Practice Essentials
Pulmonary arteriovenous malformation (PAVM) is an abnormal communication between the pulmonary artery and the pulmonary vein. PAVMs are usually congenital in origin; however, they also may be acquired in a variety of conditions, such as hepatic cirrhosis, schistosomiasis, mitral stenosis, trauma, actinomycosis, and metastatic thyroid carcinoma. [1] Approximately 10% of cases are idiopathic. [2] PAVMs are also known as pulmonary arteriovenous fistulae, pulmonary arteriovenous aneurysms, and pulmonary hemangiomas. [3]
A pathologic intrapulmonary right-to-left shunt impairs regular gas exchange and filtration of systemic venous blood. Patients who go undiagnosed may subsequently encounter complications such as ischemic stroke, myocardial infarction, cerebral abscess, massive hemoptysis, or hemothorax. [3]
Patients with PAVM may be asymptomatic or present with dyspnea, hypoxemia, or chest pain. [2] Paradoxical embolization complications, incuding stroke and brain abscess, can occur even in asymptomatic patients. [2, 4]
Approximately 70% of PAVMs are associated with Osler-Weber-Rendu disease (hereditary hemorrhagic telangiectasia [HHT]), and about 15-30% of individuals with Osler-Weber-Rendu diseases have a PAVM. [5]
(See the images below.)
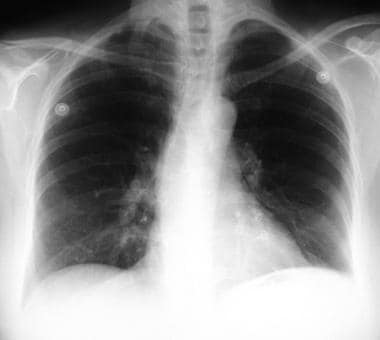
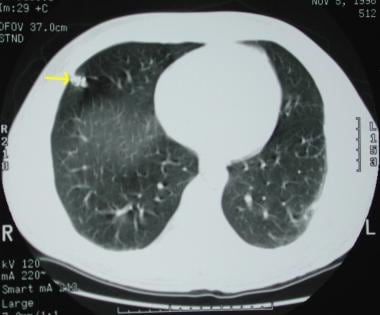
 A 60-year-old patient presented with right cerebral infarction. The chest radiograph demonstrates a left–lower-lobe nodule, which was later identified as a pulmonary arteriovenous malformation.
A 60-year-old patient presented with right cerebral infarction. The chest radiograph demonstrates a left–lower-lobe nodule, which was later identified as a pulmonary arteriovenous malformation.
Diagnostic approach in a patient with a suspected PAVM
A pulmonary arteriovenous malformation may be suspected in the following clinical situations:
-
An incidental finding of a solitary pulmonary nodule on chest radiographs
-
The presence of mucocutaneous telangiectases
-
A situation in which the patient presents with clinical findings of dyspnea, hemoptysis, hypoxemia, polycythemia, clubbing, cyanosis, cerebral embolism, or brain abscess
Whenever a PAVM is suspected, the presence of a right-to-left shunt should be confirmed by the performance of a 100% oxygen study, contrast-enhanced echocardiography, or radionuclide perfusion lung scanning. A definitive diagnosis is established by means of direct imaging of the PAVM with a contrast-enhanced study, such as a computed tomography (CT) scan or a pulmonary angiogram.
Imaging examination
Chest radiography reveals some abnormality in most patients. However, further evaluation is needed, with a test to confirm the presence of a right-to-left intrapulmonary shunt and with an imaging study to confirm the presence of PAVM.
Contrast-enhanced echocardiography is extremely sensitive in detecting clinically important PAVMs. [6] If contrast-enhanced echocardiography is not available, radionuclide perfusion lung scanning may be used.
