Practice Essentials
Small cell lung cancer (SCLC) accounts for approximately 13-15% of all cases of lung cancer. It once accounted for 20-25% of all newly diagnosed lung cancers. SCLC, previously known as oat cell carcinoma, is considered distinct from other lung cancers, which are called non–small cell lung cancers (NSCLCs) because of their clinical and biologic characteristics. SCLC is an aggressive form of primary pulmonary neuroendocrine tumor with short doubling time and a tendency for early metastasis. Median overall survival (OS) is about 12 months, and the median survival without treatment is 2-4 months. SCLC is divided into 2 stages: limited disease (LD) and extensive disease (ED). LD-SCLC is diagnosed in about 30% of patients and is confined to one hemithorax encompassed in a radiation port. ED-SCLC affects the remaining 70% of patients and extends beyond a single radiation field. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]
(Examples of SCLC are presented in the images below.)
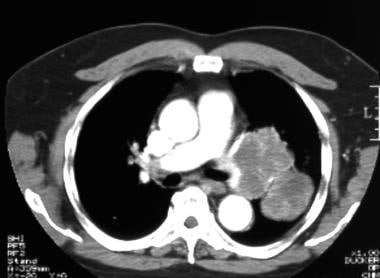 Lung cancer, small cell. Contrast-enhanced CT scan of the chest shows a large left lung and a hilar mass, with invasion of the left pulmonary artery.
Lung cancer, small cell. Contrast-enhanced CT scan of the chest shows a large left lung and a hilar mass, with invasion of the left pulmonary artery.
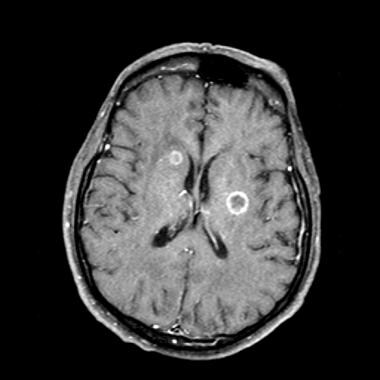 Lung cancer, small cell. Contrast-enhanced MRI of the brain in a patient with known small-cell lung cancer (SCLC). Axial section at the level of lateral ventricles shows at least 2 ring-enhancing metastatic lesions in the periventricular region. The brain is one of the predominant sites for SCLC metastasis.
Lung cancer, small cell. Contrast-enhanced MRI of the brain in a patient with known small-cell lung cancer (SCLC). Axial section at the level of lateral ventricles shows at least 2 ring-enhancing metastatic lesions in the periventricular region. The brain is one of the predominant sites for SCLC metastasis.
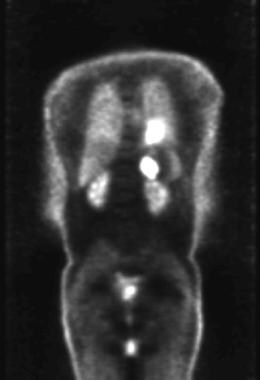 Lung cancer, small cell. Coronal positron emission tomogram shows abnormal areas of increased metabolic activity in the left hilar and left adrenal regions consistent with a hilar tumor with left adrenal metastasis.
Lung cancer, small cell. Coronal positron emission tomogram shows abnormal areas of increased metabolic activity in the left hilar and left adrenal regions consistent with a hilar tumor with left adrenal metastasis.
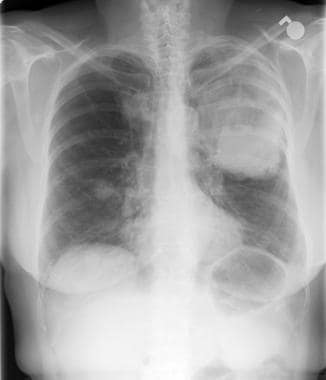 Lung cancer, small cell. Frontal chest radiograph shows extensive disease. A large mass is noted in the left mid lung with an opacity extending to the upper lung. Also present is a right lower lung nodule that suggests a metastatic deposit. Increased right paratracheal opacity indicates lymphadenopathy. A small left pleural effusion is present, with blunting of the costophrenic recess.
Lung cancer, small cell. Frontal chest radiograph shows extensive disease. A large mass is noted in the left mid lung with an opacity extending to the upper lung. Also present is a right lower lung nodule that suggests a metastatic deposit. Increased right paratracheal opacity indicates lymphadenopathy. A small left pleural effusion is present, with blunting of the costophrenic recess.
