Practice Essentials
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system, destroying the myelin and the axon in variable degrees and producing significant physical disability within 20–25 years in more than 30% of patients. White matter tracts are affected, including those of the cerebral hemispheres, infratentorium, and spinal cord. MS lesions, known as plaques, may form in CNS white matter in any location; thus, clinical presentations may be diverse. Continuing lesion formation in MS often leads to physical disability and, sometimes, to cognitive decline. [1] Magnetic resonance imaging (MRI) of the brain is useful in the diagnosis and treatment of multiple sclerosis.
Preferred examination
Radiologically, MRI has revolutionized the investigation, diagnosis, and even the treatment of MS. Usually, MRI is the only imaging modality needed for imaging patients with MS, and it far surpasses all other tests with respect to its positive predictive value. [1, 2, 3]
(See the images below.)
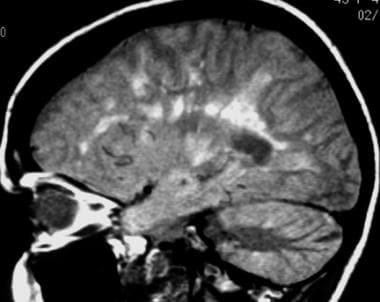
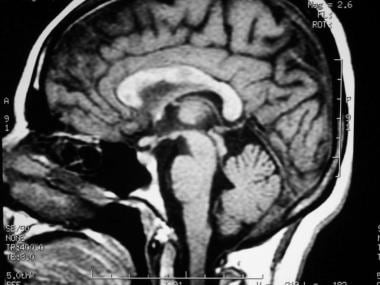 Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
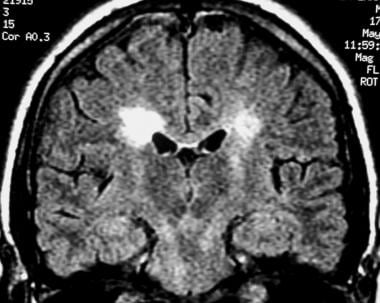 Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
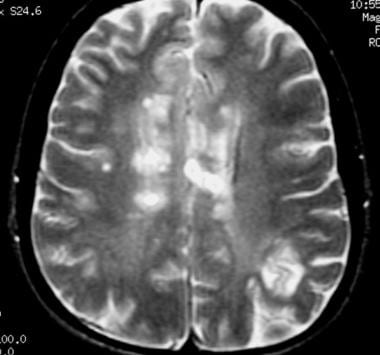 Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
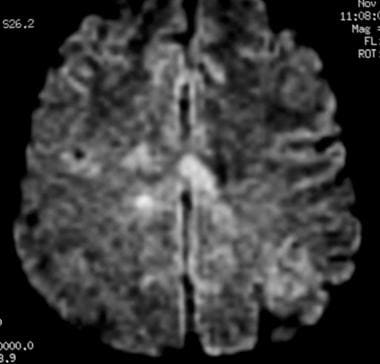 Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
One of the limitations of using MRI in patients with MS is the discordance occurring between lesion location and the clinical presentation. In addition, depending on the number and location of findings, MRI can vary greatly in terms of sensitivity and specificity in the diagnosis of MS. This is especially true of primary progressive MS, which may not show the classic discrete lesions of relapsing-remitting MS.
A clinician may be presented with an MRI report that notes nonspecific white matter lesions compatible with MS. Imaging findings need to be described in detail and, preferably, referenced to one of the published set of diagnostic criteria. [4, 5] Finally, the specific patient's neurologic history and clinical findings must be correlated with the imaging findings to establish an accurate diagnosis. [6]
Plain radiographic studies have no positive predictive value in the diagnosis of multiple sclerosis, but occasionally, plain radiographs may be used to exclude mechanical bony lesions. Angiography also has a limited role in the diagnosis and management of MS, but when central nervous system (CNS) vasculitis is considered in a patient with undifferentiated findings, angiography may occasionally be considered.
