Practice Essentials
Aortic stenosis (AS) is the obstruction of blood flow across the aortic valve. It is the most frequent type of valvular heart disease in Europe and North America. It primarily presents as calcific AS in older adults (2-7% of the population older than 65 years). [1] Symptoms of aortic stenosis usually develop gradually after an asymptomatic latent period of 10-20 years. [2] In symptomatic patients with medically treated moderate to severe aortic stenosis, death usually occurs within 5 years. [3, 4]
Degenerative, or fibrocalcific, AS is the most common native valvular heart disease encountered by cardiologists. Transthoracic echocardiography is the imaging modality of choice for noninvasive evaluation because of its widespread availability, its superior assessment of flow hemodynamics, and extensive available research data. The diagnosis of severe AS is currently based on 3 hemodynamic parameters: maximal jet velocity, mean pressure gradient across the aortic valve, and aortic valve area. [5, 6, 7, 8, 9, 10, 11]
The antegrade systolic velocity across the narrowed aortic valve, or aortic jet velocity, is measured using continuous-wave Doppler ultrasound. The mean transvalvular pressure gradient is calculated by averaging the instantaneous gradients over the ejection period, a function included in clinical instrument measurement packages using the traced velocity curve. Aortic valve area by continuity-equation calculation has been validated in clinical and experimental studies and has been reported as a valuable parameter for prediction of clinical outcome and clinical decision-making [12]
Imaging of the aortic valve is critical in establishing a diagnosis, grading severity, and informing the timing of valvular intervention. According to the American College of Radiology (ACR) in its appropriateness criteria for preintervention planning for transcatheter aortic valve displacement (TAVR), preintervention imaging with echocardiography and CT are essential for procedure planning and device selection, with MR angiography playing a complementary role. Three dimensional cross-sectional imaging has been shown to help reduce procedural complications such as vascular access injury, paravalvular regurgitation, and coronary obstruction. [3]
Echocardiography
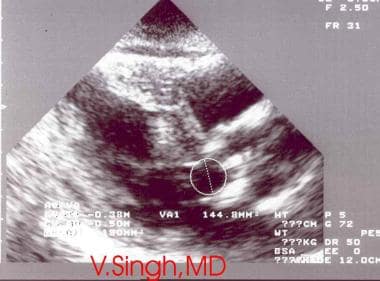
Echocardiography is the preferred imaging test for aortic stenosis. Transthoracic echocardiography is the main imaging technique used to diagnose AS. [12, 9, 10] Echocardiography is indispensable to the assessment of the extent of LV hypertrophy, systolic ejection performance, and anatomy of the aortic valve (see the image below). [13, 14, 15, 16]
A comprehensive echocardiography report should contain information on aortic valve morphology (bicuspid versus tricuspid) and mobility, cause and severity of AS (including aortic valve area, mean gradient, and peak aortic jet velocity), and its consequences on LV function (ie, stroke volume, LV ejection fraction, and diastolic function), left atrial pressure, valvuloarterial impedance, and pulmonary arterial pressure. [7]
Doppler interrogation of the aortic valve makes use of the modified Bernoulli equation (gradient = 4 × velocity2) to assess the severity of the stenosis. [15, 16] As blood flows from the body of the LV across the stenotic valve, the flow rate must accelerate for the volume to remain constant. Doppler interrogation of the valve detects this increase in velocity and helps estimate the valvular gradient. [17] In summary, echocardiography may demonstrate the following findings:
-
Concentric LV hypertrophy
-
Reduced separation of the cusp of the aortic valve
-
Mean gradients greater than 50 mm Hg in patients with severe aortic stenosis on Doppler echocardiography
Chest radiography
Chest radiographs may show several significant findings consistent with aortic stenosis. The aortic valve may appear calcified. With plain images, calcification is best detected on the lateral view. Calcification of the aortic valve is found in almost all adults with hemodynamically significant aortic stenosis.
The LV may be slightly enlarged, with a rounded apex; this is a nonspecific finding. The left atrium may be enlarged as well. Visible calcification on plain chest films usually indicates a gradient of 50 mm Hg or more across the valve, which is severe enough to require surgery.
CT scanning
CT scans may exhibit chamber enlargement and calcification of the aortic valve. This calcification is a reliable indicator of severe stenosis, particularly when it is present in a young patient.
