Practice Essentials
Osteosarcoma is the most common primary malignant tumor of bone, excluding plasma cell myeloma. High-grade intramedullary osteosarcoma is the classic, or conventional, form accounting for approximately 80% of all lesions. [1] Osteosarcoma has 2 peak ages: 18 and 60 years of age. [2] Osteosarcoma may affect any bone but most frequently occurs in the metaphyseal areas of the distal femur and proximal tibia. Osteosarcoma spreads hematogenously, with metastasis to the lung being most common. [1] It is treated by a combination of surgical excision and chemotherapy. [3, 4, 5]
For patients with classic osteosarcoma, radiography is almost always the initial imaging modality. Once the diagnosis is suspected, magnetic resonance imaging (MRI) is essential to determine the distribution of the tumor within the bone and the extent of any associated soft tissue mass. Computed tomography (CT) scanning is less sensitive than MRI in local evaluation of the tumor, but it is used in the staging of pulmonary metastases. [5, 6, 7, 8] Suspicion of osteosarcoma that is based on radiographic findings still requires a bone biopsy specimen for definitive diagnosis. CT imaging of the chest should also be performed to identify lung nodules. [2]
Ultrasonography is not routinely used in the staging of classic osteosarcoma lesions. The modality may be useful in guiding percutaneous biopsy. In patients treated with prosthetic implants, sonography may be the only imaging modality that can depict early local recurrence, because of the artifact produced by the metal on CT scans or MRIs.
Histologic confirmation of the nature of the tumor is required initially and should be performed only after baseline MRI studies are made.
(The radiologic characteristics of osteosarcoma are demonstrated in the images below.)
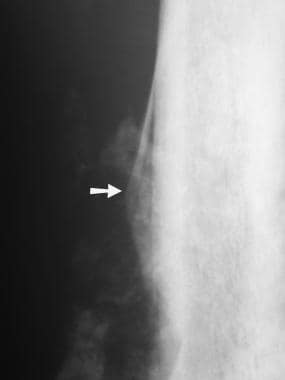 Osteosarcoma. Radiograph of the femur in a patient with osteosarcoma shows a typical Codman triangle (arrow) and more diffuse, mineralized osteoid within the soft tissues adjacent to the bone.
Osteosarcoma. Radiograph of the femur in a patient with osteosarcoma shows a typical Codman triangle (arrow) and more diffuse, mineralized osteoid within the soft tissues adjacent to the bone.
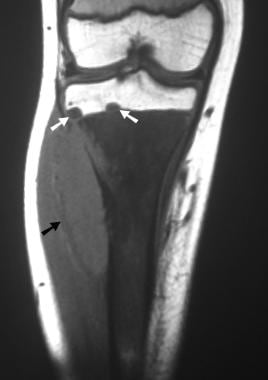 Osteosarcoma. Coronal T1-weighted MRI. Note the abnormal signal intensity in the metaphyseal marrow and the soft tissue mass (black arrow). Early tumor extension is shown beyond the growth plate into the epiphysis (white arrows).
Osteosarcoma. Coronal T1-weighted MRI. Note the abnormal signal intensity in the metaphyseal marrow and the soft tissue mass (black arrow). Early tumor extension is shown beyond the growth plate into the epiphysis (white arrows).