Cerebrospinal fluid (CSF) analysis for oligoclonal banding or immunoglobulin G (IgG) levels is no longer routine in the investigation of MS, although this test may be of use when MRI is unavailable or MRI findings are nondiagnostic. [7]
The Consortium of MS Centers Task Force, an international group of neurologists and radiologists, noted the following regarding standardized brain and spinal cord MR imaging for the diagnosis and follow-up of MS [8] :
-
A brain MR imaging with gadolinium is recommended for the diagnosis of MS.
-
A spinal cord MR imaging is recommended if the brain MR imaging is nondiagnostic or if the presenting symptoms are at the level of the spinal cord.
-
A follow-up brain MR imaging with gadolinium is recommended to demonstrate dissemination in time and ongoing clinically silent disease activity while on treatment, to evaluate unexpected clinical worsening, to reassess the original diagnosis, and as a new baseline before starting or modifying therapy.
-
A routine brain MR imaging should be considered every 6 months to 2 years for all patients with relapsing MS.
-
The brain MR imaging protocol includes 3D T1-weighted, 3D T2-FLAIR, 3D T2-weighted, post-single-dose gadolinium-enhanced T1-weighted sequences, and a DWI sequence.
-
The progressive multifocal leukoencephalopathy surveillance protocol includes FLAIR and DWI sequences only.
-
The spinal cord MR imaging protocol includes sagittal T1-weighted and proton attenuation, STIR or phase-sensitive inversion recovery, axial T2- or T2*-weighted imaging through suspicious lesions, and, in some cases, postcontrast gadolinium-enhanced T1-weighted imaging.
Clinical diagnosis
A diagnosis of MS is made on the basis of clinical findings by using supporting evidence from ancillary tests such as CSF examination for oligoclonal banding and MRI. [9, 10]
Clinically, MS has historically been diagnosed via the demonstration of white matter dysfunction disseminated in time and space. [11] With the advent of diagnostic laboratory investigations and imaging techniques, the Poser criteria were proposed to establish a degree of certainty of diagnosis in the absence of the 2 clinical attacks by using terms such as possible MS and probable MS. [12]
With increasing treatment options for MS and better imaging techniques, newer diagnostic criteria have been suggested that allow diagnosis after a single attack coupled with appropriate positive test results. These criteria have been coined the MacDonald criteria. [13] [14] Essentially, they allow for the second attack in time to be defined by a new lesion appearing on MRI. Also, the MacDonald criteria allow the dissemination in space to be established on the basis of either 9 typical white matter lesions on MRI or 1 enhancing lesion. If CSF studies show increased IgG values or oligoclonal banding, the presence of only 2 typical MRI lesions satisfy the dissemination-in-time criteria.
The new McDonald criteria include the following [9, 13, 14, 15] :
-
In a patient with a typical clinically isolated syndrome and fulfilment of clinical or MRI criteria for dissemination in space and no better explanation for the clinical presentation, demonstration of CSF-specific oligoclonal bands in the absence of other CSF findings atypical of multiple sclerosis allows a diagnosis to be made.
-
Symptomatic and asymptomatic MRI lesions can be considered in the determination of dissemination in space or time. MRI lesions in the optic nerve in a patient presenting with optic neuritis remain an exception and, owing to insufficient evidence, cannot be used in fulfilling the McDonald criteria.
-
Cortical and juxtacortical lesions can be used in fulfilling MRI criteria for dissemination in space.
-
At the time of diagnosis, a provisional disease course should be specified (relapsing-remitting, primary progressive, or secondary progressive) and whether the course is active or not, and progressive or not based on the previous year's history. The phenotype should be periodically re-evaluated based on accumulated information.