Magnetic resonance imaging
Application of cardiovascular MRI in the evaluation of AS includes anatomic assessment of the aorta and aortic valve, quantification of LV volumes, mass and function, and calculation of stenotic jet velocity. The 3 standard measures used to establish the severity of AS are valve area, peak velocity, and pressure gradient. [4]
Cine MRI may be used to depict the signal void caused by high-velocity jet flow across a narrow valvular orifice associated with the opened valve in aortic stenosis. The signal void is projected into the ascending aorta in systole. Despite the good anatomic detail obtainable with MRI, echocardiography has superseded MRI because of its improved portability.
Cardiac catheterization and angiography
During catheterization, the transvalvular pressure gradient across the aortic valve is measured by use of a catheter in the LV and another in the proximal aorta or femoral artery (see the image below). A mean pressure gradient greater than 30 mm Hg usually represents clinically significant aortic stenosis.
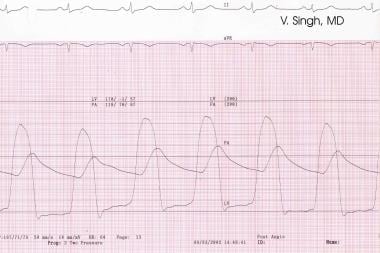 Transvalvular gradient seen across the aortic valve during simultaneous recordings of aortic and left ventricular (LV) pressures (cardiac catheterization).
Transvalvular gradient seen across the aortic valve during simultaneous recordings of aortic and left ventricular (LV) pressures (cardiac catheterization).
As a result of the dire consequences of missing the diagnosis of symptomatic aortic stenosis, the physician must have a low threshold for obtaining an echocardiogram whenever the possibility of aortic stenosis cannot be excluded during physical examination.
Asymptomatic patients with suggestive murmurs benefit from early diagnosis, which allows both the patient and the physician to be most vigilant regarding possible early signs and symptoms and to guide the use of prophylactic regimens to prevent bacterial endocarditis.
Radiography
Routine chest radiography may demonstrate normal or nondiagnostic findings in patients with critical aortic stenosis. Findings include those described below.
Enlarged cardiac chamber
The cardiac silhouette is usually normal in size or slightly enlarged on the anteroposterior view. The edge of the LV and the apex may appear rounded, presenting a boot-shaped appearance. In the presence of aortic regurgitation or heart failure, substantial cardiomegaly is noted.
The left atrium may be slightly enlarged in patients with severe aortic stenosis, and radiologic signs of pulmonary venous hypertension may be demonstrated. However, when left atrial enlargement is marked, associated mitral valvular disease should be suspected.
Valvular calcification
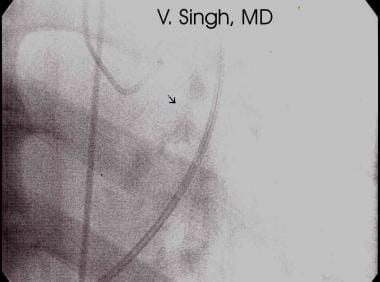
Calcification of the aortic valve is diagnostic of aortic stenosis. Calcification is detected best by using fluoroscopy or CT scans (see the mage below). If seen on plain images, calcification is detected most readily on the lateral view.
Calcification of the aortic valve is found in almost all adults with hemodynamically significant aortic stenosis. The absence of calcium in the region of the aortic valve on careful fluoroscopic examination in a patient older than 35 years essentially excludes severe valvular aortic stenosis. However, in patients older than 65 years who have degenerative aortic stenosis, severe calcification of the aortic valve may occur with no or only mild obstruction.
Dilatation of the aorta
Poststenotic dilatation of the ascending aorta is a common finding. The dilatation is characteristically located in the ascending aorta and increases convexity of the right lateral aspect of the ascending aorta. The transverse arch or aortic knob is not enlarged. LV hypertrophy and dilatation may occur in this condition and result in enlargement of the heart downward and to the left. The heart is usually not enlarged greatly unless it has begun to decompensate.
Supravalvular stenosis
Supravalvular aortic stenosis is a rare condition that is seen as an element of Williams syndrome, which consists of intellectual and physical disability, elfin facies, hypercalcemia, and peripheral pulmonary artery stenoses
In supravalvular aortic stenosis, a tight hourglass constriction of the ascending aorta is present just cephalic to the valve.
