Practice Essentials
Leptomeningeal carcinomatosis (LC) (also called leptomeningeal metastasis or carcinomatous meningitis) refers to diffuse seeding of the leptomeninges by tumor metastases. The condition was first reported in 1870 by Eberth, although the term leptomeningeal carcinomatosis was not used until the early 20th century. LC occurs in patients diagnosed with cancer and is most commonly found in breast carcinoma, lung carcinoma, and melanoma in adults and hematogenous malignancies and primitive neuroectodermal tumor (PNET) in children. Less commonly, prostate cancer can spread to the leptomeninges. LC occurs in approximately 5% of people with cancer and is usually terminal. If left untreated, median survival is 4-6 weeks; if treated, median survival is 2-3 months. [1, 2, 3, 4, 5, 6, 7]
Patients typically present with symptoms caused by the effects of tumor emboli on subarachnoid nerve roots, direct invasion into the spinal cord or brain, or cerebrospinal fluid (CSF) obstruction. MRI and CT demonstrate multiple masses within the subarachnoid space, hydrocephalus without a discernible cause, or diffuse leptomeningeal enhancement. The latter enhancement pattern has been referred to as cake icing or zuckerguss (German for sugar icing) and can be found in the brain, spine, or both, as shown in the images below.
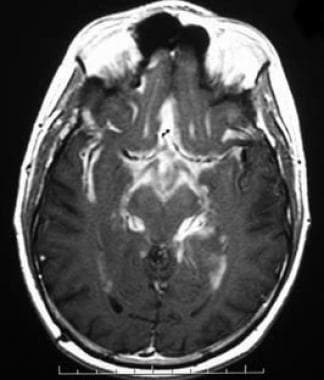 Axial T1-weighted postcontrast image demonstrates diffuse enhancement of the basal cisterns and leptomeninges from metastatic pilocytic astrocytoma. This appearance has been referred to as "zuckerguss" or sugar icing. In children, primitive neuroectodermal tumors are the most common cause of leptomeningeal carcinomatosis, but pilocytic astrocytomas (which are generally low grade) occasionally can demonstrate this type of aggressive behavior.
Axial T1-weighted postcontrast image demonstrates diffuse enhancement of the basal cisterns and leptomeninges from metastatic pilocytic astrocytoma. This appearance has been referred to as "zuckerguss" or sugar icing. In children, primitive neuroectodermal tumors are the most common cause of leptomeningeal carcinomatosis, but pilocytic astrocytomas (which are generally low grade) occasionally can demonstrate this type of aggressive behavior.
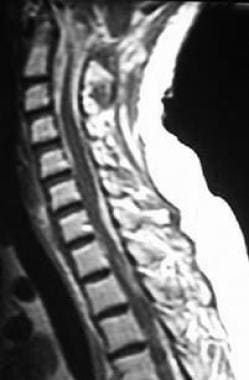 Sagittal T1-weighted postcontrast MR through the cervical spine. Note the diffuse enhancement of the subarachnoid space from extensive tumor deposits. Enhancing material surrounds the cord with no cerebrospinal fluid visible, giving the appearance of a T2 image. Marrow changes are consistent with prior radiation and/or chemotherapy.
Sagittal T1-weighted postcontrast MR through the cervical spine. Note the diffuse enhancement of the subarachnoid space from extensive tumor deposits. Enhancing material surrounds the cord with no cerebrospinal fluid visible, giving the appearance of a T2 image. Marrow changes are consistent with prior radiation and/or chemotherapy.
The patterns of growth of leptomeningeal tumor, as shown in the images below, consist of either (1) a sheetlike extension along the pial surface from direct extension, occasionally with a secondary inflammatory reaction, or (2) as multiple nodules of various sizes studding the surface of the brain, spinal cord, and nerve roots. The latter appearance typically is seen within the cerebellar folia and the cerebral sulci and easily can be mistaken for intraparenchymal metastases on MRI and CT if the association of the tumors with the deep sulci of the brain is not recognized.
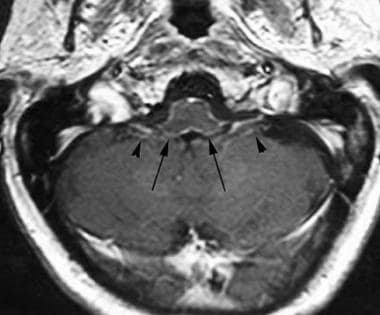 Axial T1-weighted image postcontrast at the level of the inferior cerebellar peduncles demonstrates a more subtle case of leptomeningeal carcinomatosis, with faint thin enhancing tumor covering the peduncles and medulla (arrows). Note enhancement of cranial nerves IX and X on each side (arrowheads). This was a patient with leptomeningeal spread of a glioblastoma multiforme.