Guidelines
The National Comprehensive Cancer Network (NCCN) recommends that the initial workup for osteosarcoma include imaging of the primary site by MRI with or without CT scanning and chest imaging, including chest CT and head-to-toe PET/CT scan and/or bone scan. More detailed imaging of abnormalities identified on primary imaging by CT or MRI is required when metastatic disease is suspected. Repeat imaging using pretreatment imaging modalities should be performed following chemotherapy to reassess the tumor for resectability. [1]
Guidelines of the European Society of Medical Oncology (ESMO), the European Reference Network for Paediatric Cancers (PaedCan), and the European Network for Rare Adult Solid Cancer (EURACAN) recommend radiography for the initial evaluation of osteosarcoma. When the diagnosis of malignancy cannot be excluded with certainty on radiographs, MRI of the entire compartment, along with adjacent joints, should be performed. A CT scan may be used to provide additional information on calcification, periosteal bone formation, or cortical destruction. CT scans can also be performed as the preferred imaging study for other primary sites. For disease staging of the extremities, spine, and pelvis, MRI is preferred. [9]
The Sarcoma European Latin-American Network (SELNET) published guidelines with a specific focus on patients in Latin-American countries. The recommended initial imaging test is plain radiography of the primary site and MRI of the entire compartment and nearby joints. In cases of a suspected osteosarcoma of the trunk and head and neck, a CT scan should be performed. [10]
Chest CT scan with or without abdominal and pelvic CT scan are recommended for initial staging. If available, whole-body FDG-PET or whole-body MRI may be used for staging. The SELNET guidelines note that bone scintigraphy is an adequate alternative to exclude bone involvement if PET-CT and whole-body MRI are not available. [10]
Surveillance
Because of the potential for development of local recurrence and distant metastasis after resection of a primary bone malignancy, ongoing surveillance is considered an essential aspect of osteosarcoma management. However, minimal data exist regarding the incidence and timing of these events. [11]
The NCCN guidelines recommend that after treatment, surveillance should be done every 3 months for 2 years, every 4 months for year 3, every 6 months for years 4 and 5, and annually thereafter. Surveillance includes chest imaging and imaging of the primary site as performed during the initial evaluation. Head-to-toe PET/CT and/or bone scan may also be considered. [1]
ESMO-PaedCan-EURACAN guidelines recommend surveillance with imaging of the local site and chest radiography/CT. Follow-up intervals after the completion of chemotherapy are suggested every 2-3 months for the first 2 years, every 6 months for years 3-5, every 6-12 months for years 5-10, and every 0.5 to 1-2 years thereafter according to local practice. [9]
SELNET recommends surveillance schedules for high- and low-grade tumors as follows [10] :
-
High-grade tumors: every 3-4 months for the first 2-3 years, every 6 months up to year 5, and then annually up to year 10
-
Low-grade tumors: every 6 months for 2-5 years and annually thereafter
Radiography
Radiographs are essential in the initial evaluation of bone lesions, because the results may suggest the diagnosis and ensure appropriate further investigation. Radiographic appearances are variable, as demonstrated in the images below. Most lesions show a mixture of lytic and sclerotic areas. Rarely, purely lytic or sclerotic lesions occur. Lesions appear aggressive and either moth eaten with ill-defined edges or, occasionally, permeative with multiple small cortical holes. After chemotherapy, surrounding bone may form a better-defined shell around the tumor, in which case it appears more geographic. [12]
 Osteosarcoma. Radiograph of the femur in a patient with osteosarcoma shows a typical Codman triangle (arrow) and more diffuse, mineralized osteoid within the soft tissues adjacent to the bone.
Osteosarcoma. Radiograph of the femur in a patient with osteosarcoma shows a typical Codman triangle (arrow) and more diffuse, mineralized osteoid within the soft tissues adjacent to the bone.
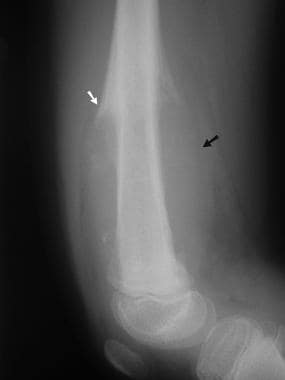 Osteosarcoma. Lateral radiograph of the distal femur in a child with osteosarcoma involving the metaphysis and metadiaphysis. Note the abnormal texture and mild sclerosis of the distal femoral shaft; the aggressive periosteal changes, including Codman triangles (white arrow); and the large soft tissue mass (black arrow).
Osteosarcoma. Lateral radiograph of the distal femur in a child with osteosarcoma involving the metaphysis and metadiaphysis. Note the abnormal texture and mild sclerosis of the distal femoral shaft; the aggressive periosteal changes, including Codman triangles (white arrow); and the large soft tissue mass (black arrow).