Degree of confidence
Chest radiograph findings are usually nondiagnostic in patients with aortic stenosis, unless valvular calcification is present. In almost one half of patients (in whom stenosis is minimal to moderate), no detectable abnormal radiograph findings are demonstrated except for the slight prominence of the ascending aorta. When present, such findings are pathognomonic.
Subaortic stenosis is particularly difficult to diagnose radiographically because little if any poststenotic dilation occurs in idiopathic hypertrophic subaortic stenosis. In the membranous type of stenosis, often no poststenotic dilatation occurs, and LV hypertrophy is often minimal.
Computed Tomography
The presence of valvular calcification is specific for aortic valve disease. Calcification is most readily detected by using CT scans. Calcification of the aortic valve is a reliable indication that stenosis is severe, particularly when it is present in a young patient. If the patient does not have decompensation, visible calcification on plain chest images usually indicates a gradient of 50 mm Hg or more across the valve. This degree of stenosis usually is treated with surgery.
CT findings in aortic stenosis may be diagnostic, but in some patients, they must be supported by clinical findings to make the diagnosis.
Subaortic stenosis is particularly difficult to diagnose radiologically because in idiopathic hypertrophic subaortic stenosis, little if any poststenotic dilation occurs. In the membranous type of stenosis, no poststenotic dilatation may be noted, and LV hypertrophy is often minimal.
Supravalvular aortic stenosis is a rare condition that is seen as an element of Williams syndrome, which consists of intellectual and physical disability, elfin facies, hypercalcemia, and peripheral pulmonary artery stenoses. In supravalvular aortic stenosis, a tight hourglass constriction of the ascending aorta occurs just cephalic to the valve. [18, 19, 20]
Magnetic Resonance Imaging
MRI is uniquely advantageous for imaging the cardiovascular structures. High contrast is demonstrated between the moving blood pool and the static cardiovascular structures. [21] Imaging techniques include spin-echo (SE) and cine gradient echo (GRE) imaging. [22]
Various planes may be used for imaging. A long-axis view through the LV apex and aortic outflow tract in a coronal plane is most useful in assessing aortic stenosis.
Spin-echo MRI
Spin-echo (SE) imaging can clearly demonstrate the structural details of the aortic valve cusps, supravalvular and subvalvular structures, and dimensions of the LV and aortic root. It can show a bicuspid valve, thickened or bulging leaflets, reduced valve excursion, LV hypertrophy, and ascending aortic dilatation caused by the impact of the stenotic jet.
On SE MRI, the blood appears black, whereas the static internal cardiac structures, such as chamber walls and valves, appear bright. In contrast, high signal intensity is noted on cine GRE MRI, on which the blood pool appears white and has signal intensity higher than that of the myocardium.
Cine GRE MRI
Cine GRE images may be used to determine the severity of aortic stenosis by measuring the size and extent of the stenotic jet into the ascending aorta imaged in the coronal plane centered on the LV outflow tract. Velocity-encoded MRI is one of the best ways to quantify the transvalvular pressure gradient and valvular area. The valvular area may also be directly traced on the transverse axial cine GRE images obtained with a velocity-encoded sequence.
The maximal velocity in the stenotic jet may be determined on planes perpendicular to the flow (through-plane measurement) or parallel to the flow (in-plane measurement). As with Doppler echocardiography, the pressure gradient across the stenotic aortic valve may be calculated by using the modified Bernoulli equation: P = 4 × (Vmax)2, where P = pressure gradient, and Vmax = maximal velocity.
In addition, the area of the aortic valve (AAo) may be calculated by determining the area of the aortic outflow tract (AOT), the maximum velocity in the aortic outflow tract (VOT), and the maximum velocity in the aortic stenotic jet (VAo) by using the following continuity equation: AAo = (AOT X VOT)/VAo.
The calculated area of the aortic valve and the pressure gradient are well correlated with the data obtained from Doppler echocardiography and hemodynamic monitoring in the catheterization laboratory.
Degree of confidence
MRI is useful, and the degree of confidence is high. During cine GRE imaging, the echo time (TE) must be kept long because with shorter TEs, the signal void produced by the stenotic jet well may be missed or may not be apparent.
Certain potential sources of error are associated with the use of velocity-encoded MRI. The imaging plane must be as close to perpendicular and as parallel to the jet as possible. Also, because of the cyclic quality of phase shift, aliasing may appear, especially when the velocity range is lowered.