With respect to the initial clinical presentation in MS, it may vary with the white matter tract involved, and it may include somatic sensory changes, optic neuritis, or weakness. After only a single attack, the diagnosis of MS is suggested if the first impairment is coupled with positive paraclinical test results, such as those on imaging or CSF studies. Furthermore, the attack must be compatible with the pattern of impairment found in patients with MS, which typically means that the duration of deficit is days to weeks. Worsening of vision due to optic neuritis and subsequent exercise is known as the Uhthoff phenomenon.
Stankiewicz et al correlated brain lesions and clinical status with 1.5T and 3T MRI in 32 patients with MS by use of MRI fluid-attenuated inversion-recovery (FLAIR) sequences, and the authors found that MRI at 3T may provide increased sensitivity and validity in assessment of MS brain lesions. The study showed that FLAIR lesion volume (FLLV) at 3T was higher than at 1.5T. While 3T FLLV correlated moderately and significantly, 1.5T FLLV correlated poorly. When controlling for age and depression, correlations between FLLV and cognitive measures were significant at 1.5T for the Judgment of Line Orientation Test, the Symbol Digit Modalities Test, and the California Verbal Learning Test Delayed Free Recall, but at 3T, correlations were significant and of greater magnitude. [16]
Clinical course
The clinical course of MS can follow different patterns, and this observation has led to the classification of distinct types of MS. The most common form of MS is termed relapsing-remitting MS, in which progression involves symptoms of neurologic dysfunction frequently followed by partial or complete clinical recovery. In relapsing-remitting MS, global clinical deterioration has traditionally been attributed to cumulative deficit due to incomplete recovery from repeated occurrences of individual relapses. However, this cumulative deficit has been questioned, because evidence increasingly suggests an ongoing background neurologic deterioration that is independent of the relapses.
Occasionally, the course of MS may be more indolent and exhibit a chronic, persistent neurologic deficit without apparent ongoing deterioration or further impairment. Sometimes, this course of MS is called inactive or benign MS, and this form is often observed in patients with prior relapsing-remitting disease.
Another potentially complicating matter clinically is that highly active MS lesions may sometimes demonstrate significant mass effect. Rarely, mass effect can lead to midline shift, herniations, infarctions, and even death. Such a drastic clinical and radiologic presentation can lead to an incorrect preliminary diagnosis and inappropriate neurosurgical intervention. When MS presents in a more fulminant, aggressive manner, it is frequently known as malignant MS or the Marburg variant.
In a prospective study, Lebrun et al followed 70 patients who had their first brain MRI for a variety of medical symptoms not suggestive of MS and found the mean time between the first brain MRI and the first clinically isolated syndrome to be 2.3 years (range, 0.8-5 yr). Diagnostic studies of the blood, CSF, and visual evoked potentials were conducted, and clinical conversion occurred in 23 patients: 6 to optic neuritis, 6 to myelitis, 5 to brainstem symptoms, 4 to sensitive symptoms, 1 to cerebellar symptoms, and 1 to cognitive deterioration. [17] For patient education information, see Multiple Sclerosis.
In a study of cognitive impairment in MS patients by using brain MRI sequences, cognitively impaired MS patients had gray matter atrophy of the left thalamus, right hippocampus, and parietal regions. They also showed atrophy of several white matter tracts, mainly located in posterior brain regions, and widespread white matter diffusivity abnormalities. Of 61 patients with relapsing-remitting MS, 23 (38%) had cognitive impairment. [18]
A large multicenter, longitudinal study of high-resolution T1-weighted MRI scans in 1214 MS patients showed that deep gray matter volume loss drives disability accumulation in MS, and the rate of temporal cortical gray matter atrophy in secondary-progressive MS was significantly faster than in relapsing-remitting MS. [19]
Progress has been made in the development of more specific MRI markers for monitoring progressive multiple sclerosis (PMS); longitudinal studies have explored the sensitivity of these markers, such as brain atrophy. [20, 21, 22]
See Multiple Sclerosis, a Critical Images slideshow, for more information on incidence, presentation, intervention, and additional resources.