Transthoracic contrast echocardiography (TTCE) has a sensitivity of 95-100%. In TTCE, agitated saline is introduced into the peripheral circulation, and its course is observed through the cardiac system. A positive result is the presence of bubbles in the left cardiac chamber after 3-8 cardiac cycles. [3]
Contrast-enhanced CT scanning remains the criterion standard in the diagnosis of PAVM. Although pulmonary angiography is less sensitive than contrast-enhanced CT scanning, it is performed to accurately define the anatomy, specifically before therapeutic embolization is performed. Although pulmonary angiography may also be a criterion standard for confirmation of a PAVM, angiography is required only when further intervention is planned. Otherwise, in most situations, contrast-enhanced CT scanning is sufficient to confirm the diagnosis. [7] Generally, if the CT scan shows one or more PAVMs with a feeding artery diameter of 2-3 mm or larger, pulmonary angiography should be performed. [3]
Although magnetic resonance imaging (MRI) is reportedly useful in the diagnosis of PAVM, it may be less useful than contrast-enhanced CT scanning. Therefore, if the plain chest radiographs suggest a PAVM, contrast-enhanced CT scan remains the preferred examination for confirming its presence. [8]
Parra et al compared the results of transthoracic contrast echocardiography (TTCE; divided into 4 grades depending on degree of opacification of the left ventricle after administration of contrast) with the results of thoracic CT scanning in 95 patients with HHT who were examined with both modalities to detect PAVMs. Of the 95 patients, none with normal or grade 1 TTCE had detectable PAVMs on thoracic CT. Grades 2, 3, and 4 were associated with PAVMs according to thoracic CT in 25, 80, and 100% of cases, respectively. [9] The authors therefore concluded that there is a significant association between the TTCE grade and the detection of a PAVM by thoracic CT. They additionally noted that there were significant associations between TTCE grade and the cardiac cycle when the contrast was first visible in the left atrium. [10]
Al-Saleh et al prospectively evaluated the diagnostic yield of a screening protocol for pulmonary and cerebral AVMs in children with a definite or potential diagnosis of HHT. According to the authors, their data showed that children with a definite HHT diagnosis have a high frequency of pulmonary AVMs, even if they are clinically asymptomatic. In contrast, there were no AVMs detected in patients who did not fulfill the diagnostic criteria for HHT. Screening tests for AVMs included chest CT and brain MRI. Of 61 children with a definite clinical or genetic diagnosis of HHT who were asymptomatic for visceral AVMs at first baseline assessment, 15 (25%) had a pulmonary or cerebral AVM on initial screening tests, with pulmonary AVMs predominating in pediatric HHT patients. Of 55 children who fulfilled only 1 or 2 HHT clinical diagnostic criteria, none had pulmonary or cerebral AVMs on initial screening tests. [11]
Brinjikji et al rescreened 172 adults with HHT who were negative for PAVM on initial CT screening and reported 9 newly detected PAVM cases (5.2%) over a median course of 7 years (range, 3 to ≥10 yr). The overall rate of newly detected PAVMs was 0.7%/patient-year. The investigators suggested that longer screening intervals may be warranted. [12]
Tokunaga et al, in a study of 59 patients suspected of PAVM who underwent contrast-enhanced CT, found that all patients with confirmed PAVM had parallel, straight-running, paired abnormal vessels, with most of the vessels running through the identical segment. [13]
The American College of Radiology in its Appropriateness Criteria for clinically suspected PAVM noted the following [14] :
-
Although transthoracic echocardiographic bubble studies and radionuclide perfusion detect right-to-left shunts, they do not provide all of the information needed for treatment planning and may remain positive after embolization.
-
Pulmonary angiography is appropriate for preintervention planning but not as an initial test.
-
MR angiography has a potential role in younger patients with HHT who may require lifelong surveillance, despite lower spatial resolution compared with CT.
-
Diagnostic pulmonary angiography remains the reference standard for inconclusive cases and remains a critical component of the treatment of PAVMs when performed with concurrent transcatheter embolization.
