Practice Essentials
Aortic dissection is an acute large vessel disease characterized by sudden onset, rapid disease progression, and high mortality. [1] The imaging evaluation of a patient with aortic dissection can be undoubtedly complex, requiring that the interpreting physician understand the classification systems and vocabulary used. This can be made all the more challenging by advances in medical imaging that reshape our understanding of aortic dissection. [2]
Classic aortic dissection is seen as a longitudinal split or partition in the media of the aorta. An intimal tear connects the media with the aortic lumen, and an exit tear creates a true lumen and a false lumen. The smaller true lumen is lined by intima, and the false lumen is lined by media. Typically, flow in the false lumen is slower than in the true lumen, and the false lumen often becomes aneurysmal when subjected to systemic pressure.
Acute aortic dissection is considered chronic at 2 weeks. Typical symptoms of acute aortic dissection include severe chest pain, hypotension, and syncope; hence, they mimic acute myocardial infarction or pulmonary embolism. [3] The dissection usually stops at an aortic branch vessel or at the level of an atherosclerotic plaque. [4, 5, 6]
(See the images of aortic dissection below.)
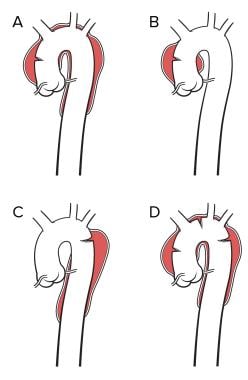 Image A represents a Stanford A or a DeBakey type 1 dissection. Image B represents a Stanford A or DeBakey type II dissection. Image C represents a Stanford type B or a DeBakey type III dissection. Image D is classified in a manner similar to A but contains an additional entry tear in the descending thoracic aorta. Note that a primary arch dissection does not fit neatly into either classification.
Image A represents a Stanford A or a DeBakey type 1 dissection. Image B represents a Stanford A or DeBakey type II dissection. Image C represents a Stanford type B or a DeBakey type III dissection. Image D is classified in a manner similar to A but contains an additional entry tear in the descending thoracic aorta. Note that a primary arch dissection does not fit neatly into either classification.
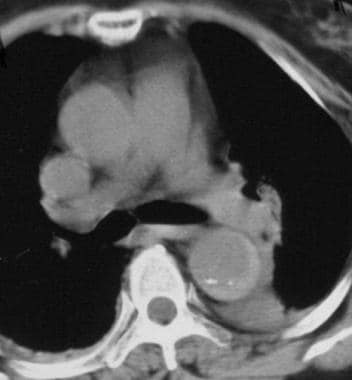
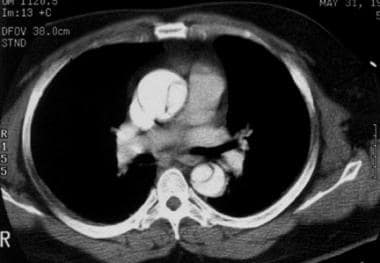 Contrast-enhanced axial CT image demonstrates an intimal flap that separates the 2 channels in the ascending and descending aorta diagnostic of a Stanford type A dissection. Courtesy of Joel L Fishman, MD.
Contrast-enhanced axial CT image demonstrates an intimal flap that separates the 2 channels in the ascending and descending aorta diagnostic of a Stanford type A dissection. Courtesy of Joel L Fishman, MD.
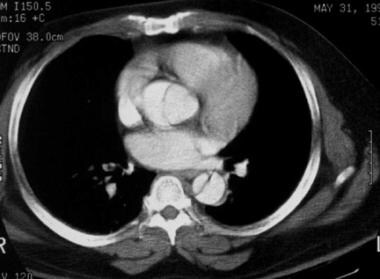
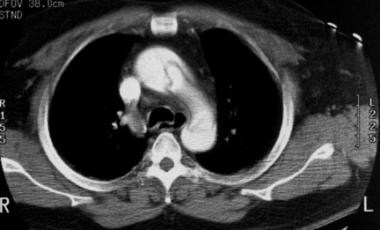 Contrast-enhanced CT scan obtained at the level of the aortic arch demonstrates an aortic dissection with almost complete separation of the aortic intima. The slight prolapse may be the beginning of a configuration at risk for intimo-intimo intussusception, a potentially fatal event. Courtesy of Joel L Fishman, MD.