Axial T1-weighted image postcontrast at the level of the inferior cerebellar peduncles demonstrates a more subtle case of leptomeningeal carcinomatosis, with faint thin enhancing tumor covering the peduncles and medulla (arrows). Note enhancement of cranial nerves IX and X on each side (arrowheads). This was a patient with leptomeningeal spread of a glioblastoma multiforme.
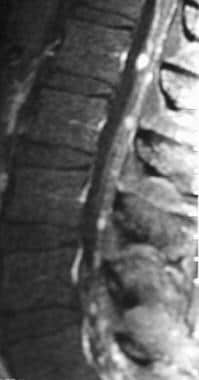 Sagittal T1-weighted postcontrast image of the lumbar spine with fat saturation reveals diffuse tumor seeding of the cauda equina from metastatic squamous cell carcinoma. Tiny tumor foci give a "string of beads" appearance to some of the nerve roots.
Sagittal T1-weighted postcontrast image of the lumbar spine with fat saturation reveals diffuse tumor seeding of the cauda equina from metastatic squamous cell carcinoma. Tiny tumor foci give a "string of beads" appearance to some of the nerve roots.
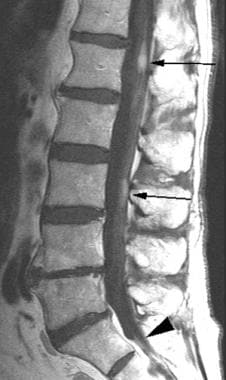 Sagittal T1 postcontrast image through the lumbar spine in this patient with small cell lung carcinoma demonstrates enhancing foci in the cauda equina and adjacent to the conus (arrows). In addition, notice the finely enhancing nerve root at the S1-2 level (arrowhead). This patient was experiencing lower extremity weakness.
Sagittal T1 postcontrast image through the lumbar spine in this patient with small cell lung carcinoma demonstrates enhancing foci in the cauda equina and adjacent to the conus (arrows). In addition, notice the finely enhancing nerve root at the S1-2 level (arrowhead). This patient was experiencing lower extremity weakness.
Early diagnosis is important, as it can alert the oncologist to begin therapy prior to neurologic deterioration. While there are clinical signs and radiologic findings that strongly suggest LC, most cases are diagnosed by CSF cytology or leptomeningeal biopsy. [8] As the diagnostic accuracy of lumbar puncture (LP) is only 50-60% after a single LP and 90% after 3 LPs, MRI is considered complementary and can be invaluable, detecting up to 50% of cases with false-negative LPs.
Without appropriate therapy, the outlook is grim; untreated patients are unlikely to survive more than 4-6 weeks. Intrathecal chemotherapy and/or radiation can increase survival to some extent, depending partly on the cell type of tumor involved, but most patients succumb to their disease within 6-8 months.
Preferred examination
Contrast-enhanced MRI of the brain and spine is the imaging modality of choice because of its safety, excellent contrast resolution, and multiplanar abilities. [9, 10] A wide range of the sensitivity of MRI in detecting leptomeningeal carcinomatosis has been reported. Some of this discrepancy is from the difference in sensitivity between solid tumors and hematologic malignancies, with one study reporting a sensitivity of 90% in patients with solid tumors but only 55% in patients with lymphoma and leukemia. [11, 12, 13, 14, 15, 16, 17]
Sensitivity rates have risen with advances in MRI technology, particularly better T1-weighted images as well as the advent of 3-dimensional T1-weighted sequences and postcontrast fluid attenuated inversion recovery (FLAIR). [15, 17] Collie et al reported a 100% sensitivity for intracranial LC in 25 patients evaluated with gadolinium-enhanced MRI. [18] MRI sensitivity is likely in the 70-90% range in detecting intracranial LC.
Leptomeningeal disease can include the entire neuraxis, so images should be of both the brain and the spine. [9, 10, 2, 19]
When the patient has a contraindication to MRI, CT myelography is the next best test to evaluate the spine and has the added benefit of allowing CSF sampling at the same time as the diagnostic test is performed. The physician must exclude obstructive hydrocephalus prior to beginning the procedure, as removal of CSF below the obstruction may result in downward herniation and death.
Plain myelography demonstrates the thickened nerve roots, subarachnoid masses, and blockage of the subarachnoid space, but it has not been used as a primary diagnostic tool since the increased availability of good quality CT and MRI. Plain myelography is still used to provide additional imaging during a CT myelogram.