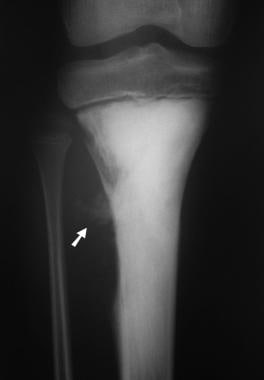 Osteosarcoma. Anteroposterior (AP) radiograph of the proximal tibia in a child with osteosarcoma involving the metaphysis. The tumor is densely sclerotic, but an area of lucency and cortical destruction is shown proximally on its lateral margin. Scalloping of the cortex is observed inferior to this area, with amorphous mineralized osteoid shown in the soft tissues (arrow). Note that the tumor appears to be superiorly confined by the growth plate.
Osteosarcoma. Anteroposterior (AP) radiograph of the proximal tibia in a child with osteosarcoma involving the metaphysis. The tumor is densely sclerotic, but an area of lucency and cortical destruction is shown proximally on its lateral margin. Scalloping of the cortex is observed inferior to this area, with amorphous mineralized osteoid shown in the soft tissues (arrow). Note that the tumor appears to be superiorly confined by the growth plate.
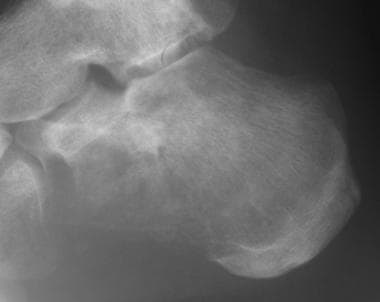 Osteosarcoma. Lateral radiograph of the calcaneum in an adult with osteosarcoma shows a predominantly lucent lesion in the anteroinferior part of the bone and cortical destruction.
Osteosarcoma. Lateral radiograph of the calcaneum in an adult with osteosarcoma shows a predominantly lucent lesion in the anteroinferior part of the bone and cortical destruction.
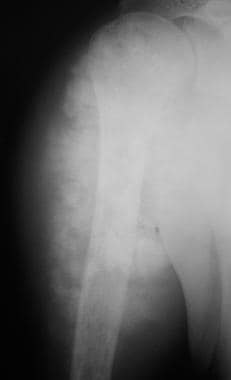 Osteosarcoma. Anteroposterior (AP) radiograph in a patient with osteosarcoma of the proximal humerus. Note the extensive soft tissue mass containing a considerable amount of mineralized osteoid.
Osteosarcoma. Anteroposterior (AP) radiograph in a patient with osteosarcoma of the proximal humerus. Note the extensive soft tissue mass containing a considerable amount of mineralized osteoid.
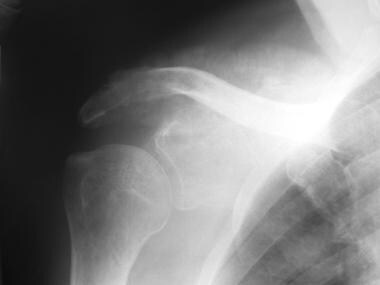 Osteosarcoma. Anteroposterior (AP) radiograph of the shoulder in a patient with osteosarcoma of the scapula. Note the extensive cortical destruction, aggressive periosteal changes, and soft tissue ossification of the acromion and upper scapula.
Osteosarcoma. Anteroposterior (AP) radiograph of the shoulder in a patient with osteosarcoma of the scapula. Note the extensive cortical destruction, aggressive periosteal changes, and soft tissue ossification of the acromion and upper scapula.
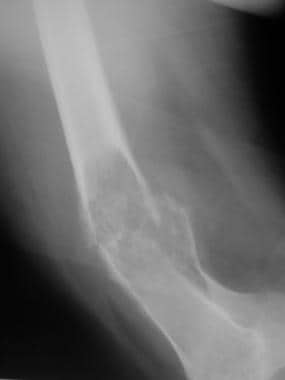 Osteosarcoma. Lateral radiograph of the distal femur in an adult patient with osteosarcoma appearing as a pathologic fracture.