Contrast-enhanced CT scan obtained at the level of the aortic arch demonstrates an aortic dissection with almost complete separation of the aortic intima. The slight prolapse may be the beginning of a configuration at risk for intimo-intimo intussusception, a potentially fatal event. Courtesy of Joel L Fishman, MD.
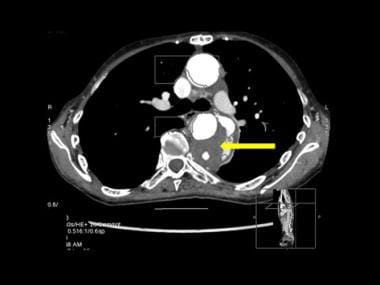
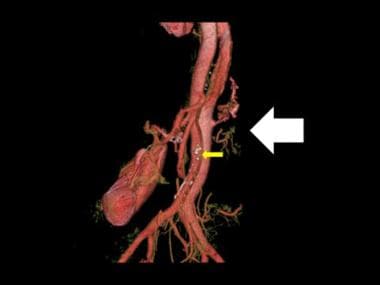 3-D color reconstruction of CTA in descending thoracic aortic dissection shows severance of the left renal artery due to dissection (white arrow). Note the few spidery collaterals at the point of left renal artery severance and the atheromatous calcified plaques (yellow arrow).
3-D color reconstruction of CTA in descending thoracic aortic dissection shows severance of the left renal artery due to dissection (white arrow). Note the few spidery collaterals at the point of left renal artery severance and the atheromatous calcified plaques (yellow arrow).
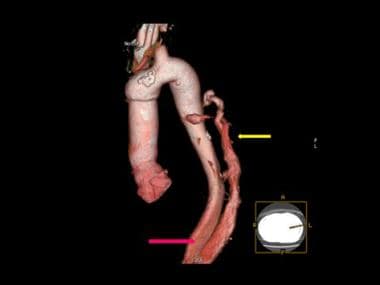
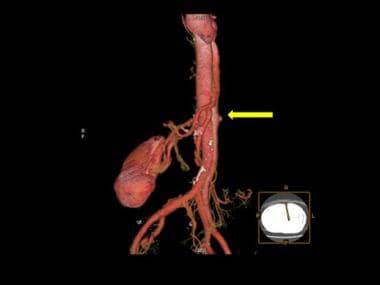 3-D color reconstruction of CTA in descending thoracic aortic dissection shows severance of the left renal artery due to dissection (yellow arrow).
3-D color reconstruction of CTA in descending thoracic aortic dissection shows severance of the left renal artery due to dissection (yellow arrow).
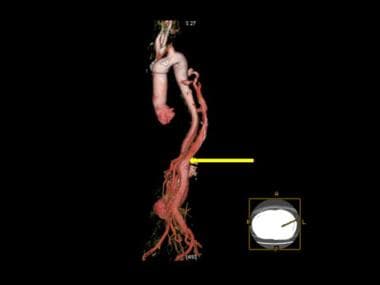 3-D color reconstruction of CTA in descending thoracic aortic dissection shows resumption/re-entry of normal distal aortic circulation, past the point of the false lumen (yellow arrow).
3-D color reconstruction of CTA in descending thoracic aortic dissection shows resumption/re-entry of normal distal aortic circulation, past the point of the false lumen (yellow arrow).
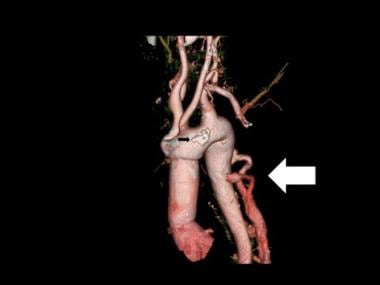
 3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (yellow arrow). The dissection and the false lumen are demonstrated by the red arrow.
3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (yellow arrow). The dissection and the false lumen are demonstrated by the red arrow.
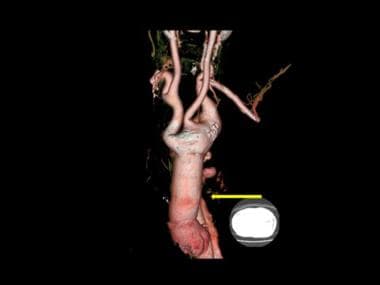
 3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (white arrow). The point of dissection is not shown. Note the aortic calcified plaque (black arrow).