Historically, the histologic identification of small cell cancer dates back to the 1920s, when the small cell/oat cell tumor was shown to be a carcinoma of the lung and not an oat cell sarcoma of the mediastinum, as thought earlier. This cancer was also noted to occur in patients younger (27-66 yr) than those with the other cancers.
Since that time, many histologic subtypes of SCLC have been found, and attempts have been made to classify this tumor. However, disagreement regarding the classifications still exists. In 1998, the International Association for the Study of Lung Cancer (IASLC) classified SCLC into 3 general histologic subtypes: small, mixed, and combined. According to this classification, the small-cell subtype includes the previous World Health Organization (WHO) variety of oat cell and intermediate types. The mixed variety encompasses mixed cell and large cell cancers. (In the WHO classification, this is considered a combined variety.) The combined variety includes a significant proportion of squamous cell or adenocarcinoma cell cancers in addition to small cell cancers (1-3% of cases of SCLC). [14]
The British Medical Research Council reported that patients with SCLC had a poor prognosis and that SCLC was considered a distinct clinicopathologic entity. After that report, different treatment options were considered, and surgery alone was found to be an insufficient method of treatment. A better response was obtained with the addition of chemotherapy and irradiation.
Because SCLC is considered a systemic disease, the clinical course, prognosis, and treatment options are clearly different from those of other lung cancers. Clinically, lung cancers are often categorized into SCLC and non-SCLC (NSCLC).
Patients with SCLC are rarely surgical candidates, and they are usually treated with irradiation and/or chemotherapy. Patients with NSCLC are usually evaluated for possible surgical excision, and their disease is staged by using the common tumor, nodes, and metastases (TNM) staging system.
Guidelines
According to the American College of Radiology (ACR) Appropriateness Criteria, chest CT with IV contrast and MRI without and with IV contrast are usually appropriate for the noninvasive initial clinical staging of SCLC. FDG PET/CT from skull base to mid-thigh or abdominal or pelvic CT with IV contrast is usually appropriate; however, FDG-PET/CT is found to be more sensitive for lymph node and adrenal metastases, and it is superior to CT for bone marrow metastases. [15]
The National Comprehensive Cancer Network (NCCN) guidelines recommend CT scan with IV contrast of the chest/abdomen and brain imaging using MRI (preferred) or CT scan with IV contrast for staging of SCLC. If limited-stage disease is suspected, a PET/CT scan from skull base to mid-thigh can be performed to assess for distant metastases. A bone scan can be considered if PET/CT is equivocal or not available. [16]
The European Society of Medical Oncology (ESMO) recommends the following [6] :
-
A CT scan with contrast of the chest and abdomen.
-
In localized disease or if symptoms or clinical findings suggest involvement, additional bone scintigraphy and CT or MRI of the brain.
-
FDG-PET CT is optional in localized disease. PET findings, which modify treatment decisions, should be pathologically confirmed.
Additional guidelines on SCLC and NSCLC have been published by ESMO, ACS, ACR, ASCO, ACCP, ASCO, NCI, USPSTF, and NCCN. [15, 16, 6, 7, 17, 13, 18, 19]
Preferred examination
The most common appearance of SCLC on imaging studies is a centrally located lung mass or mediastinal mass with hilar involvement. In two thirds of patients, tumor tissue encases mediastinal structures, including vessels, airways, and the esophagus. The usefulness of the various imaging examinations largely depends on the clinical findings at the time of presentation and on the stage of the disease. Many imaging modalities are used to further evaluate the findings seen on the previous imaging and to determine the stage of the disease. [20, 21, 22, 23]
Conventional radiography is not helpful in finding early disease. When the mass or mass effect is visible on a radiograph, the disease is almost invariably in an advanced stage.
Chest CT is the modality of choice for initial evaluation of the SCLC. For patients with known or suspected SCLC, chest CT with IV contrast is recommended. If concurrent abdominal CT is not obtained, the adrenal glands should be covered. Chest CT without IV contrast may also be used. Chest CT with IV contrast can help identify tumor invasion in the chest wall, assess mediastinal invasion, evaluate other mediastinal and hilar lymph nodes, distinguish central obstructing tumor from surrounding atelectasis, and identify liver metastases. [15, 24, 10, 11, 25]
Brain MRI with IV contrast is recommended in all SCLC patients and has been shown to identify metastatic lesions in 10-15% of newly diagnosed SCLC patients without neurologic symptoms. Although most centers do not routinely use MRI to evaluate the primary lesion in the chest, it may provide useful information in problematic cases of mediastinal invasion. MRI does have a role in ruling out brain metastatic lesions and in differentiating questionable adrenal masses. In pregnant patients, MRI can also be used instead of CT scanning, to avoid the potential effects of ionizing radiation. [26] MRI has a greater sensitivy than CT for intracranial metastases. [24, 10, 11, 25]
CT scanning is generally used to guide biopsy of suspicious lesions. It can be used to guide transbronchial biopsy by demonstrating the location of the lesions, or it can be used to direct CT scan–guided percutaneous transthoracic biopsy. Similarly, ultrasonography can also be used to guide biopsy of suspicious intra-abdominal or pelvic lesions.