Limitations of techniques
Chest radiographs may suggest a PAVM, but these images are not helpful in distinguishing between the various causes of a lung nodule. Contrast-enhanced echocardiography may be useful for distinguishing between intracardiac and intrapulmonary shunts, although this identification may be difficult at times.
Contrast-enhanced CT scans may not depict microscopic PAVMs, but the diagnostic yield with spiral CT scanning has been improving. Pulmonary angiography is less sensitive in identifying small or microscopic PAVMs, and MRI has significant limitations in screening for small lesions and in differentiating PAVM from lesions of other causes.
Radiography
Chest radiography is recommended for the initial evaluation of patients with heredtary hemorrhagic telangiectasia (Osler-Weber-Rendu disease). Pulmonary arteriovenous malformation may well be an incidental finding on a chest radiograph. A common radiographic finding is a round or oval mass of uniform opacity. The opacity may have sharply defined borders with occasional lobulation. The mass is usually 1-5 cm, and linear shadows are adjacent to the opacity; these are the feeding vessels. PAVMs are commonly present in the lower lobes, and approximately 50% of patients have 2-8 lesions.
(See the images below.)
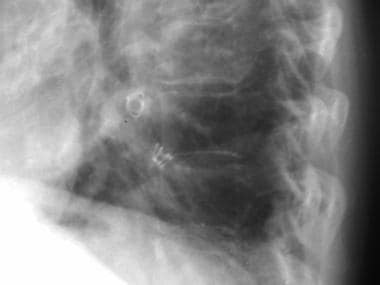 Lateral chest radiograph shows a pulmonary arteriovenous malformation in the left lower lobe that was occluded with coil embolization.
Lateral chest radiograph shows a pulmonary arteriovenous malformation in the left lower lobe that was occluded with coil embolization.
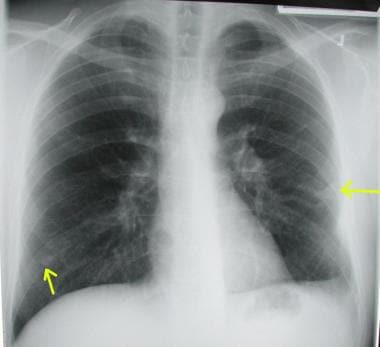 Chest radiograph shows bilateral pulmonary arteriovenous malformations in a patient who presented with dyspnea and pulmonary hemorrhage.
Chest radiograph shows bilateral pulmonary arteriovenous malformations in a patient who presented with dyspnea and pulmonary hemorrhage.
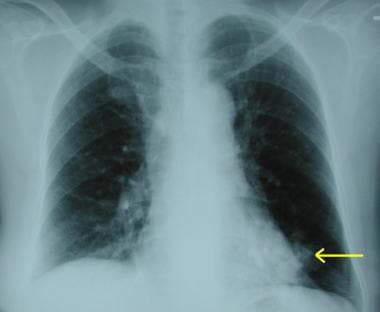 Chest radiograph demonstrating a peripheral opacity with feeding vessels radiating to the hilum. These features strongly suggest the presence of a pulmonary arteriovenous malformation.
Chest radiograph demonstrating a peripheral opacity with feeding vessels radiating to the hilum. These features strongly suggest the presence of a pulmonary arteriovenous malformation.
Degree of confidence
When the classic radiographic features are present on the chest radiograph in a patient with suspected PAVM, the diagnosis is certain. However, chest CT scanning is invariably required to confirm the finding.
Patients with microvascular telangiectases may have normal chest radiographic results. Of 27 patients in one series, the plain chest radiographic findings were strongly suggestive of PAVM in 6 patients, somewhat suggestive in 5 patients, and not suggestive in 6 patients. CT scanning may be more accurate than plain chest radiography in diagnosing PAVM. In a patient who has clinical features suggestive of PAVM but has a normal chest radiograph, further evaluation using other imaging modalities should be undertaken.