Ultrasonography
The evaluation of patients with known or suggested aortic valve disease requires integration of anatomic information from 2-dimensional echocardiography and physiologic information from Doppler studies. [23, 24, 25, 26]
Bicuspid valve
Transthoracic echocardiography is a reliable method for detecting the bicuspid aortic valve. With this technique, the hallmark of the bicuspid valve is eccentric closure of the leaflets in the aorta. In approximately 80% of patients, 2 rather than 3 leaflets may be visualized directly. On close scrutiny with transesophageal echocardiography, what at first appears to be a true bicuspid valve is often found to be a 3-leaflet valve in which the leaflets are of different sizes and in which fusion of 1 of the 3 commissures has resulted in a functional bicuspid valve. Coarctation of the aorta is strongly associated with a bicuspid valve. When clinical findings suggest either of these conditions, the other should be looked for as well.
Calcific aortic stenosis
Degenerative calcific valves appear as 3-leaflet structures with marked thickening of the leaflets. Thickening and calcification may be more prominent at the base of the leaflets than at the tips. The range of immobility and stenosis is broad and depends on the duration and severity of disease.
Rheumatic stenosis
Rheumatic aortic stenosis typically results in thickening of the leaflet along the commissural edges. It is seen almost exclusively in association with rheumatic mitral stenosis.
Secondary effects of aortic stenosis
After aortic stenosis is defined anatomically, secondary effects may be evaluated. These include poststenotic dilatation of the aorta and LV hypertrophy. LV systolic function also should be assessed.
Assessment of the severity of aortic stenosis
Continuous Doppler echocardiography is essential for assessing the physiologic significance of aortic stenosis. In clinically significant aortic stenosis, the gradient is likely to exceed 50 mm Hg. This value corresponds to a Doppler velocity of approximately 3.5 m/s, which is out of the range for accurate quantitation using pulsed-wave Doppler study. For this reason, use of continuous-wave Doppler imaging is essential for quantitation. Doppler interrogation of the aortic valve makes use of the modified Bernoulli equation (gradient = 4 × velocity2) to assess the severity of the stenosis. As blood flows from the body of the LV across the stenotic valve, the flow rate must accelerate for the volume to remain constant. Doppler interrogation of the valve depicts this increase in velocity and helps in estimating the valvular gradient.
An additional method of determining the area of the aortic valve relies on the continuity equation with pulsed Doppler echocardiography. In aortic stenosis, the LV outflow tract area may typically be derived from the diameter of the annulus if a circular geometry is assumed. Then, pulsed Doppler echocardiography is used to determine the velocity of flow at that site. The product of the 2 values represents the volumetric flow in the outflow tract. At the stenotic orifice, continuous-wave Doppler imaging is used to determine the mean velocity. Then, the algebraic equation may be solved for the area of the aortic valve. In a modification of this technique, mitral-valve flow is used instead of LV outflow. Because the velocity of flow increases at the restrictive orifice, several investigators have suggested using the ratio of the V1/V2 leads as a marker for clinically significant aortic stenosis.
Variations in the valve area and gradients
From a practical standpoint, determining the area of the aortic valve is often unnecessary. In a patient with thickened, restricted leaflets in whom the mean gradient exceeds 50 mm Hg, severe aortic stenosis is clinically ensured. Likewise, in patients with normal ventricular function in whom the gradients are low, the likelihood of clinically significant aortic stenosis becomes negligible.
Low ejection fraction
A serious case is when the LV function is reduced, typically involving ejection fractions of 25-35%, and when there is a modest transvalvular gradient of 25-30 mm Hg. This situation may represent either mild disease of the aortic valve and unrelated LV dysfunction or critical aortic stenosis with secondary LV dysfunction. While LV function and valvular gradients are being monitored, an infusion of dobutamine may be helpful in differentiating these 2 entities. If LV function improves with dobutamine infusion and if the gradient increases, aortic stenosis is severe and is associated with secondary LV dysfunction. Patients with this condition benefit from aortic valve replacement. If ventricular function improves without a change in the gradient, aortic stenosis is unlikely to be the limiting factor. Patients with this condition may be treated medically.
Degree of confidence
The gradients determined using Doppler echocardiography are very well correlated with simultaneously determined invasive measurements.
By using transthoracic echocardiography, the orifice of the valve is usually not visualized to a reliable degree. By using transesophageal echocardiography and planimetry, a direct measurement of the aortic valve orifice may be obtained in many patients with aortic stenosis.