Computed Tomography
Similar to radiography, computed tomography (CT) scanning has had a limited role in the diagnosis of MS and in the treatment of patients since the advent of MRI. CT scans may be used to exclude other causes of neurologic impairment, but they have a low positive predictive value in the diagnosis of MS; thus, the false-negative rate is high.
Prior to the use of MRI, CT scanning, with the injection of double or triple doses of intravenous contrast material, was used in attempts to identify active MS lesions. However, the scans were insensitive for the detection of chronic lesions. CT scans can help assess the degree of cerebral atrophy associated with advanced MS, but given the plethora of additional information provided by MRI, CT is no longer used for this purpose.
An acute MS lesion may enhance and appear simply as an enhancing white matter lesion on CT scans, but the appearance is highly nonspecific. When a highly active MS lesion is observed to enhance and possibly exerts mass effect, it can be termed tumefactive (due to the potential for misidentification as a tumor). Because CT scans typically do not help to identify the more chronic lesions, the tumefactive MS lesion may appear as a solitary enhancing mass, which leads to neurosurgical intervention. Fortunately, this situation is relatively uncommon.
In a cohort of 200 patients, Paty et al found that of the 19 who went on to develop clinically definite MS (CDMS), abnormal CT findings were demonstrated in only 9 (47%). In contrast, abnormal MRI findings were demonstrated in 18 (95%). All of the abnormal CT findings were also demonstrated on MRIs. [4]
Magnetic Resonance Imaging
The advent of MRI has revolutionized the diagnosis and monitoring of MS. MRI is well established as the preferred imaging modality for depicting MS lesions. In patients with clinically definite MS (CDMS), MRI demonstrates a high rate of abnormal findings compatible with the diagnosis. In a study by Lukes et al, lesions were demonstrated in 10 patients with CDMS. [23] In a larger study by Robertson et al, MRI findings were abnormal in 124 of 133 patients with CDMS. Ormerod et al found that 112 of 114 patients with CDMS had abnormal MRI findings and that 102 of 114 had discrete white matter lesions. [24]
Another major use of MRI has been the evaluation of patients who have had only 1 episode of neurologic impairment and who do not meet the clinical criteria for the diagnosis. The overall risk of developing MS after a single episode of neurologic impairment is estimated to be as low as 12% (2-yr follow-up study by Beck et al) to as high as 45% (12.9-yr follow-up study by Sandberg-Wollheim et al [25] ) or 58% (14.9-yr follow-up study by Rizzo et al [26] ).
MRI has been proven to be the most useful investigation for predicting the progression to MS. In a 10-year follow-up study of patients with a clinically isolated event, 45 (83%) of 54 patients with abnormal MRI findings went on to develop clinical MS, whereas only 3 of 27 with normal MRI findings developed MS. [27]
Tintoré et al followed up 70 patients for an average of 28.3 months after an isolated neurologic event and compared various MRI criteria for the diagnosis MS, as defined by Paty et al, Fazekas et al, and Barkhof et al. [4, 5, 28, 29] With the method of Paty et al, which requires 3 or 4 lesions (1 of which is periventricular), the authors reported a sensitivity of 86% but a specificity of only 54%. The criteria of Fazekas et al resulted in the same sensitivity and specificity. These criteria require 3 lesions with 2 of the 3 following characteristics: infratentorial location, periventricular location, and lesion greater than 6 mm. The criteria of Barkhof require 1 infratentorial lesion, 1 juxtacortical lesion, 3 periventricular lesions, and either 1 gadolinium-enhanced lesion or more than 9 lesions on T2-weighted MRI scans. These criteria resulted in a sensitivity of 73% and a specificity of 73%. Thus, as the MRI criteria become more stringent in the diagnosis of MS, specificity increases at the expense of decreasing sensitivity.