Computed Tomography
Contrast-enhanced CT scanning is the preferred imaging modality for confirming the diagnosis of PAVM. [11] One study reported better sensitivity for pulmonary arteriovenous malformations with ultrafast, contrast-enhanced CT scanning than with pulmonary angiography. CT scans depicted 98% of PAVMs in 20 patients, whereas angiography depicted only 60% of PAVMs.
Three-dimensional (3D), spiral CT scanning produces images of vascular structures that are continuously reconstructed. In one study, spiral CT scanning proved to be a better investigative tool than unilateral pulmonary angiography. The accuracy of 3D spiral CT scanning is reported to be more than 95%, and it may also be useful in identifying smaller PAVMs. [15]
(See the CT scans below.)
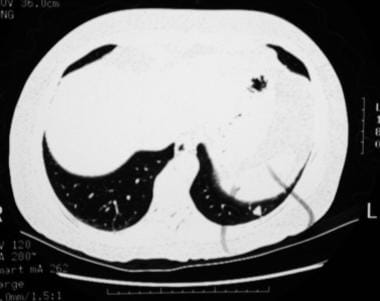
 A 60-year-old patient presented with right cerebral infarction. The chest radiograph demonstrates a left–lower-lobe nodule, which was later identified as a pulmonary arteriovenous malformation.
A 60-year-old patient presented with right cerebral infarction. The chest radiograph demonstrates a left–lower-lobe nodule, which was later identified as a pulmonary arteriovenous malformation.
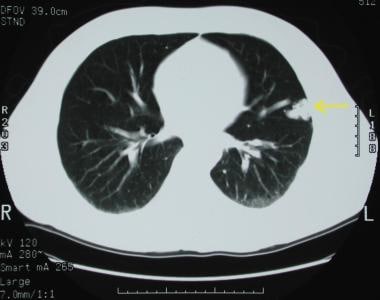 A pulmonary arteriovenous malformation in the left upper lobe. This PAVM has already been embolized with a coil.
A pulmonary arteriovenous malformation in the left upper lobe. This PAVM has already been embolized with a coil.
Multidetector-row CT scanning
The development of multidetector-row CT (MDCT) scanning has resulted in a revolution in imaging capabilities. [16] When compared with single–detector-row CT (SDCT) scanning, CT angiography with MDCT scanning provides superior imaging capabilities. The advantages include an increased per-second scanning area, the ability to retrospectively select the scanning width, the ability to routinely provide 1-mm collimation, and the possibility of higher resolution with no blooming of section thickness. [17, 18, 19, 20]
MDCT scanning also has several practical advantages in the evaluation of PAVM. These include volume imaging, with 3D displays of the volume data, and retrospective reconstruction of the CT scanning data in any plane with high resolution. [18]
Maximum-intensity projection (MIP) and surface rendering were the 3D techniques that were primarily applied to CT angiographic applications in the past. However, volume rendering is becoming the preferred 3D rendering technique for CT angiography.
Surface rendering
Surface rendering, the earliest method for 3D display, is commonly available and utilizes each voxel in the data set. The voxel intensity is compared with a threshold value, thereby defining the surface of the object, and the remaining data are discarded. Surface contours are typically modeled with surface shading; therefore, the resulting image is simplified and may provide a misleading representation of the structure. Conversion of data from a volume to a surface sacrifices a large portion of the data and limits the usefulness of surface-rendered medical images.
Maximum-intensity projection
MIP has been extensively utilized in creating angiographic images from CT scanning and MRI data. Each voxel is evaluated along a line through the image, and the value of the corresponding display pixel is selected based on the value the maximum voxel. The resulting images are typically not displayed with surface shading and increase the background mean of the image, thereby enhancing the structures. The problem with MIP is that volume averaging along with the algorithm may lead to artifacts. Despite its artifacts and deficiencies, MIP has been extensively evaluated and usually provides accuracy superior to that of surface rendering for CT angiography.
