancreatic tumors that express somatostatin receptors (eg, neuroendocrine tumors), because this agent is avid for these receptors. However, serous cystadenomas do not typically express these receptors.
The ability of positron emission tomography (PET) scanning to depict these tumors will likely be proven because it is sensitive to hypermetabolic processes. However, PET is not yet approved for use in the workup of pancreatic lesions. Thallium 201–chloride single-photon emission computed tomography (SPECT) can depict malignant, as well as some benign, pancreatic processes. [22]
Latest Developments
Developments in the diagnosis and treatment of pancreatic cystic neoplasms include the following [9] :
-
Ex vivo optical coherence tomography (OCT) of freshly resected pancreatectomy specimens has shown that mucinous cysts could be differentiated from nonmucinous cysts. OCT is an interferometric technique that typically uses near-infrared light. It allows noninvasive assessment of biological tissues by measuring their optical reflections.
-
Confocal laser endomicroscopy (CLE) is a novel imaging technology that uses a low-power laser. Needle-based CLE (nCLE) is used for intra-abdominal organs under endoscopic ultrasound (EUS) guidance. The nCLE mini probe is introduced into the lesion through a fine-needle aspiration (FNA) needle to obtain a biopsy. Pancreatitis has been reported as an adverse complication in a few cases.
-
EUS-guided pancreatic cyst ablation uses EUS guidance to inject a cytotoxic agent—either ethanol or paclitaxel—after puncture of the pancreatic cyst. Injection of a cytotoxic agent may ablate the cyst epithelium.
-
Serous cystadenoma on a contrast-enhanced CT scan. Note the Swiss cheese–like enhancement and the gentle external lobulation.
-
Serous cystadenoma on a nonenhanced CT scan. Note central calcification, attenuation similar to that of water, and external lobulation.
-
MRIs of serous cystadenoma. Top left, T1-weighted image; top right, T2-weighted image; bottom left, T1-weighted gadolinium-enhanced image; bottom right, fat-suppressed T1-weighted gadolinium-enhanced image. The mass is externally lobulated and hypointense on the T1-weighted image and is hyperintense on the T2-weighted image with septal enhancement and, atypically, some larger cysts. Image courtesy of Arnold C. Friedman, MD, FACR.
-
Sonogram of serous cystadenoma. The large mass in the head of the pancreas is externally lobulated, with some cystic-appearing regions, some solid-appearing regions, and increased through transmission. Image courtesy of Arnold C. Friedman, MD, FACR.