In a cohort of the BENEFIT study (a multicenter, randomized, clinical study of 468 patients), the modified Barkhof criteria showed moderate predictive value for conversion to CDMS over 3 years, despite the fact that all patients received interferon beta-1b therapy for at least 1 year. Follow-up MRI was found to be most informative after 9 months in patients without dissemination in space at baseline. The overall conversion rate to CDMS was 42%. Barkhof criteria with the strongest prognostic value were the presence at baseline of at least 9 T2-weighted lesions and at least 3 periventricular lesions. [30]
According to a study of postmortem MS tissue by Pitt et al, 3-dimensional (3-D), T2*-weighted, gradient-echo (T2*GRE) and white matter–attenuated, turbo-field-echo (TFE) sequences at a 7T field strength can detect most cortical lesions. The 3-D T2*GRE and white matter–attenuated TFE sequences retrospectively detected 93% and 82% of all cortical lesions, respectively. [31]
Susceptibility MRI contrast variations reflect alterations in brain iron and myelin content. In 24 MS patients (306 white matter lesions) who underwent 7T MRI of the brain, most lesions were hypointense on R2*. Hyperintense lesions on quantitative susceptibility mapping were more frequent in relapsing-remitting MS than in progressive MS. Hyperintense lesion rims on quantitative susceptibility maps were more common in progressive MS and in patients with higher levels of disability and fatigue. Mean lesion R2* was inversely related to disability and fatigue and significantly reduced in progressive MS. Relative susceptibility was lower in lesions in progressive MS than in relapsing-remitting MS. [32]
Typical findings and pulse sequences
Because of the inflammation and breakdown of the blood-brain barrier in MS lesions, the presence of extravascular fluid leads to hyperintensity on T2-weighted images. Thus, in a patient with MS, MRI scans typically demonstrate more than 1 hyperintense white matter lesion. [33, 34, 35, 36, 37]
Lesions may be observed anywhere in the CNS white matter, including the supratentorium, infratentorium, and spinal cord; however, more typical locations for MS lesions include the periventricular white matter, brainstem, cerebellum, and spinal cord. Ovoid lesions perpendicular to the ventricles are common in MS and occasionally are called Dawson bars or fingers, which occur along the path of the deep medullary veins. Perhaps the most specific lesions in MS are noted in the corpus callosum at the interface with the septum pellucidum. [38]
(See the MRI scans below.)
 Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
 Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
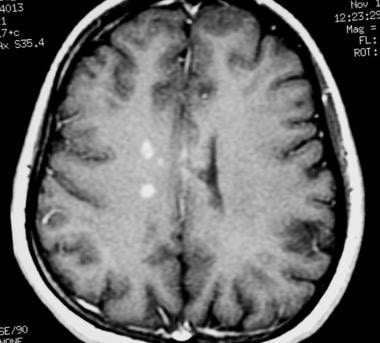 Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease.
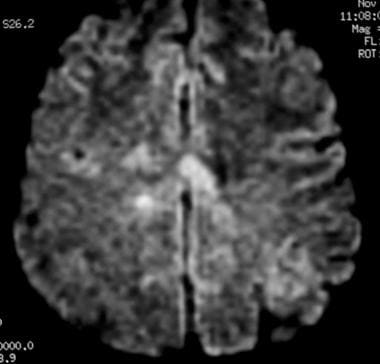 Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
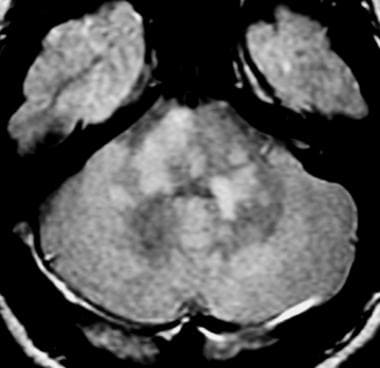 Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa.
Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa.
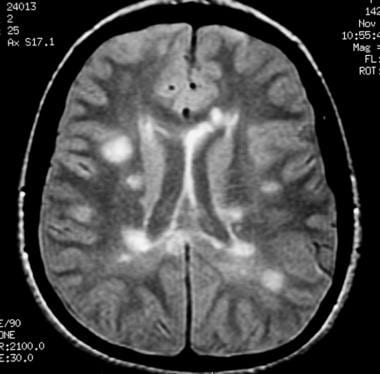 Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis.
Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis.
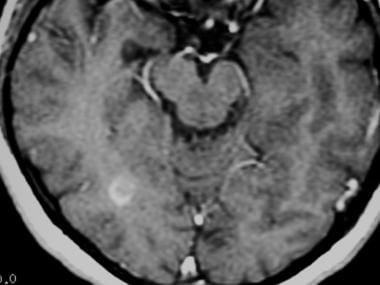 Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis.
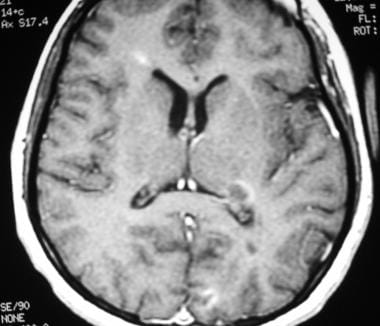
 Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions.
Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions.
 Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.
 Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
Proton density (PD)–weighted MRI has an advantage over standard T2 imaging, because on PD series, MS lesions remain hyperintense, while the CSF signal is suppressed. Therefore, the lesions are easily identified. Depending on the PD technique, the CSF signal is suppressed to a variable degree, rendering it isointense to hypointense relative to the brain parenchyma. This sequence results in substantial suppression of Virchow-Robin spaces, which are perivascular CSF spaces that may penetrate to the subcortical white matter. These spaces may appear as hyperintense spots on standard T2-weighted MRI scans.
Compared with other techniques, nonenhanced T1-weighted MRI is far less sensitive in detecting MS lesions. Acute lesions usually are not depicted at all. With T1-weighted MRI, the clinician can gain a general appreciation of the global cerebral atrophy that occurs with advanced chronic MS. Global atrophy has been suggested to have the strongest imaging correlation with disability.
Chronic MS lesions usually result in localized leukomalacia, and they may appear as hypointense lesions that represent loss of tissue.
Gadolinium-enhanced T1-weighted MRI scans can depict acute, active MS lesions. These appear as enhancing white matter lesions; the presence of an enhancing lesion has been shown to increase the specificity for MS. [5, 28]
FLAIR MRI
Newer MRI pulse sequences and techniques, including fluid-attenuated inversion recovery (FLAIR) MRI and MR spectroscopy, have emerged that are potentially useful in the evaluation of patients with MS.
FLAIR MRI is a heavily T2-weighted technique that dampens the ventricular (ie, free-water) CSF signal. Thus, the highest signals on the sequence are from certain brain parenchymal abnormalities, such as MS lesions, while the CSF appears black. This appearance is different from that on PD-weighted MRIs, on which periventricular MS lesions may appear nearly isointense to the adjacent CSF.
MTR-based imaging or multi-compartment diffusion imaging might be useful to investigate the effect of drugs promoting remyelination, while post-contrast FLAIR could be applied to investigate the monitoring response to drugs targeting B cells. [39]
(See the image below.)
 Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
The greater relative suppression of CSF on FLAIR images compared with PD-weighted series increases the contrast between periventricular lesions and CSF, enhancing their detection. FLAIR has been shown to be superior to PD-weighted sequences in the detection of MS lesions in the cerebral hemispheres. However, PD-weighted imaging remains the investigation of choice for infratentorial lesions. [40]
MR spectroscopy
Magnetic resonance (MR) spectroscopy uses the characteristic spectra of specific biochemical markers to quantitate organic compounds in vivo. N -acetylaspartate (NAA) is a relatively specific neuronal marker that is present in sufficient concentrations in the brain to be revealed on MR spectroscopic images. By comparing the spectral signal of NAA with that of creatinine (Cr), MR spectroscopy can be useful in assessing neuronal and axonal loss.