Volume rendering
This technique renders the entire volume of data rather than just the surfaces, with the total contributions of each voxel being utilized; therefore, more information is displayed than would be provided in a surface model. Volume technique is the most advanced form of 3D rendering currently available for creating accurate, clinically useful medical images and has revolutionized the field of vascular imaging.
False positives/negatives
It may be difficult to differentiate a PAVM from a vascular tumor on a CT scan. In this situation, a false-positive diagnosis of PAVM is possible. Because prolonged breath holding is required, a large PAVM may be difficult to visualize on 3D spiral CT scans. Furthermore, CT scans may not depict microscopic or small PAVMs.
Magnetic Resonance Imaging
Conventional MRI may be useful in detecting PAVMs. However, compared with other techniques, it has reduced sensitivity and specificity. Because rapidly flowing blood results in an absent or minimal MRI signal, a PAVM may be indistinguishable from normal vascular structures or an air-filled lung.
Various techniques have been used to improve the sensitivity of MRI. These include a rotating, gated MRI technique and gradient recalled-echo MRI. Some investigators have suggested that phase-contrast cine sequences are most accurate in detecting PAVM. Magnetic resonance angiography (MRA) has been used to define the vascular anatomy of a PAVM. [11]
Although a combination of MRI techniques may be useful in differentiating PAVMs from other lesions, more studies are required before the routine use of MRI can be recommended.
Ultrasonography
Contrast-enhanced echocardiography is used to confirm a right-to-left intrapulmonary shunt. Cardiac imaging with 2-dimensional echocardiography is performed while 5-10 mL of agitated sodium chloride solution is injected into a peripheral vein. In a healthy person, microbubbles are rapidly visualized in the right atrium before they gradually dissipate. When an intracardiac shunt is present, the contrast material is visualized in the left heart chambers within 1 cardiac cycle after it appears in the right atrium. The visualization of contrast material in the left atrium after a delay of 3-8 cardiac cycles confirms the presence of an intrapulmonary shunt (ie, PAVM). [21]
Degree of confidence
Contrast-enhanced echocardiography has high sensitivity in detecting right-to-left intrapulmonary shunts, such as PAVMs. This finding should be confirmed by using other tests. The finding of an intrapulmonary shunt during contrast-enhanced echocardiography still warrants further anatomic evaluation with contrast-enhanced CT or pulmonary angiography.
Contrast-enhanced echocardiography is generally not used as a first-line screening test because of its cost, limited availability, and possible overdetection of clinically insignificant PAVMs.
The sensitivity of contrast-enhanced echocardiography approaches 100%; therefore, false-negative test results are unlikely. Contrast-enhanced echocardiography may depict microscopic or clinically insignificant PAVMs.
Nuclear Imaging
Radionuclide perfusion lung scanning is used in the diagnosis of PAVMs, and the findings usually confirm a right-to-left shunt. This test is particularly useful if contrast-enhanced echocardiography or the 100% oxygen method is not available. The test involves the peripheral, intravenous injection of macroaggregated albumin labeled with technetium-99m (99mTc). In healthy persons, these particles are filtered by pulmonary capillaries. However, in the presence of true right-to-left shunts, radiolabeled particles pass through the lungs and are trapped in the brain and kidneys. The shunt fraction may be calculated by quantifying the renal uptake as a percentage of the total dose given or lung uptake.
Although radionuclide scanning is comparable to the 100% oxygen method in detecting PAVMs, its expense and limited availability prohibit its widespread use. Nonetheless, although the radionuclide method of shunt calculation is not routinely available at most hospitals, it has the following advantages over the 100% oxygen method: (1) radionuclide scanning does not require arterial blood gas sampling, (2) the 100% oxygen method may cause overestimation of the intrapulmonary shunt, and (3) the radionuclide method is more suitable for shunt measurement during exercise.