FDG-PET or PET/CT is recommended in patients with clinical stage I or II LS-SCLC who are being considered for curative treatment. If ES-SCLC is established, FDG-PET or PET/CT is optional for further staging. [21, 22, 23] It is less commonly used in patients with SCLC than in patients with NSCLC because most SCLC patients are not candidates for surgery. PET is also useful for evaluating cases in which recurrent disease is questionable. [27, 28, 29]
Technetium-99m bone scintigraphy may be used as an alternative imaging modality to evaluate for extrathoracic bone metastasis in SCLC patients if FDG-PET or PET/CT is not performed.
Bone scanning is routinely used to evaluate bony metastatic disease.
Limitations of techniques
Small cell lung cancer (SCLC) is a histologic diagnosis that is always based on findings in tissue biopsy samples. Imaging only shows suspicious abnormalities that are invariably examined at subsequent biopsy to establish the tissue diagnosis. [30, 31]
Chest radiography has limited usefulness in detecting early SCLC. In most cases in which an abnormality is visible on a radiograph, the cancer has already metastasized. Radiography has poor sensitivity and specificity, and almost all suspicious abnormalities require further evaluation with other modalities, most often CT.
CT shows the anatomic details of the lesion well. CT may have lower sensitivity than MRI in detecting mediastinal invasion. Because CT staging involves criteria based on the size of the lymph nodes, CT has an inherent limitation. Disease may be overstaged if enlarged benign lymph nodes are measured, or disease may be understaged if the microscopically involved normal-sized nodes are classified as being benign.
The spatial resolution of MRI is generally considered to be lower than that of CT. For this reason, a group of nodes may sometimes be falsely mistaken as a single large node. Also, because of the inability to detect calcium with MRI, enlarged and calcified benign nodes may be mistaken for pathologic nodes. The cost of MRI and the artifacts due to cardiac and vascular pulsation and respiratory movements limit its usefulness in evaluating primary lung cancer in most cases; however, MRI may be useful in special circumstances.
Although PET is emerging as a popular modality for evaluating many cancers, the usefulness of PET is limited because of its cost and unavailability in many clinical practices. Generally, the resolution of PET is not considered good for lesions smaller than 1 cm. The PET results can also overlap with the standard uptake values (SUVs) in some benign lesions and malignant lesions. [32]
Radiography
Chest radiographs may show unilateral hilar enlargement, increased hilar opacity, a perihilar mass, mediastinal mass, or a combination of these. Less commonly, small cell lung cancer (SCLC) may appear as a solitary pulmonary nodule. (See the images below.) [33]
 Lung cancer, small cell. Frontal chest radiograph shows extensive disease. A large mass is noted in the left mid lung with an opacity extending to the upper lung. Also present is a right lower lung nodule that suggests a metastatic deposit. Increased right paratracheal opacity indicates lymphadenopathy. A small left pleural effusion is present, with blunting of the costophrenic recess.
Lung cancer, small cell. Frontal chest radiograph shows extensive disease. A large mass is noted in the left mid lung with an opacity extending to the upper lung. Also present is a right lower lung nodule that suggests a metastatic deposit. Increased right paratracheal opacity indicates lymphadenopathy. A small left pleural effusion is present, with blunting of the costophrenic recess.
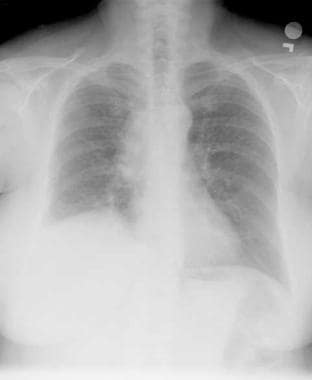 Lung cancer, small cell. Frontal chest radiograph shows increased opacity in the right hilar and paratracheal region, with thickening of the right paratracheal stripe. Some volume loss is also shown in the right lower lobe. Small-cell lung cancer frequently appears as a hilar or mediastinal mass.
