Practice Essentials
Giant cell arteritis (GCA) is a systemic obliterative vasculitis mainly involving the arteries that originate from the arch of the aorta. However, any vessel in the body can be affected. The inflammation is a necrotizing obliterative vasculitis of large and medium-sized vessels. [1] The superficial temporal, vertebral, ophthalmic, and posterior ciliary arteries are more commonly affected than the internal and external carotid arteries. Intracranial arteries other than those involving the orbits are less commonly affected. Cases involving the proximal distal aorta and the subclavian and abdominal arteries have been reported. The inflammation is segmental, and therefore, skip lesions are seen. [2]
GCA is a disease of elderly persons, the incidence of which increases with increasing age. The mean age of onset is 70 years.
GCA is a medical emergency that can lead to irreversible blindness and other ischemic vascular events, if left untreated. Prompt access to specialist assessment, a fast-track diagnostic pathway, and appropriate treatment are key factors in preventing morbidity associated with this disease. [3]
Substantial improvement in the diagnosis and treatment of GCA is mainly attributed to the introduction of highly sensitive diagnostic tools, incorporation of modern imaging modalities for diagnosis and monitoring of large-vessel vasculitis, and the introduction of highly effective novel biologic therapies, such as the interleukin-6 inhibitor tocilizumab. [4, 5]
Research in the 2000s has shed new light on the clinical paradigm of this condition, expanding its spectrum beyond cranial vessel inflammation. GCA can now be considered a multifaceted vasculitic syndrome encompassing inflammation of cranial and extracranial arteries and girdles, isolated or combined. Such heterogeneity often leads to diagnostic delays and increases the likelihood of acute and chronic GCA-related damage. On the other hand, the approach to suspected GCA has been revolutionized by the introduction of vascular ultrasound, which allows a rapid, cost-effective, and noninvasive GCA diagnosis. [6]
Many unanswered questions remain about the optimal approach to imaging in GCA. For example, it is uncertain how best to monitor disease activity, given the frequent discordance between imaging findings and conventional disease activity measures; also, imaging changes typically fail to resolve completely with treatment. [7]
Preferred examination
The etiopathogenesis of GCA may involve a genetic background triggered by unknown environmental factors (eg infections), activation of dendritic cells, and inflammatory and vascular remodeling. However, its pathogenetic mechanism remains unclear, although progress has been made in this topic. In the past, inflammatory markers and arterial biopsy were considered the gold standard for the diagnosis of GCA. However, emerging imaging methods have been made more sensitive and specific for GCA diagnosis. [5]
Imaging is increasingly being used to guide clinical decision-making for patients with GCA. Although ultrasound has been rapidly adopted in fast-track clinics worldwide as an alternative to temporal artery biopsy for the diagnosis of cranial disease, whole-body positron emission tomography (PET)/computed tomography (CT) is emerging as a potential gold standard test for establishing large-vessel involvement. However, many unanswered questions remain about the optimal approach to imaging in GCA. [7]
Color Doppler ultrasonography (CDUS) is a method of assessing blood flow qualitatively and quantitatively. In the presence of arteritis, these sonograms may show a hypoechoic halo due to edema of the arterial wall. [8] A systematic review and meta-analysis found that, compared with a clinical diagnosis of GCA, the ‘halo’ sign at temporal arteries (8 studies, 605 patients) had a pooled sensitivity of 77% (95% CI, 62% to 87%) and specificity of 96% (95% CI, 62% to 87%). [9]
Although the presence of a halo sign on CDUS can used in the diagnosis of GCA, the sign is not seen in all patients with GCA, and it may also be seen in healthy persons. The halo has also been reported in polyarteritis nodosa. Therefore, temporal artery biopsy remains the criterion standard. In one study, however, performing US in all patients with suspected GCA and limiting biopsy to patients with negative scans increased the sensitivity of biopsy from 39% to 65% while maintaining specificity at 81%. [10]
The advantages of CDUS are that it is simple, it is noninvasive, and it can be used to examine several vessels, superficial and deep. Another advantage of this technique is that follow-up scans can be obtained to assess the response to steroids. The hypoechoic shadow becomes mid-echoic in about 2 weeks. With fibrosis, the shadow becomes hyperechoic. [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]
CDUS of temporal and axillary arteries is the most reliable imaging technique for the diagnosis of GCA, displaying high sensitivity and specificity. Nevertheless, CDUS is poorly performed in common clinical practice and generally is performed only by rheumatologists with relevant expertise.