Arnold et al noted that the NAA-Cr ratio in the CNS was decreased in moderate to advanced MS. White matter that appeared normal on T1- and T2-weighted images also demonstrated the reduction. [41] In addition, a normal ratio was noted in the area of a recently active lesion associated with clinical deficits that subsequently resolved. The findings led the authors to propose that MR spectroscopic findings may be able to help identify irreversible axonal damage.
In a study involving 88 patients with MS, De Stefano et al found a strong correlation between disability scores and NAA-Cr ratios. [42] The ratio exhibited a stronger correlation in patients with MS patients who had milder disability scores. Because MR spectroscopy appears to be capable of depicting changes in white matter that are not detected with routine pulse sequences and because the findings are correlated with disability scores, the use of MR spectroscopy may prove valuable in monitoring patients after treatment and in formulating their prognosis.
Nonstandard MRI sequences
Beyond the standard MRI sequences that are used in clinical practice (T1 +/- Gad, T2, diffusion-weighted imaging, FLAIR), more advanced MRI techniques have been used for research purposes. Many of these series require greater magnetic field strengths over the popular 1.5T, but with the increasing availability of 3T MRI, these sequences will likely find their way more and more into standard clinical practice. [43]
Diffusion tensor imaging (DTI) can utilize diffusion-weighted imaging techniques in different orientations to establish pathology along white matter tracts in the CNS. DTI can identify demyelination and loss of axons along tracts that would otherwise go undetected by conventional techniques. [44, 45, 46] DTI can also identify disease activity in and injury to gray matter structures, which in turn can be used as markers of disease activity and severity. [47, 48, 49, 50]
Double inversion recover (DIR) sequences can also detect cortical lesions with increased sensitivity over standard MRI sequences, with higher MRI field strengths improving sensitivity. [51, 52]
Magnetization transfer imaging (MTI) is capable of identifying MS lesions before they can be detected by conventional MRI techniques. [53, 54] Magnetization transfer ratio (MTR)—based imaging or multi-compartment diffusion imaging might be useful to investigate the effect of drugs promoting remyelination, while post-contrast FLAIR could be applied to investigate the monitoring response to drugs targeting B cells.
Limitations
In virtually all patients with clinically well-established MS, MRI scans demonstrate the corresponding changes. False-negative findings occur more frequently in patients with early MS and a minimal clinical history of neurologic impairment than in other patients.
O'Riordan et al prospectively found that in 3 of 27 patients with normal MRI findings, MS subsequently developed. [27] However, the patients with normal MRI findings all developed lesions detectable on MRI scans when the disease became established. Similarly, as patients are followed for longer periods, the rate of false-positive findings decreases, because in many patients with abnormal MRI findings after a single neurologic event, the clinical criteria for MS eventually develop.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF), also called nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MR angiography scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Ultrasonography
Ultrasonography is not currently used in the investigation of MS. Berg et al, however, used transcranial sonography to determine the size of the ventricles in patients with MS and found that an increasing size is correlated with the MRI-determined brain volume, as well as with cognitive dysfunction and clinical disability. Further studies may establish a role for ultrasonography in the prognosis and treatment of patients with MS. [55]
According to Walter et al, in patients with MS, hyperechogenicity of the substantia nigra and lenticular nucleus correlates with more pronounced MRI T2 hypointensity, which is thought to reflect iron deposition, and a larger bilateral substantia nigra echogenic area is related to a higher rate of disease progression. In addition, a small echogenic area predicts a disease course without further progression over 2 years. [56] Walter et al performed the study to determine whether transcranial ultrasonography can identify lesions in deep gray matter in patients with MS and whether such findings can identify the severity and progression of MS. Of 75 patients followed, abnormal hyperechogenicity of the substantia nigra occurred in 41%; of the lenticular nucleus, in 54%; of the caudate nucleus, in 40%; and of the thalamus, in 8%, with similar frequency in patients with relapsing-remitting and primary or secondary progressive MS if corrected for disease duration.