3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (white arrow). The point of dissection is not shown. Note the aortic calcified plaque (black arrow).
Aortic dissection is a life-threatening event that is associated with a very poor outcome. A number of complex phenomena are involved in its initiation and propagation. Advances in comprehension of the mechanisms leading to dissection are the result of improvements in imaging and experimental techniques. However, the micromechanics involved in triggering such rupture events remains poorly described and understood. [7]
Most classic aortic dissections begin at 1 of 3 distinct anatomic locations: the aortic root; 2 cm above the aortic root; and just distal to the left subclavian artery. Ascending aortic involvement may result in death from wall rupture, hemopericardium and tamponade, occlusion of the coronary ostia with myocardial infarction, or severe aortic insufficiency.
 3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (yellow arrow). The point of dissection is not shown.
3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (yellow arrow). The point of dissection is not shown.
Aortic intramural hematoma (AIH) is a more recently described entity in which no intimal flap is present. It results in a spontaneous medial hematoma that may be secondary to an infarction of the vasa vasorum of the adventitia. Aortic intramural hematoma accounts for approximately 25% of aortic dissections. Involvement of the ascending aorta, especially if the overall aortic diameter is greater than 5 cm, should be treated surgically to prevent rupture or progression to a classic dissection with intimal tear. Conservative management is indicated for AIH of the descending aorta. [8, 9, 10, 11, 12, 13]
Aortic dissection remains a serious cardiovascular emergency with significant early and late mortality and morbidity. Improving outcomes are directly linked to early clinical diagnosis, swift confirmation by appropriate imaging, and management by dedicated teams with high levels of expertise in a complex clinical condition. [14]
Despite improvements in diagnostic measures, including imaging modalities and biomarkers, misdiagnosis of acute aortic dissection remains commonplace and current guidelines are relatively limited in preventing its occurrence. Measures proposed to improve diagnosis of acute aortic dissection and limit the risk of misdiagnosis include early use of acute aortic dissection risk scoring, focused echocardiography, and fast-tracking patients to cross-sectional imaging when suspicion of acute aortic dissection is high. [15]
Imaging modalities
Several factors determine the best modality for initial evaluation and postoperative follow-up. These factors include the following: stability of the patient's condition, the patient's renal function, suspected postoperative complications, and the availability of each imaging modality.
Preferred examinations for aortic dissection include contrast-enhanced spiral computed tomography (CT), transesophageal echocardiography (TEE) in the emergency setting, and magnetic resonance (MR) imaging for hemodynamically stable patients. TEE has an advantage over CT and magnetic resonance imaging (MRI) in its ability to reveal the status of the aortic valve and the ostia of the coronary arteries. CT and MR angiography have largely replaced conventional diagnostic angiography for assessment of aortic dissection. [6, 16, 17, 18, 19, 20, 21, 22]
Maffei et al performed a randomized, controlled trial in which 44 patients (252 evaluations) were examined with TEE and CT. [23] The authors concluded that both TEE and CT are atraumatic, safe, and accurate techniques for serial follow-up studies of patients treated for aortic dissection.
Three noninvasive studies are associated with high specificity and sensitivity for aortic dissection. CT and MRI are associated with a sensitivity and a specificity of 94-100% and 95-100%, respectively. TEE is less sensitive and specific than spiral CT or MRI, and TEE is operator-dependent. In addition, because of tracheal interposition, there is a 2-cm "blind spot" for TEE just proximal to the innominate arteries. Note that TEE is contraindicated in approximately 1% of patients (eg, patients with esophageal varices).
Radiography
Mediastinal widening is the most common plain radiographic finding in aortic dissection; it is noted in 80% of patients (see the image below).
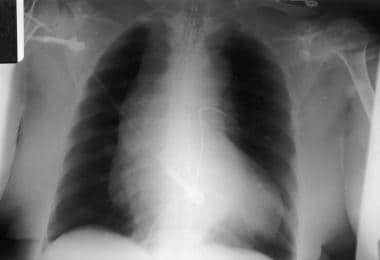 Plain anteroposterior view of the chest demonstrates a wide mediastinum. Courtesy of Jorge J Guerra, Jr, MD.