Osteosarcoma. Lateral radiograph of the distal femur in an adult patient with osteosarcoma appearing as a pathologic fracture.
Soft tissue extension of osteosarcoma is common; on radiographs, such extension is seen as a soft tissue mass. Near joints, this extension may sometimes be difficult to differentiate from an effusion. Cloudlike areas of sclerosis, resulting from malignant osteoid production and calcification, may be seen within the mass. Periosteal reactions are commonly seen once the tumor extends through the cortex. A spectrum of changes occur, including Codman triangles and multilaminated, spiculated, and sunburst reactions, all of which indicate an aggressive process.
Although essential in establishing the diagnosis of classic osteosarcoma, the use of radiographs often leads to an underestimation of the tumor's extent within and outside of the bone. Other tumors, such as Ewing sarcoma, chondrosarcoma, and fibrosarcoma, as well as other aggressive conditions, such as infection or Langerhans cell histiocytosis, are part of the differential diagnosis.
Computed Tomography
CT scanning may be helpful locally when the radiographic appearances are confusing, particularly in areas of complex anatomy. Cross-sectional images provide a clearer indication of bone destruction, as well as the extent of any soft tissue mass, than do radiographs. It may depict small amounts of mineralized osseous matrix not seen on radiographs. The modality may be particularly helpful in visualizing flat bones, in which periosteal changes may be more difficult to appreciate.
CT scanning rarely alters the differential diagnosis when it is used to image conventional osteosarcoma, except when it leads to the detection of small amounts of mineralized osseous matrix that are undetectable on radiographs.
The modality is infrequently required in local evaluation of tumors in the long tubular bones, but it is the most accurate modality for staging pulmonary metastases. MRI more accurately shows soft tissue extension and medullary bone involvement.
Magnetic Resonance Imaging
MRI is the modality of choice in evaluating the local extent of disease because of its excellent bone marrow and soft tissue contrast and multiplanar capabilities. The MRI characteristics of osteosarcoma are demonstrated in the images below. [6, 13, 14]
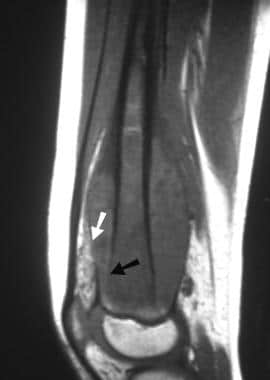 Osteosarcoma. Sagittal T1-weighted MRI. The signal intensity of the bone marrow within the distal femoral epiphysis is normal, but abnormal signal intensity is present throughout the visible shaft. The growth plate has limited extension of the tumor. Cortical destruction (arrow) and the soft tissue mass may be readily appreciated. Note that the fat deep to the quadriceps tendon has rather heterogeneous signal intensity.
Osteosarcoma. Sagittal T1-weighted MRI. The signal intensity of the bone marrow within the distal femoral epiphysis is normal, but abnormal signal intensity is present throughout the visible shaft. The growth plate has limited extension of the tumor. Cortical destruction (arrow) and the soft tissue mass may be readily appreciated. Note that the fat deep to the quadriceps tendon has rather heterogeneous signal intensity.
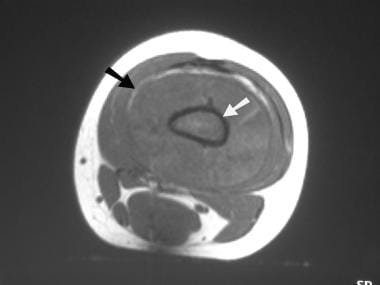 Osteosarcoma. Axial T1-weighted MRI. The abnormal medullary signal intensity (white arrow) and the soft tissue mass (black arrow) are identified.
Osteosarcoma. Axial T1-weighted MRI. The abnormal medullary signal intensity (white arrow) and the soft tissue mass (black arrow) are identified.