Lung cancer, small cell. Frontal chest radiograph shows increased opacity in the right hilar and paratracheal region, with thickening of the right paratracheal stripe. Some volume loss is also shown in the right lower lobe. Small-cell lung cancer frequently appears as a hilar or mediastinal mass.
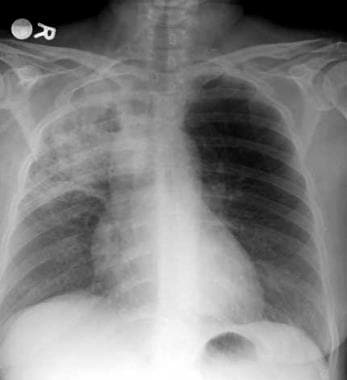 Lung cancer, small cell. Frontal chest radiograph shows obstructive pneumonitis with atelectasis of the right upper lobe. Increased opacity in the right tracheobronchial and paratracheal region suggests a mass or lymphadenopathy in that region.
Lung cancer, small cell. Frontal chest radiograph shows obstructive pneumonitis with atelectasis of the right upper lobe. Increased opacity in the right tracheobronchial and paratracheal region suggests a mass or lymphadenopathy in that region.
Compression of the bronchi is relatively common in SCLC because of the central location of the tumor in most cases. About 30-50% of SCLCs show evidence of obstructive pneumonitis on the initial presentation. SCLC can appear as segmental or lobar atelectasis with or without an obvious hilar mass. The S sign of Golden is seen when a collapsed upper lobe forms a meniscus concave toward the hilum and when an enlarged hilar mass forms the convex meniscus of the S. Occasionally, endobronchial growth or bronchial compression may be appreciated as a bronchial cutoff or filling defect.
Thickening of the right paratracheal stripe may be an indication of right paratracheal lymphadenopathy. With massive subcarinal lymphadenopathy, widening of the carinal angle may occasionally be observed. Subtle changes of hilar asymmetry, increased opacity, a convex or lobulated outer hilar border, or any change from a previous radiograph should be viewed with suspicion.
Involvement of pleura or pericardium may result in pleural or pericardial effusions. Rarely, involvement of a pulmonary artery may result in compression of the artery with oligemia in the area of distribution. Invasion of pulmonary artery may result in pulmonary metastatic lesions. Large mediastinal masses may lead to lymphatic obstruction, which may result in reticulonodular opacities in the lung. Lateral views are complementary to the frontal views and help in assessing the mediastinal abnormalities, especially in the retrosternal and hilar regions. Paratracheal masses and thickening of the posterior wall of the bronchus intermedius may be seen on the lateral view.
The degree of confidence in radiography is low, because a bulky mediastinal mass may also be seen in a variety of conditions other than small cell lung cancer.
Computed Tomography
CT scanning is the modality most commonly used for the evaluation and characterization of an abnormality depicted on a chest radiograph. CT is used to assess the size or volume of the tumor, mediastinal involvement, pathologically enlarged lymph nodes, and vascular invasion. It is also sensitive in detecting pleural and pericardial effusion or thickening. Nodularity of pleura or pericardium is the hallmark of metastatic involvement. (See the images below.)
 Lung cancer, small cell. Contrast-enhanced CT scan of the chest shows a large left lung and a hilar mass, with invasion of the left pulmonary artery.
Lung cancer, small cell. Contrast-enhanced CT scan of the chest shows a large left lung and a hilar mass, with invasion of the left pulmonary artery.
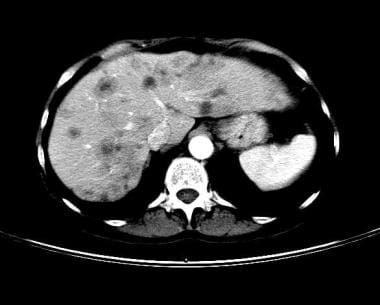 Lung cancer, small cell. Contrast-enhanced CT scan of the abdomen. Axial section through the liver shows multiple hypoattenuating areas in the liver. Poorly defined margins, attenuation greater than that of water, and scattered distribution in a patient with known lung cancer is most consistent with metastatic disease.
Lung cancer, small cell. Contrast-enhanced CT scan of the abdomen. Axial section through the liver shows multiple hypoattenuating areas in the liver. Poorly defined margins, attenuation greater than that of water, and scattered distribution in a patient with known lung cancer is most consistent with metastatic disease.