Plain anteroposterior view of the chest demonstrates a wide mediastinum. Courtesy of Jorge J Guerra, Jr, MD.
Other radiographic findings include the following:
-
Double aortic knob sign (present in 40% of patients)
-
Diffuse enlargement of the aorta with poor definition or irregularity of the aortic contour
-
Inward displacement of aortic wall calcification by more than 10 mm
-
Tracheal displacement to the right
-
Pleural effusion (more common on the left side; suggests leakage)
-
Pericardial effusion
-
Cardiac enlargement
-
Displacement of a nasogastric tube
-
Left apical opacity
All findings on plain images are nonspecific but may help in determining the need for further workup. Mediastinal fat commonly causes widening of the mediastinum, which may lead to a false-positive diagnosis of aortic dissection.
Computed Tomography
Since its introduction in the 1970s, CT has become a widely used technology, particularly in the ED. With the advent of spiral CT, studies may be performed in less time than before, with less patient discomfort, greater accuracy, and lower iodine load. Spiral CT permits patient translation and data acquisition simultaneously. This technology is useful in the evaluation of thoracic trauma, which enables the rapid diagnosis of thoracic injury. Multislice or multidetector CT may be used to perform faster imaging or to acquire thinner slices that may be reconstructed in multiple planes. [24, 25, 26, 27, 28, 29, 30, 31, 32]
The sensitivity of CT for aortic dissection is 87-94%, and the specificity is 92-100%. A typical helical scanning protocol for aortic dissection includes the following parameters: 5-mm collimation, 1.5 pitch, and 7.5-mm imaging spacing.
Because cardiac output is variable in patients with aortic dissection, a test injection of contrast should be used to determine circulation time; alternatively, an automated bolus detection scheme may be used. One advantage of the test injection method is that one may visually differentiate the true lumen from the false lumen on the basis of contrast arrival time.
Usually, scanning is performed from the thoracic inlet to the common femoral arteries. When dissection is identified, repeat scanning may be performed to obtain delayed images of the false lumen and aortic branches. Multiplanar reformation images are obtained in sagittal, coronal, oblique sagittal, and curved projections generated at an independent workstation. Use of volume rendering may be helpful in planning surgery.
Typical CT findings in acute dissection or intramural hematoma include the following:
-
Aortic intramural hematoma: Crescentic high-attenuating clot within the media, with internally displaced calcification (see the image below)
-
Intimal flap separating the 2 aortic channels (see the images below)
 Contrast-enhanced axial CT image demonstrates an intimal flap that separates the 2 channels in the ascending and descending aorta diagnostic of a Stanford type A dissection. Courtesy of Joel L Fishman, MD.
Contrast-enhanced axial CT image demonstrates an intimal flap that separates the 2 channels in the ascending and descending aorta diagnostic of a Stanford type A dissection. Courtesy of Joel L Fishman, MD.
Hemorrhagic pleural and pericardial effusions and mediastinal hemorrhage may be seen.
CT is helpful in postoperative follow-up. It may accurately depict associated complications, including the following:
-
Thrombosis
-
Hemorrhage
-
Infection
-
Pseudoaneurysm
-
Aortoenteric fistula
-
Ureteral obstruction
Inadequate contrast opacification may lead to false-negative findings of aortic dissection. Aortic intramural hematoma may be misinterpreted as an aneurysm with thrombus or arteritis.
Spiral CT artifacts include perivenous streaks and motion artifacts. Perivenous streaks are caused by beam hardening and motion resulting from transmitted pulsation in a vein that carries undiluted contrast medium into the heart. Some authors recommend injecting the contrast agent at a rate of 2 mL/s via a peripheral intravenous site in the right arm. Aortic motion artifact is produced by aortic wall motion from the end of diastole to the end of systole. Typically, this artifact is seen in the left anterior and right posterior margins of the aortic circumference.
In some patients, especially those with cystic medial necrosis, the intimal flap may be subtle.
CT scanning helps assess predictive factors associated with better endovascular prognosis and treatment.
(See the images below.)