 Osteosarcoma. Coronal T1-weighted MRI. Note the abnormal signal intensity in the metaphyseal marrow and the soft tissue mass (black arrow). Early tumor extension is shown beyond the growth plate into the epiphysis (white arrows).
Osteosarcoma. Coronal T1-weighted MRI. Note the abnormal signal intensity in the metaphyseal marrow and the soft tissue mass (black arrow). Early tumor extension is shown beyond the growth plate into the epiphysis (white arrows).
MRI is the most important imaging technique for the accurate local staging of osteosarcoma; in addition, it assists in determining the most appropriate surgical management. For the purposes of staging, assessment of the relationship of a tumor to the anatomic compartment in which it originated and to other adjacent compartments is of vital importance. Compartments include individual bones, joints, and clearly defined, fascially enclosed soft tissue spaces. Disease confined to its original compartment is associated with a better prognosis than is disease that has spread into other compartments.
The intraosseous and extraosseous extent of the tumor must be assessed. Important features of intraosseous disease are the longitudinal distance of bone containing tumor, the involvement of adjacent epiphyses, and the presence or absence of skip metastases.
The most accurate sequence for determining the longitudinal extent of disease is the T1-weighted spin-echo sequence. The use of short-tau inversion recovery (STIR), seen in the images below, may significantly lead to an overestimation of disease, because edema and marrow hyperplasia may show high signal intensity similar to that of tumor. The maximum longitudinal extent of the tumor should be measured, and its maximal distance from the articular surface of the nearest joint should be recorded. The longitudinal extent is usually maximal within medullary bone, but occasionally, intracortical extension is more extensive.
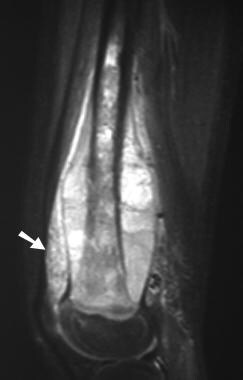 Osteosarcoma. Sagittal short-tau inversion recovery (STIR) MRI. Note the increased signal intensity (arrow) throughout the reactive zone within fat deep to the quadriceps tendon. Microinvasion by tumor and reactive edema cannot be differentiated in these areas.
Osteosarcoma. Sagittal short-tau inversion recovery (STIR) MRI. Note the increased signal intensity (arrow) throughout the reactive zone within fat deep to the quadriceps tendon. Microinvasion by tumor and reactive edema cannot be differentiated in these areas.
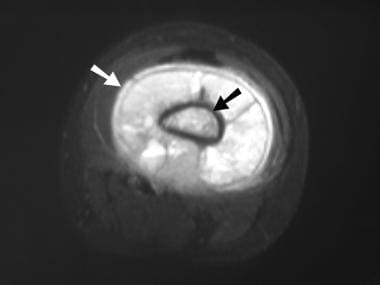 Osteosarcoma. Axial short-tau inversion recovery (STIR) MRI. The abnormal medullary signal intensity (black arrow) and the soft tissue mass (white arrow) are identified.
Osteosarcoma. Axial short-tau inversion recovery (STIR) MRI. The abnormal medullary signal intensity (black arrow) and the soft tissue mass (white arrow) are identified.
Epiphyseal extension of tumor is now known to occur considerably more frequently than was previously believed; it commonly is radiographically occult. Epiphyseal involvement may be diagnosed when abnormal signal intensity similar to that of the metaphyseal tumor is seen within the epiphysis in association with focal destruction of the growth plate. STIR and T1-weighted sequences are accurate in depicting epiphyseal tumor extension. STIR sequences are slightly more sensitive; T1 sequences are slightly more specific.
Skip metastases are synchronous foci of tumor that are anatomically separate from the primary lesion and that occur within the same bone. Secondary deposits on the other side of a joint are termed transarticular skip metastases. Patients with skip lesions are more likely to have distant metastatic disease and shorter periods of disease-free survival.