Tromba et al used Italian researcher Paolo Zamboni’s ultrasound protocol, along with M-mode ultrasound, and found that about 60% of 112 patients with MS had venous insufficiency, [57] but in a Canadian study using catheter venography, Traboulsee et al found that only 1 of 65 patients (2%) with MS had extracranial venous narrowing. [58] The Canadian study also utilized the ultrasound criteria proposed by Zamboni and found that 35 of 79 (44%) MS patients met the criteria for cerebrospinal venous insufficiency. The Canadian researchers concluded that the ultrasound criteria are neither sensitive nor specific for narrowing on catheter venography. [58]
Macgowan et al looked at venous cerebral outflow in 26 MS patients and 26 controls using ultrasound and found no difference between the 2 groups. They also used phase-contrast MRI and found no significant difference in venous flow in the vertebral arteries, internal jugular veins, and epidural veins. [59]
Questions & Answers
Overview
What is multiple sclerosis (MS)?
What is the role of imaging in the diagnosis of multiple sclerosis (MS)?
How is multiple sclerosis (MS) diagnosed?
What is the characteristic clinical course of multiple sclerosis (MS)?
What is the role of CT scanning in the workup of multiple sclerosis (MS)?
What is the role of MRI in the diagnosis and follow-up of multiple sclerosis (MS)?
Which findings on brain MRI are characteristic of multiple sclerosis (MS)?
What is the role of FLAIR MRI in the diagnosis of multiple sclerosis (MS)?
What is the role of MR spectroscopy in the diagnosis of multiple sclerosis (MS)?
Which nonstandard MRI sequences are used in the evaluation of multiple sclerosis (MS)?
What are the limitations of MRI in the evaluation of multiple sclerosis (MS)?
What is the role of ultrasonography in the evaluation of multiple sclerosis (MS)?
-
Sagittal T1-weighted MRI depicts multiple hypointense lesions in the corpus callosum; this finding is characteristic of multiple sclerosis.
-
Axial T2-weighted MRI in a patient with multiple sclerosis demonstrates numerous white matter plaques in a callosal and pericallosal white matter distribution.
-
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis demonstrates several intensely enhancing pericallosal white matter lesions compatible with active disease.
-
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.
-
Axial proton density–weighted MRI through the posterior fossa in a patient with multiple sclerosis demonstrates multiple bright foci in the brainstem and cerebellum. Proton density–weighted sequences are highly sensitive for the detection of plaques in multiple sclerosis, especially in the posterior fossa.
-
Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with multiple sclerosis demonstrates periventricular high–signal intensity lesions, which exhibit a typical distribution for multiple sclerosis. FLAIR MRI is a highly sensitive sequence for lesion detection, particularly supratentorially.
-
Axial proton density–weighted MRI demonstrates multiple lesions in a distribution characteristic of multiple sclerosis. Specifically, the periventricular lesions and the more peripheral white matter lesions near the gray matter–white matter junction are typical MRI findings in multiple sclerosis.
-
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts enhancement of a plaque in the right temporo-occipital lobe, signifying disease activity. Note the C-shaped, or arclike, enhancement, which is fairly characteristic of multiple sclerosis.
-
Point-resolved spectroscopic study performed in a patient with multiple sclerosis demonstrates a slightly decreased N-acetylaspartate peak and a mildly elevated choline peak; these findings are compatible with demyelination with neuronal loss and increased cell membrane turnover.
-
Sagittal proton density–weighted MRI in a patient with multiple sclerosis demonstrates the characteristic corpus callosal and pericallosal white matter lesions.
-
Axial T1-weighted, gadolinium-enhanced MRI in a patient with multiple sclerosis depicts several enhancing lesions, at least 2 of which show characteristic C-shaped, or arclike, peripheral enhancement.
-
Axial diffusion-weighted MRI in a patient with multiple sclerosis shows several hyperintense lesions, a feature of inflammatory disease activity.