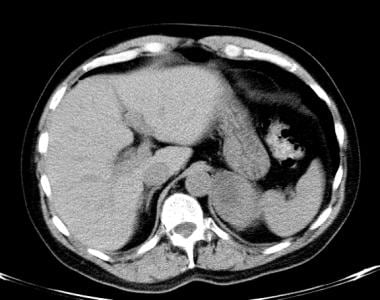 Lung cancer, small cell. Nonenhanced CT scan of the abdomen at the level of adrenal gland shows a large adrenal mass on the left side. The high attenuation values on this image and the large size of the adrenal mass suggest a malignant lesion. The adrenal glands are a common site for metastatic small-cell lung cancer.
Lung cancer, small cell. Nonenhanced CT scan of the abdomen at the level of adrenal gland shows a large adrenal mass on the left side. The high attenuation values on this image and the large size of the adrenal mass suggest a malignant lesion. The adrenal glands are a common site for metastatic small-cell lung cancer.
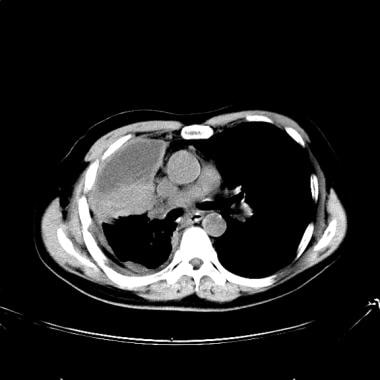 Lung cancer, small cell. CT scan of the chest at the level of hila shows a large hilar tumor on the right side, with loculated pleural effusion. Nodular thickening of the pleura suggests pleural metastasis. The tumor mass is difficult to differentiate from the adjacent atelectatic lung.
Lung cancer, small cell. CT scan of the chest at the level of hila shows a large hilar tumor on the right side, with loculated pleural effusion. Nodular thickening of the pleura suggests pleural metastasis. The tumor mass is difficult to differentiate from the adjacent atelectatic lung.
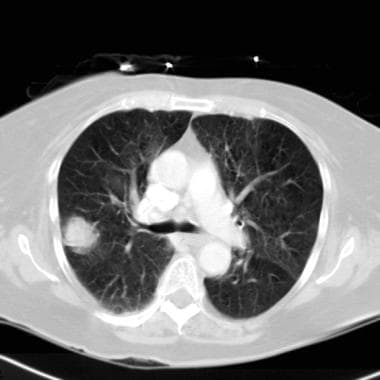 Lung cancer, small cell. Axial CT scan though the lungs show a solitary pulmonary nodule in the peripheral part of the right lung. Small-cell lung cancer occasionally appears as a peripheral lung nodule.
Lung cancer, small cell. Axial CT scan though the lungs show a solitary pulmonary nodule in the peripheral part of the right lung. Small-cell lung cancer occasionally appears as a peripheral lung nodule.
Contrast-enhanced CT can sometimes be used to differentiate a tumor mass from the adjacent collapsed lung or pneumonitis, which usually enhances more than the tumor. Sometimes, air bronchograms are observed. Three-dimensional (3D) images reconstructed from thin sections through the mass improve the sensitivity in detecting invasion of adjacent organs. Chest wall invasion can be demonstrated with evidence of rib destruction (the most specific finding), pleural thickening, and obliteration of the extrapleural fat line. An obtuse angle of the mass with the chest wall may also suggest invasion. Pain in the chest wall is a more specific sign of involvement.
Similarly, contact with the mediastinum of more than 3 cm, contact with aorta of more than 90°, invasion of the mediastinal fat, and pleural or pericardial thickening are considered signs of mediastinal invasion. CT scans can also show endobronchial growth and the degree of compression of the bronchi or vessels.
The CT scans of patients with SCLC of less than 3.0 cm in size may show notching more frequently than those of patients with adenocarcinoma, whereas surrounding ground-glass opacity, air bronchogram, pleural indentation, and spiculation are observed less frequently in SCLC than in adenocarcinoma. [34]
The size of lymph nodes is generally estimated for staging purposes by measuring the short axis of the lymph nodes. Compared with the long axis, the short axis is a more accurate predictor of the volume. For practical purposes, a short-axis measurement greater than 1 cm is generally considered abnormal in the chest. However, some have observed different measurements in different groups of patients.