The assessment of the extent of extraosseous tumor involves a determination of which muscle compartments are involved and of the relationship of the tumor to neurovascular structures and adjacent joints. Radiologists must be aware of the compartmental anatomy of the affected area to clearly communicate with the patient's orthopedic oncologist. This is particularly important in planning routes for biopsy to avoid the contamination of previously uninvolved compartments, which would result in the patient's undergoing more extensive surgical resection.
The relationship of extraosseous tumor to neurovascular structures is well shown on STIR and fat-suppressed, T2-weighted images or on proton density–weighted sequences. The neurovascular bundle may be classified as free from tumor (when an intervening plane of muscle or fat is clearly seen), abutting tumor (when this tissue plane is abolished), or unequivocally involved (when infiltration or encasement by tumor is seen).
Joint involvement may be diagnosed when tumor tissue is seen to extend directly into the joint through subarticular bone and cartilage. In tumors of the knee joint, extension along the cruciate ligaments is also diagnostic.
The performance of dynamic contrast-enhanced (DCE) MRI has been evaluated in a number of these areas. DCE MRI is performed with a bolus injection of a gadolinium chelate (0.1 mmol/kg), which is ideally delivered by use of an automatic pump injector at a rate of 3 mL/sec to standardize the results. Ultrafast T1-weighted imaging is performed with either spin-echo or gradient-echo sequences.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
An evaluation of the images during the first seconds after injection provides information about the speed with which signal intensity changes in particular areas of tissue. Increases in signal intensity reflect the vascularity of different areas of tissue. The characteristics of the enhancement pattern differ in viable tumor, as compared to other tissues.
The use of DCE MRI adds extra cost and time to the examination, in addition to requiring intravenous access. Although it may have a role in the staging of osteosarcoma, many centers do not currently consider it a standard part of the staging protocol.
Degree of confidence
MRI is insensitive to small amounts of calcium. Calcium usually returns no signal, and large calcified areas appear hypointense on images obtained with all sequences. Small deposits of calcium surrounded by other tissue within the same small volume (voxel) may not be easily identified, because the signal from the entire voxel represents the average of contributions from all tissues within the voxel. This is termed the partial-volume effect.
The presence of a joint effusion alone has a low positive predictive value for intra-articular tumor.
DCE MRI has been shown to demonstrate occult, microscopic interosseous disease as far as 3.5 cm beyond the edge of tumor identifiable on images obtained with standard sequences. Its overall accuracy in detecting epiphyseal involvement is less than that of T1-weighted or STIR imaging. DCE MRI may be used to differentiate tumor-infiltrated muscle from edematous muscle on the basis of their different enhancement rates.
CT is the most accurate modality for detecting small amounts of calcification, although ultrasonography may be helpful in evaluating soft tissue extension when such extension is superficial.
The precise accuracy of MRI in detecting skip metastases is not clear, but one longitudinal sequence covering the entire bone should be performed to detect such lesions. The author prefers T1-weighted images; STIR images may also be used, although other abnormalities, such as focal marrow hyperplasia, may cause false-positive findings.
Nuclear Imaging
Osteosarcomas typically show increased uptake of radioisotope on bone scans obtained by use of technetium-99m (99mTc) methylene diphosphonate (MDP). Because osteosarcomas typically show increased uptake, bone scans are sensitive but are not specific. Increased isotope uptake is demonstrated in the image below. [6, 7]
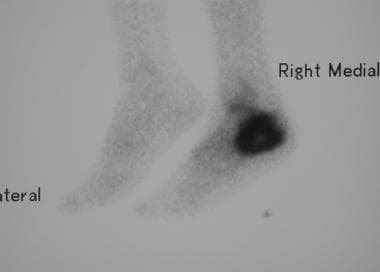 Osteosarcoma. Lateral isotope bone scan reveals intense uptake in the calcaneal region. The remainder of the skeleton appeared normal.
Osteosarcoma. Lateral isotope bone scan reveals intense uptake in the calcaneal region. The remainder of the skeleton appeared normal.
Bone scans are most useful in excluding multifocal disease. Skip lesions and pulmonary metastases may also take up the radioisotope, but skip lesions are most reliably excluded by MRI.