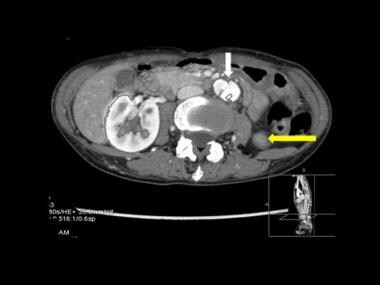 Axial CT scan through the upper abdomen in mid-descending thoracic aortic dissection shows the dissection flap entering the abdominal aorta (white arrow). Note the normally perfused right renal artery and poorly perfused upper pole of the left kidney at the point of dissection.
Axial CT scan through the upper abdomen in mid-descending thoracic aortic dissection shows the dissection flap entering the abdominal aorta (white arrow). Note the normally perfused right renal artery and poorly perfused upper pole of the left kidney at the point of dissection.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is an accurate tool for the diagnosis of aortic dissection, but it may not be readily available in the acute setting. Unstable patients with Swan-Ganz catheters should not undergo MRI. [19, 22, 25, 28, 30, 32, 33, 34, 35, 36, 37, 38, 39] MRI has greater than 90% sensitivity and specificity for aortic dissection.
MRI findings of aortic dissection include the following:
-
An intimal flap of medium signal intensity surrounded by a signal void of fast-flowing blood is evident on "black blood" echocardiogram-gated spin-echo or double inversion recovery single-shot fast spin-echo MRI (see the image below).
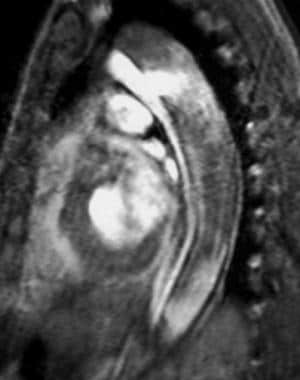 Sagittal gradient-echo MRI image obtained in early systole shows a jet of blood flowing through the intimal tear from the smaller anterior true lumen into the larger posterior false lumen. The intimal flap is recognized as the medium signal intensity linear structure that divides the true and false lumens. An aortic stent-graft could be placed across the intimal/entry tear in the descending thoracic aorta to redirect blood into the anterior true lumen. Courtesy of Joel L Fishman, MD.
Sagittal gradient-echo MRI image obtained in early systole shows a jet of blood flowing through the intimal tear from the smaller anterior true lumen into the larger posterior false lumen. The intimal flap is recognized as the medium signal intensity linear structure that divides the true and false lumens. An aortic stent-graft could be placed across the intimal/entry tear in the descending thoracic aorta to redirect blood into the anterior true lumen. Courtesy of Joel L Fishman, MD.
-
With cine gradient echo imaging, the intimal flap appears as a dark line against the high signal intensity of flowing blood; its configuration may change during the cardiac cycle. It is important that a careful examination of the aortic flap be conducted during the cardiac cycle with cine MRI so as to detect the presence of "true lumen collapse," which may be associated with end-organ ischemia. In some cases, the intima is stripped 360° from the media and is essentially "free floating"; this may result in catastrophic intimo-intimo intussusception, a potentially fatal event.
Newer pulse sequences such as true FISP or FIESTA offer very fast cine imaging.
Basic MRI sequences for evaluating aortic dissection include spin-echo T1-weighted or breath-hold double inversion recovery sequences, cardiac-gated gradient-echo sequences, and 3-dimensional (3-D) thin-section MR angiography (MRA) with a bolus injection of a single or double dose of gadolinium-based contrast agent.
MRI findings of acute intramural hematoma (AIH) include a crescent of blood surrounding but not compressing the aorta. The signal intensity of the crescent varies with age on T1-weighted imaging: It is isointense to muscle in the acute setting and is markedly hyperintense after 3-7 days.
MRI is helpful in postoperative follow-up. It may accurately depict associated complications, including the following:
-
Thrombosis
-
Hemorrhage
-
Infection
-
Pseudoaneurysm
-
Aortoenteric fistula
-
Ureteral obstruction
Potential drawbacks of MRI include reported artifacts on cardiac-triggered thoracic spin-echo phase images. These may appear as an artifactual borderlike feature across the aorta (caused by helical flow in the aorta) that may be interpreted as a dissection. Other potential causes of misinterpretation include an atypical configuration of the intimal flap seen in short dissections and multiple false channels in cases in which the flaps are complex.