CT of the chest routinely includes imaging of the adrenal glands, which are common sites for small cell lung cancer metastases. A lesion with an attenuation value less than 10 HU (Hounsfield units) on a nonenhanced CT scan most likely represents an adenoma (90% accuracy). CT of the abdomen and pelvis is also generally indicated in staging of small cell lung cancer to rule out metastases to the liver, nodes, or other organs. CT of the head helps rule out brain metastasis, which is also common in SCLC. [20] CT is also routinely used to follow up patients with SCLC after irradiation and chemotherapy.
In one study by Kobayashi et al, high-resolution CT (HRCT) features of peripherally located SCLC were retrospectively reviewed in 33 patients with peripherally located SCLC measuring 30 mm or less, and it was determined that a non-round shape and thickening of the bronchovascular bundle (BVB) were common, while marginal ground-glass opacity (GGO) and air bronchogram were less common in small-sized, peripherally located SCLC. In addition, the vermiform/branching shape and thickening of the BVB suggested relatively advanced disease. [35]
Magnetic Resonance Imaging
MRI is not routinely used for detecting the primary tumor or for staging. However, it may sometimes help in problematic cases because MRI offers improved tissue contrast resolution and a multiplanar imaging capability (see the image below). [36] Brain MRI with IV contrast is recommended in all SCLC patients and has been shown to identify metastatic lesions in 10-15% of newly diagnosed SCLC patients without neurologic symptoms. Although most centers do not routinely use MRI to evaluate the primary lesion in the chest, it may provide useful information in problematic cases of mediastinal invasion. MRI does have a role in ruling out brain metastatic lesions and in differentiating questionable adrenal masses. In pregnant patients, MRI can also be used instead of CT scanning to avoid the potential effects of ionizing radiation. [26]
MRI has a greater sensitivy than CT for intracranial metastases. In primary tumors, MRI can sometimes help differentiate tumor from surrounding atelectasis or pneumonitis, which has relatively high signal intensity on T2-weighted images, as opposed to the relatively low signal intensity of the tumor. [10, 11, 20, 24, 25] Because of the relatively low spatial resolution of MRI compared with that of CT, a cluster of small lymph nodes may occasionally be mistaken for a single enlarged node. This observation can lead to a false-positive finding. Also, calcifications may be missed on MRIs.
 Lung cancer, small cell. Contrast-enhanced MRI of the brain in a patient with known small-cell lung cancer (SCLC). Axial section at the level of lateral ventricles shows at least 2 ring-enhancing metastatic lesions in the periventricular region. The brain is one of the predominant sites for SCLC metastasis.
Lung cancer, small cell. Contrast-enhanced MRI of the brain in a patient with known small-cell lung cancer (SCLC). Axial section at the level of lateral ventricles shows at least 2 ring-enhancing metastatic lesions in the periventricular region. The brain is one of the predominant sites for SCLC metastasis.
Gadolinium-enhanced MRI may also be helpful because the lung enhances rapidly, whereas the tumor usually enhances relatively slowly. MRI is also good for detecting nodes in the aortopulmonary window or for detecting subcarinal nodes, because it can provide images in the sagittal and coronal planes. With chemical shift imaging, MRI is reliable in differentiating adrenal adenomas from possible metastasis because it shows decreases in signal intensity on out-of-phase images as compared with in-phase images.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). NSF/NFD has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
In a study in which diffusion-weighted imaging (DWI) and short tau inversion recovery (STIR) turbo spin-echo imaging were compared in differentiating SCLC from NSCLC, DWI was found to be more useful than STIR. The specificity and accuracy were each 85.7% for DWI, whereas for STIR, specificity was 63.3% and accuracy was 66.1%. However, the accuracy was 94.6% when the 2 modalities were combined. [37]
Nuclear Imaging
Bones are the most common sites for metastatic disease in SCLC. Metastatic disease is usually seen as multiple asymmetric areas of increased uptake, mostly in the axial skeleton.Bone scanning is used as one of the tools for staging the SCLC, as well as for follow-up. A normal bone scan result usually excludes metastatic disease; however, in rare cases, aggressive osteolytic lesions with little bone reaction may not be evident. Similarly, benign conditions such as fractures or degenerative disease may occasionally be confused with metastatic disease.
(See the image below.)
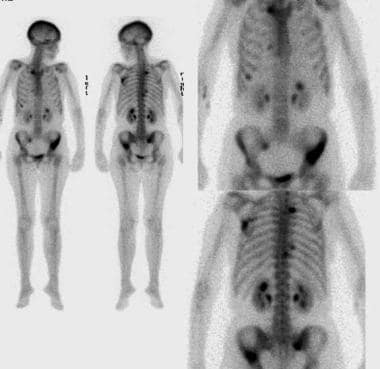 Lung cancer, small cell. Whole-body nuclear medicine bone scanning with anterior and posterior images reveal multiple abnormal areas of increased radiotracer activity in the pelvis, spine, ribs, and left scapula. These findings are consistent with bony metastatic disease. The bones are commonly affected in patients with small-cell lung cancer.