Radionuclide perfusion scanning depicts only right-to-left shunts and is not useful in determining a shunt's intracardiac or intrapulmonary location.
Angiography
Pulmonary angiography has been the criterion standard for defining the anatomy of PAVMs. However, spiral CT scanning is now considered more appropriate for diagnosis. Pulmonary angiography has high specificity, but it has a sensitivity lower than that of contrast-enhanced ultrafast CT scanning or 3D spiral CT scanning.
Contrast-enhanced pulmonary angiography should be used in the treatment of PAVM using embolization or before surgical treatment. This test is usually necessary to study the detailed anatomy of PAVM, particularly if the resection or obliterative therapy is planned. Furthermore, angiography may depict other unsuspected PAVMs, as well as unsuspected intrathoracic or extrathoracic vascular communications.
Digital subtraction angiography has replaced conventional angiography in the diagnostic imaging of PAVM. Because nonselective pulmonary angiograms may have difficulty depicting the anatomy of a PAVM, superselective pulmonary angiography is recommended.
(See the image below.)
-
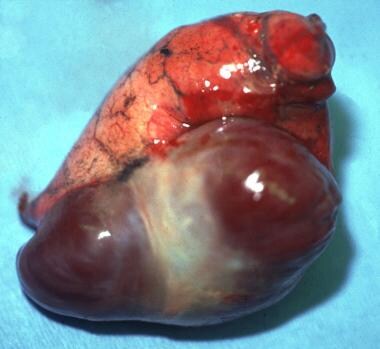
Gross anatomy of a large pulmonary arteriovenous malformation.
-
Pulmonary arteriovenous malformations in the left lower lobe were treated with coil embolization.
-
Lateral chest radiograph shows a pulmonary arteriovenous malformation in the left lower lobe that was occluded with coil embolization.
-
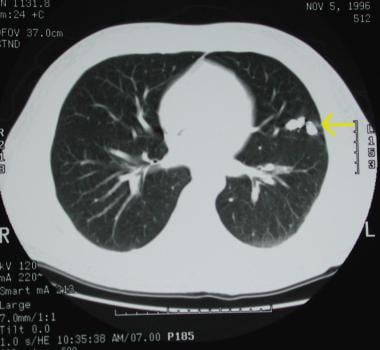
A small pulmonary arteriovenous malformation is depicted in the left lower lobe.
-
A 60-year-old patient presented with right cerebral infarction. The chest radiograph demonstrates a left–lower-lobe nodule, which was later identified as a pulmonary arteriovenous malformation.
-
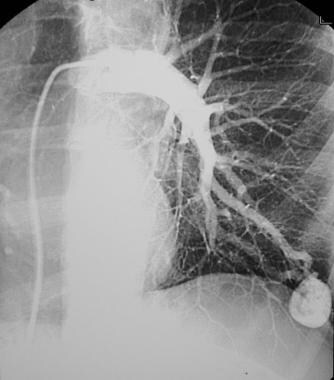
Pulmonary angiogram confirms a large pulmonary arteriovenous malformation with feeding vessels.
-
Three-dimensional computed tomography angiographic reconstruction of a pulmonary arteriovenous malformation. The reconstruction utilized the volume rendering technique to enhance the image quality
-
Chest radiograph shows bilateral pulmonary arteriovenous malformations in a patient who presented with dyspnea and pulmonary hemorrhage.
-
Pulmonary arteriovenous malformation in the left upper lobe.
-
A pulmonary arteriovenous malformation in the left upper lobe. This PAVM has already been embolized with a coil.
-
Pulmonary arteriovenous malformation in the right lower lobe. This PAVM was previously embolized.
-
Chest radiograph demonstrating a peripheral opacity with feeding vessels radiating to the hilum. These features strongly suggest the presence of a pulmonary arteriovenous malformation.