On occasion, the use of Doppler echocardiography may lead to an underestimation of the gradient. This is common with nonsimultaneous recordings, but it also occurs when the angle of interrogation exceeds approximately 20°. Off-angle interrogation is the most common cause of underestimation of a gradient in aortic stenosis.
Nuclear Imaging
Myocardial perfusion imaging may be helpful for assessing concomitant atherosclerosis of the coronary arteries. [27] Treadmill stress testing or pharmacologic imaging can usually be performed safely in patients with mild to moderate aortic stenosis, though it may be contraindicated in patients with severe aortic stenosis.
Radionuclide ventriculography may be used to determine LV function in patients with aortic stenosis. Nuclear medicine findings are reliable for the assessment of myocardial ischemia and for the assessment of LV function, if needed.
Angiography
Cardiac catheterization and hemodynamics
When echocardiography demonstrates severe aortic stenosis and when the patient has 1 or more of the classic symptoms of the disease, AVR should be performed. Because most patients with aortic stenosis are at the age at which coronary disease is common, cardiac catheterization to perform coronary arteriography is usually accomplished before surgery. When the hemodynamic diagnosis is unclear, right- and left-sided heart catheterization should be performed to obtain the transaortic valvular pressure gradient and cardiac-output readings. This information is used to calculate the aortic valve area with the Gorlin equation, as follows: Aortic valve area = [CO/(SEP X HR)]/(44.3 × h1/2), where CO = cardiac output (in milliliters per minute), SEP = systolic ejection period (in seconds), HR = the heart rate, and h = the mean gradient.
Methods
In patients with aortic stenosis, the transvalvular pressure gradient should be measured, whenever possible, by using a catheter in the LV and another in the proximal aorta. Although measuring the gradient between the LV and the femoral artery is convenient, downstream augmentation of the pressure signal and delay in pressure transmission between the proximal aorta and the femoral artery may alter the pressure waveform substantially and introduce errors into the measured gradient.
Nevertheless, in many patients, LV–femoral artery pressure gradients may suffice for estimating the severity of aortic stenosis in confirming a severely stenotic valve. If the side port of the arterial introducing sheath is used to monitor femoral pressure, the inner diameter of the sheath should be 1F larger than the outer diameter of the catheter used. The LV–femoral artery pressure gradient may not always be reliable when calculating the area of the valve orifice in patients with equivocal valve gradients.
A careful single-catheter pullback from the LV to the aorta often is preferred to simultaneous measurement of LV and femoral artery pressures. As an alternative, a single catheter with distal and proximal lumina or a micromanometer catheter with distal and proximal transducers may be used for simultaneous measurement of LV and central aortic pressures. In patients with atrial fibrillation, several beats should be taken and averaged. The preferred method may be to obtain simultaneous pressure recordings from the LV and the aorta, with the averaging of several beats, to reduce errors caused by beat-to-beat variations resulting from changes in stroke volume. Another possibility that may be considered is the use of temporary transvenous pacing to regularize the R-R interval and therefore reduce this error.
The mean pressure gradient across the aortic valve is determined by means of planimetry of the area separating the LV and aortic pressures through the use of multiple beats. This gradient is applied to the calculation of the area of the valve orifice. The peak-to-peak gradient, measured as the difference between peak LV pressure and peak aortic pressure, is commonly used to quantify the valve gradient because this measurement is rapidly obtained and can be visually estimated.
Degree of confidence
Some risk is associated with the rapid injection of a large volume of contrast material into a high-pressure LV; therefore, this procedure is usually not advisable for patients with aortic stenosis and critical obstruction. In patients in this situation, angiographic studies of the LV and the aortic valve are best performed by injecting contrast material into the pulmonary artery and by imaging in the 30° right anterior oblique and 60° left anterior oblique projections. These examinations often make it possible to ascertain the number of cusps of the stenotic valve and to demonstrate doming of a thickened valve and a systolic jet.
In patients with very severe aortic stenosis, the LV catheter may reduce the effective area of the orifice, resulting in an increase in artifacts in the measured pressure gradient. This is usually of no importance because the diagnosis of severe aortic stenosis is usually already apparent in these patients.
-
Aortic stenosis is seen on 2-dimensional echocardiography. Note thickened calcified leaflets.
-
Valvular calcification of aortic stenosis seen with cardiac fluoroscopy during catheterization.
-
Transvalvular gradient seen across the aortic valve during simultaneous recordings of aortic and left ventricular (LV) pressures (cardiac catheterization).