Lung cancer, small cell. Whole-body nuclear medicine bone scanning with anterior and posterior images reveal multiple abnormal areas of increased radiotracer activity in the pelvis, spine, ribs, and left scapula. These findings are consistent with bony metastatic disease. The bones are commonly affected in patients with small-cell lung cancer.
Positron emission tomography
PET is one of the most rapidly emerging modalities for the evaluation, staging, and posttherapeutic follow-up of cancer. [21] PET combines the functional and anatomic aspects of the lesions. PET primarily depends on the metabolism of glucose, which is usually high in tumor cells. By measuring the standardized uptake values (SUVs) within the lesions, the malignant lesions (which usually have values greater than 2.5) can be reliably differentiated from benign lesions in most cases. However, the values can overlap.
(See the images below.)
 Lung cancer, small cell. Coronal positron emission tomogram shows abnormal areas of increased metabolic activity in the left hilar and left adrenal regions consistent with a hilar tumor with left adrenal metastasis.
Lung cancer, small cell. Coronal positron emission tomogram shows abnormal areas of increased metabolic activity in the left hilar and left adrenal regions consistent with a hilar tumor with left adrenal metastasis.
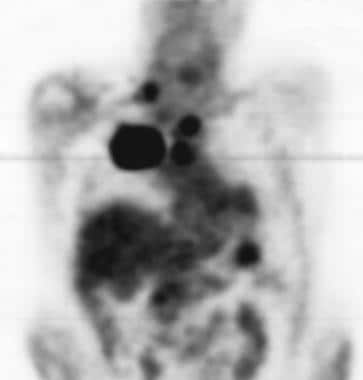 Lung cancer, small cell. Coronal positron emission tomogram shows a large focal hypermetabolic area on the right consistent with a large mass in the central portion of the right upper lobe. Multiple other smaller hypermetabolic areas suggest lymph-node metastatic disease in the chest, abdomen, and right supraclavicular region.
Lung cancer, small cell. Coronal positron emission tomogram shows a large focal hypermetabolic area on the right consistent with a large mass in the central portion of the right upper lobe. Multiple other smaller hypermetabolic areas suggest lymph-node metastatic disease in the chest, abdomen, and right supraclavicular region.
PET can be used for staging purposes by determining nodal involvement and distant metastasis. [22] CT is usually used along with PET for anatomic comparison. Some studies have shown that PET is more sensitive than CT for staging purposes. Other studies have also shown that PET has good sensitivity in detecting bony metastasis.
FDG-PET or PET/CT is recommended in patients with clinical stage I or II LS-SCLC who are being considered for curative treatment. If ES-SCLC is established, FDG-PET or PET/CT is optional for further staging. [21, 22, 23] It is less commonly used in patients with SCLC than in patients with NSCLC because most patients with SCLC are not candidates for surgery. PET is also useful for evaluating cases in which recurrent disease is questionable. [27, 28, 29]
According to an Agency for Healthcare Research and Quality (AHRQ) study of imaging for the pretreatment staging of SCLC, FDG PET/CT is more sensitive than MDCT for detecting osseous metastases; FDG PET/CT is more sensitive than bone scintigraphy for detecting osseous metastases; and standard staging plus FDG PET/CT is more sensitive than standard staging alone for detecting any distant metastases. [25]
The main limitations of PET use are its cost, its limited availability, and the lack of expertise in performing the examination.
Ventilation/perfusion scanning
Infrequently, when surgery is being considered in the treatment of a peripheral SCLC with limited disease, lung ventilation/perfusion scanning is sometimes helpful for surgical planning to assess the respiratory reserve and perfusion distribution after lobectomy or pneumonectomy.
Monoclonal antibody scanning
Monoclonal antibody study with technetium-99m NRLU-10 whole-body scanning has been shown to be sensitive in detecting SCLC. However, this study has not been widely used in clinical practice.
Iodine-131 6-beta-iodomethyl-19-norcholesterol nuclear imaging
Iodine-131 6-beta-iodomethyl-19-norcholesterol (NP-59) nuclear imaging is sometimes used to differentiate adrenal metastasis from adrenal adenomas (which take up the agent).
Questions & Answers
Overview
What is the prevalence and prognosis of small cell lung cancer (SCLC)?