Color Doppler eye ultrasound (CDEUS) is variously employed in ophthalmology; preliminary findings have shown a possible role in the diagnostic workup of GCA. Conticini et al in 2022 provided preliminary data displaying good reliability of CDEUS in distinguishing between arteritic and nonarteritic central retinal artery occlusion (CRAO) but not between arteritic and nonarteritic anterior ischemic optic neuropathy (AION). Given that AION represents the most common presentation of cranial GCA, CDEUS does not seem a reliable procedure in the diagnostic workup of GCA and its use should be restricted to excluding thromboembolic occlusions within the territory of the central retinal artery. [22]
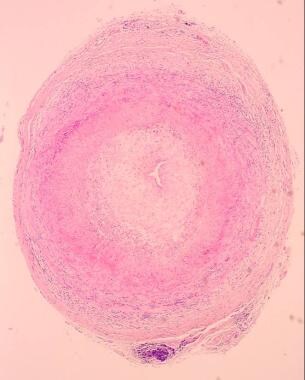
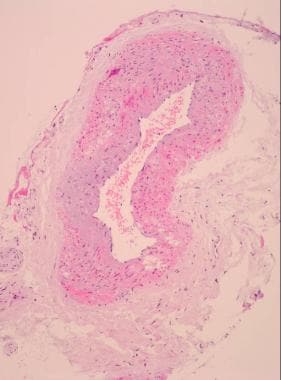
Other nonradiologic investigations include measurement of the erythrocyte sedimentation rate and temporal artery biopsy (see the images below). [23]
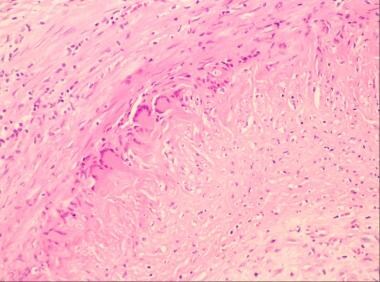 Giant cell arteritis. High-power view shows disruption of the intima with a collection of multinucleated giant cells.
Giant cell arteritis. High-power view shows disruption of the intima with a collection of multinucleated giant cells.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not useful in diagnosing GCA; however, they may be used to diagnose complications due to GCA, such as stroke.
A 2023 mini-review offers a critical appraisal of current imaging or histopathologic tools used to diagnose and monitor GCA. In this article, Monti et al provide an overview of the most updated evidence and current application of CDUS, temporal artery biopsy, 18-fluorodeoxyglucose [18F] FDG-PET/CT, MRI, and CT angiography. This review highlights the complementary value of available modalities to ensure a correct diagnosis of GCA and to provide valuable prognostic information. Novel evidence is accumulating to support the role of imaging, particularly US, as a monitoring tool for this disease, opening new perspectives for future management of large-vessel vasculitis. [24]
For patient education information, see Temporal Arteritis.
EULAR guidelines
European League Against Rheumatism (EULAR) guidelines, published in 2018, include the following recommendations on imaging in GCA [25] :
-
In patients with suspected GCA, early imaging is recommended to complement the clinical diagnostic criteria, assuming high expertise and prompt availability of the imaging technique. Imaging should not delay initiation of treatment.
-
In patients in whom there is a high clinical suspicion of GCA and a positive imaging study, the diagnosis of GCA may be made without biopsy or further imaging. In patients with a low clinical probability and a negative imaging result, the diagnosis of GCA can be considered unlikely. In all other situations, additional efforts toward a diagnosis are necessary.
-
Temporal artery ultrasound (US), with or without axillary artery US, is recommended as the first imaging modality in patients with suspected GCA with predominantly cranial manifestations (eg, headache, visual symptoms, jaw claudication, temporal artery swelling and/or tenderness). A non-compressible ‘halo’ sign is the US finding most suggestive of GCA.
-
If US is not available or the results are inconclusive, high-resolution MRI of cranial arteries to investigate mural inflammation may be used as an alternative for diagnosis of GCA.
-
CT and PET are not recommended for the assessment of inflammation of cranial arteries.
-
US, PET, MRI, and/or CT may be used for detection of mural inflammation and/or luminal changes in extracranial arteries to support the diagnosis of large-vessel GCA. US is of limited value for assessment of aortitis.
-
Conventional angiography is not recommended for the diagnosis of GCA, as it has been superseded by the above-mentioned imaging modalities.
-
In patients with GCA in whom a flare is suspected, imaging might be helpful to confirm or exclude it. Imaging is not routinely recommended for patients in clinical and biochemical remission.
-
MRA, CTA, and/or US may be used for long-term monitoring of structural damage in patients with GCA, particularly to detect stenosis, occlusion, dilatation, and/or aneurysms. The frequency of screening as well as the imaging method applied should be decided on an individual basis.
-
Imaging examination should be done by a trained specialist using appropriate equipment, operational procedures, and settings. The reliability of imaging, which has often been a concern, can be improved by specific training.
-
Giant cell arteritis. Low-power view of a temporal artery biopsy sample shows giant cell arteritis.
-
Giant cell arteritis. Low-power view of a normal temporal artery biopsy sample.
-
Giant cell arteritis. High-power view shows disruption of the intima with a collection of multinucleated giant cells.