Multiple-gated acquisition (MUGA) cardiac scans may be required to monitor the toxic effects of certain chemotherapeutic agents by identifying changes in the left ventricular ejection fraction from a baseline prechemotherapy scan.
FDG PET/CT has been shown to be useful for staging, detecting recurrence, monitoring response to neoadjuvant chemotherapy, and predicting prognosis in patients with osteosarcoma. [15, 16] In a study by Quartuccio et al, FDG PET/CT had a higher accuracy than CT or dedicated MRI for detection of bone metastases in pediatric osteosarcoma (accuracy, 95% vs 67% for CT and 86% for MRI). [17]
-
Osteosarcoma. Radiograph of the femur in a patient with osteosarcoma shows a typical Codman triangle (arrow) and more diffuse, mineralized osteoid within the soft tissues adjacent to the bone.
-
Osteosarcoma. Lateral radiograph of the distal femur in a child with osteosarcoma involving the metaphysis and metadiaphysis. Note the abnormal texture and mild sclerosis of the distal femoral shaft; the aggressive periosteal changes, including Codman triangles (white arrow); and the large soft tissue mass (black arrow).
-
Osteosarcoma. Sagittal T1-weighted MRI. The signal intensity of the bone marrow within the distal femoral epiphysis is normal, but abnormal signal intensity is present throughout the visible shaft. The growth plate has limited extension of the tumor. Cortical destruction (arrow) and the soft tissue mass may be readily appreciated. Note that the fat deep to the quadriceps tendon has rather heterogeneous signal intensity.
-
Osteosarcoma. Sagittal short-tau inversion recovery (STIR) MRI. Note the increased signal intensity (arrow) throughout the reactive zone within fat deep to the quadriceps tendon. Microinvasion by tumor and reactive edema cannot be differentiated in these areas.
-
Osteosarcoma. Axial T1-weighted MRI. The abnormal medullary signal intensity (white arrow) and the soft tissue mass (black arrow) are identified.
-
Osteosarcoma. Axial short-tau inversion recovery (STIR) MRI. The abnormal medullary signal intensity (black arrow) and the soft tissue mass (white arrow) are identified.
-
Osteosarcoma. Anteroposterior (AP) radiograph of the proximal tibia in a child with osteosarcoma involving the metaphysis. The tumor is densely sclerotic, but an area of lucency and cortical destruction is shown proximally on its lateral margin. Scalloping of the cortex is observed inferior to this area, with amorphous mineralized osteoid shown in the soft tissues (arrow). Note that the tumor appears to be superiorly confined by the growth plate.
-
Osteosarcoma. Coronal T1-weighted MRI. Note the abnormal signal intensity in the metaphyseal marrow and the soft tissue mass (black arrow). Early tumor extension is shown beyond the growth plate into the epiphysis (white arrows).
-
Osteosarcoma. Lateral radiograph of the calcaneum in an adult with osteosarcoma shows a predominantly lucent lesion in the anteroinferior part of the bone and cortical destruction.
-
Osteosarcoma. Sagittal T1-weighted MRI. The true extent of the lesion within the calcaneum may be better appreciated on this image than on the preceding radiograph. Extension into the sinus tarsi and calcaneocuboid joint is also shown.
-
Osteosarcoma. Lateral isotope bone scan reveals intense uptake in the calcaneal region. The remainder of the skeleton appeared normal.
-
Osteosarcoma. Anteroposterior (AP) radiograph in a patient with osteosarcoma of the proximal humerus. Note the extensive soft tissue mass containing a considerable amount of mineralized osteoid.
-
Osteosarcoma. Anteroposterior (AP) radiograph of the shoulder in a patient with osteosarcoma of the scapula. Note the extensive cortical destruction, aggressive periosteal changes, and soft tissue ossification of the acromion and upper scapula.
-
Osteosarcoma. Lateral radiograph of the distal femur in an adult patient with osteosarcoma appearing as a pathologic fracture.