How is small cell lung cancer (SCLC) classified?
How is small cell lung cancer (SCLC) treated?
What are the guidelines on imaging for small cell lung cancer (SCLC)?
What is the role of imaging studies in the workup of small cell lung cancer (SCLC)?
What are the limitations of imaging studies in the diagnosis of small cell lung cancer (SCLC)?
What are the limitations of radiography in the diagnosis of small cell lung cancer (SCLC)?
What are the limitations of CT scans in the diagnosis of small cell lung cancer (SCLC)?
What are the limitations of MRI in the diagnosis of small cell lung cancer (SCLC)?
What are the limitations of PET scans in the diagnosis of small cell lung cancer (SCLC)?
Which finding on chest radiography are characteristic of small cell lung cancer (SCLC)?
Which finding on CT scans are characteristic of small cell lung cancer (SCLC)?
What is the role of MRI in the workup of small cell lung cancer (SCLC)?
What is the role of bone scanning in the workup of small cell lung cancer (SCLC)?
What is the role of PET scans in the workup of small cell lung cancer (SCLC)?
What is the role of ventilation/perfusion scans in the workup of small cell lung cancer (SCLC)?
What is the role of monoclonal antibody scans in the workup of small cell lung cancer (SCLC)?
What is the role of NP-59 nuclear imaging in the workup of small cell lung cancer (SCLC)?
-
Lung cancer, small cell. Contrast-enhanced CT scan of the chest shows a large left lung and a hilar mass, with invasion of the left pulmonary artery.
-
Lung cancer, small cell. Coronal positron emission tomogram shows abnormal areas of increased metabolic activity in the left hilar and left adrenal regions consistent with a hilar tumor with left adrenal metastasis.
-
Lung cancer, small cell. Contrast-enhanced CT scan of the abdomen. Axial section through the liver shows multiple hypoattenuating areas in the liver. Poorly defined margins, attenuation greater than that of water, and scattered distribution in a patient with known lung cancer is most consistent with metastatic disease.
-
Lung cancer, small cell. Whole-body nuclear medicine bone scanning with anterior and posterior images reveal multiple abnormal areas of increased radiotracer activity in the pelvis, spine, ribs, and left scapula. These findings are consistent with bony metastatic disease. The bones are commonly affected in patients with small-cell lung cancer.
-
Lung cancer, small cell. Nonenhanced CT scan of the abdomen at the level of adrenal gland shows a large adrenal mass on the left side. The high attenuation values on this image and the large size of the adrenal mass suggest a malignant lesion. The adrenal glands are a common site for metastatic small-cell lung cancer.
-
Lung cancer, small cell. CT scan of the chest at the level of hila shows a large hilar tumor on the right side, with loculated pleural effusion. Nodular thickening of the pleura suggests pleural metastasis. The tumor mass is difficult to differentiate from the adjacent atelectatic lung.
-
Lung cancer, small cell. Contrast-enhanced MRI of the brain in a patient with known small-cell lung cancer (SCLC). Axial section at the level of lateral ventricles shows at least 2 ring-enhancing metastatic lesions in the periventricular region. The brain is one of the predominant sites for SCLC metastasis.
-
Lung cancer, small cell. Coronal positron emission tomogram shows a large focal hypermetabolic area on the right consistent with a large mass in the central portion of the right upper lobe. Multiple other smaller hypermetabolic areas suggest lymph-node metastatic disease in the chest, abdomen, and right supraclavicular region.
-
Lung cancer, small cell. Axial CT scan though the lungs show a solitary pulmonary nodule in the peripheral part of the right lung. Small-cell lung cancer occasionally appears as a peripheral lung nodule.
-
Lung cancer, small cell. Frontal chest radiograph shows extensive disease. A large mass is noted in the left mid lung with an opacity extending to the upper lung. Also present is a right lower lung nodule that suggests a metastatic deposit. Increased right paratracheal opacity indicates lymphadenopathy. A small left pleural effusion is present, with blunting of the costophrenic recess.
-
Lung cancer, small cell. Frontal chest radiograph shows increased opacity in the right hilar and paratracheal region, with thickening of the right paratracheal stripe. Some volume loss is also shown in the right lower lobe. Small-cell lung cancer frequently appears as a hilar or mediastinal mass.
-
Lung cancer, small cell. Frontal chest radiograph shows obstructive pneumonitis with atelectasis of the right upper lobe. Increased opacity in the right tracheobronchial and paratracheal region suggests a mass or lymphadenopathy in that region.