Aortic anomalies also may cause confusion. False-positive findings seen in gadolinium-enhanced MRA include a central line or "maki" artifact. This occurs when the acquisition is performed too early, as intra-aortic gadolinium concentration is rising. This artifact may be readily differentiated from an aortic dissection because it does not take a spiral course in contrast to a true intimal flap.
Electrocardiography and Ultrasonography
Electrocardiography is helpful in the diagnosis of aortic dissection. It is particularly helpful in cases of ascending thoracic dissection, cardiac tamponade, and aortic regurgitation; transesophageal echocardiography (TEE) has a greater sensitivity and specificity than CT or MRI in detecting coronary arterial occlusion, aortic insufficiency, and cardiac tamponade, with a sensitivity of 97-99% and a specificity of 97-100%. [40, 41]
According to a study by Mastrogiovanni and colleagues, TEE was the favored study for the evaluation of aortic dissection. [42] In their report of 54 patients, TEE findings confirmed diagnostic dissection in all but 1 patient. TEE noted the site of the intimal tear, as well as the extension of the dissection, pericardial effusion, aortic incompetence, and left ventricular function. Because of the high level of correspondence between the diagnosis made at TEE and surgical anatomic findings, these authors favored the use of TEE—for many cases, as the sole diagnostic modality.
A tortuous aorta may result in a false-positive diagnosis of dissection. In cases involving a massive dilated ascending aorta (usually occurring as a result of cystic medial necrosis), it may be difficult to identify a small intimal flap.
Intravascular ultrasonography (IVUS) plays an essential role in diagnosis and classification of aortic dissection, as well as in evaluation of the dissection flap and assessment of whether or not it exhibits dynamic or static obstruction of the aortic branches. IVUS provides imaging guidance for fenestration in treatment of aortic dissection and in endograft placement.
Angiography
Aortography was the reference standard for preoperative evaluation and diagnosis of aortic dissection. With the advent of TEE, CT, and MRI, its role has become important only if nonsurgical interventional procedures are indicated. Aortographic findings are less sensitive than those of newer noninvasive techniques, especially for aortic intramural hematoma (AIH), which is almost impossible to diagnose with aortography because no compression of the lumen exists. [21]
Chest CT angiography (CTA) is essential in the diagnosis of acute aortic syndromes. Acute aortic syndromes are associated with high morbidity and mortality, particularly when involving the ascending aorta, and include classic aortic dissection, penetrating atherosclerotic ulcer, and acute intramural hematoma. An understanding of the pathogenesis and distinguishing imaging features of acute aortic syndromes and aortic rupture and some of the less common manifestations is helpful when one is interpreting imaging findings. [43]
It is controversial whether coronary angiography should be performed before sternotomy in a stable patient with aortic dissection, inasmuch as concomitant coronary bypass grafting may be performed if diseased vessels are present.
Diagnostic criteria include visualization of a lucent flap and delayed filling and washout of the false lumen. The expanding false lumen may compress the true lumen, causing it to become narrowed. A dual-lumen aorta is noted when both the true lumen and the false lumen are opacified (see the image below).
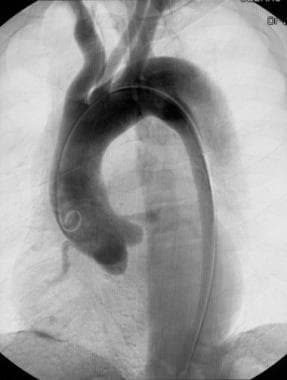 Oblique arteriogram of the thoracic aorta demonstrates the double-barrel aorta sign of aortic dissection. Both the true and false lumina are opacified. Courtesy of Joel L Fishman, MD.
Oblique arteriogram of the thoracic aorta demonstrates the double-barrel aorta sign of aortic dissection. Both the true and false lumina are opacified. Courtesy of Joel L Fishman, MD.
Pitfalls of angiography include lack of visualization of the false lumen due to thrombosis or inadequate opacification with contrast material. Streak artifacts secondary to aortic or cardiac motion or opacification of the sinus of Valsalva may be confused with thrombus. Pitfalls also include missed diagnosis of an intramural hematoma (frequently associated with progression to frank dissection) and misdiagnosis when the false lumen is thrombosed.
Future Directions
Aortic dissection remains a serious cardiovascular emergency with significant early and late mortality and morbidity. Improving outcomes is directly linked to early clinical diagnosis, swift confirmation by appropriate imaging, and management by dedicated teams with high levels of expertise in a complex clinical condition. [14] New concepts regarding the diagnosis, classification, and treatment of aortic dissection have emerged. [44] Evolving imaging modalities (such as 4-dimensional [4-D] flow MRI and hybrid positron-emission tomography [PET]-CT) afford more comprehensive insight into anatomic, hemodynamic, and molecular features of the aorta and have shown promise for detecting vessel wall instability at an early stage. [45] Finally, new treatment options for aortic dissection include the frozen elephant trunk approach, thoracic endovascular repair, and the endo-Bentall concept. [44, 46, 47]
-
Image A represents a Stanford A or a DeBakey type 1 dissection. Image B represents a Stanford A or DeBakey type II dissection. Image C represents a Stanford type B or a DeBakey type III dissection. Image D is classified in a manner similar to A but contains an additional entry tear in the descending thoracic aorta. Note that a primary arch dissection does not fit neatly into either classification.
-
Plain anteroposterior view of the chest demonstrates a wide mediastinum. Courtesy of Jorge J Guerra, Jr, MD.
-
Nonenhanced CT scan of the chest demonstrates a type B acute aortic intramural hematoma with displacement of intimal calcification and a crescentic high-attenuating clot without mass effect on the aortic lumen. Courtesy of Joel L Fishman, MD.
-
Contrast-enhanced axial CT image demonstrates an intimal flap that separates the 2 channels in the ascending and descending aorta diagnostic of a Stanford type A dissection. Courtesy of Joel L Fishman, MD.
-
Contrast-enhanced axial CT image demonstrates an intimal flap that separates the 2 channels in the ascending aorta and descending aorta and begins at the level of the aortic root. Courtesy of Joel L Fishman, MD.
-
Sagittal gradient-echo MRI image obtained in early systole shows a jet of blood flowing through the intimal tear from the smaller anterior true lumen into the larger posterior false lumen. The intimal flap is recognized as the medium signal intensity linear structure that divides the true and false lumens. An aortic stent-graft could be placed across the intimal/entry tear in the descending thoracic aorta to redirect blood into the anterior true lumen. Courtesy of Joel L Fishman, MD.
-
Unenhanced CT scan of the chest depicts a complex dissection with multiple false channels in an aneurysmal descending aorta. Aortic rupture could not be excluded, as there is a large aorta with hyperintense areas of fresh hematoma and a pleural effusion. Left lower lobe lung disease is present. Courtesy of Joel L Fishman, MD.
-
Contrast-enhanced CT scan obtained at the level of the aortic arch demonstrates an aortic dissection with almost complete separation of the aortic intima. The slight prolapse may be the beginning of a configuration at risk for intimo-intimo intussusception, a potentially fatal event. Courtesy of Joel L Fishman, MD.
-
Oblique arteriogram of the thoracic aorta demonstrates the double-barrel aorta sign of aortic dissection. Both the true and false lumina are opacified. Courtesy of Joel L Fishman, MD.
-
3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (yellow arrow). The point of dissection is not shown.
-
3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (white arrow). The point of dissection is not shown. Note the aortic calcified plaque (black arrow).
-
3-D color reconstruction of CTA in descending thoracic aortic dissection shows the opening up of collateral circulation proximal to the point of dissection (yellow arrow). The dissection and the false lumen are demonstrated by the red arrow.
-
3-D color reconstruction of CTA in descending thoracic aortic dissection shows resumption/re-entry of normal distal aortic circulation, past the point of the false lumen (yellow arrow).
-
3-D color reconstruction of CTA in descending thoracic aortic dissection shows severance of the left renal artery due to dissection (yellow arrow).
-
3-D color reconstruction of CTA in descending thoracic aortic dissection shows severance of the left renal artery due to dissection (white arrow). Note the few spidery collaterals at the point of left renal artery severance and the atheromatous calcified plaques (yellow arrow).
-
Axial CT scan through the mid-descending aorta in aortic dissection shows the false lumen (yellow arrow).
-
Axial CT scan through the upper abdomen in mid-descending thoracic aortic dissection shows the dissection flap entering the abdominal aorta (white arrow). Note the normally perfused right renal artery and poorly perfused upper pole of the left kidney at the point of dissection.