It is important to remember that CSF cytology will detect some cases that are not visible on MRI, and vice versa. Both tests should be performed if LC is clinically suspected and the initial test is negative. [20]
Obstructed flow of CSF will be present in 30-70% of patients with leptomeningeal carcinomatosis. CSF flow studies utilize indium-111 DTPA or technetium-99m and are more sensitive than conventional MRI studies. [19, 15]
CSF biomarker analysis can identify leptomeningeal disease earlier than MRI or CSF flow analysis. Groves et al found that CSF VEGF had a 75% senstivity, 97% specificity, and 94% negative predictive value in detecting leptimeningeal disease in breast cancer patients. [4, 21, 22, 23]
(See the MRI and CT images of leptomeningeal carcinomatosis below.)
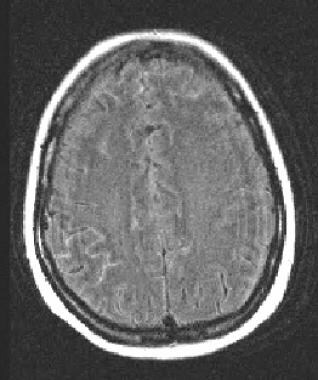 Postcontrast fluid attenuated inversion recovery image demonstrates the use of this sequence in leptomeningeal carcinomatosis. Enhancement of the subarachnoid space is present scattered over both hemispheres, but most pronounced along the right parietal lobe in this patient with metastatic lung carcinoma.
Postcontrast fluid attenuated inversion recovery image demonstrates the use of this sequence in leptomeningeal carcinomatosis. Enhancement of the subarachnoid space is present scattered over both hemispheres, but most pronounced along the right parietal lobe in this patient with metastatic lung carcinoma.
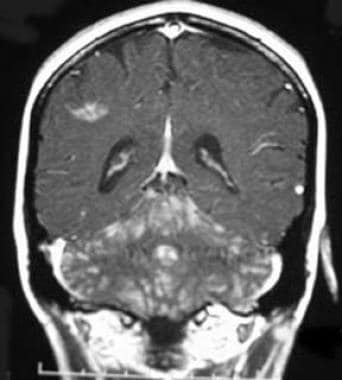 Coronal T1 postcontrast image demonstrates metastatic disease in this patient whose breast cancer was thought to be cured. Metastatic spread can occur decades after the initial tumor has been treated. Leptomeningeal carcinomatosis from a second primary is another possibility, and central nervous system spread of melanoma or lung cancer could have this appearance. Sarcoid also can demonstrate leptomeningeal spread.
Coronal T1 postcontrast image demonstrates metastatic disease in this patient whose breast cancer was thought to be cured. Metastatic spread can occur decades after the initial tumor has been treated. Leptomeningeal carcinomatosis from a second primary is another possibility, and central nervous system spread of melanoma or lung cancer could have this appearance. Sarcoid also can demonstrate leptomeningeal spread.
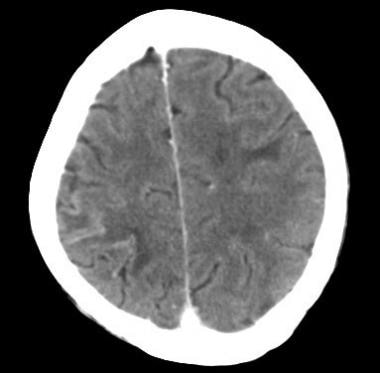 Postcontrast CT of a patient with metastatic lung cancer and headache demonstrates contrast enhancement along the right parietal sulci with bilateral cortical edema.
Postcontrast CT of a patient with metastatic lung cancer and headache demonstrates contrast enhancement along the right parietal sulci with bilateral cortical edema.
Limitations of techniques
CT continues to be used as a screening tool in the metastatic workup of many cancer patients but is relatively insensitive compared with MRI, particularly in the detection of LC. Contrast-enhanced CT may miss tumor in up to one third of cases and mischaracterize leptomeningeal tumor deposits as intraparenchymal in another third. CT may be necessary when a contraindication to MRI exists, such as the presence of a pacemaker or certain aneurysm clips, or when the patient is unable to hold still long enough for an MRI. With the advent of newer and faster techniques and MRI scanners, as well as MRI-compatible aneurysm clips, these situations have become less frequent.
MRI depicts leptomeningeal tumor well, particularly when magnetization transfer or postcontrast FLAIR techniques are used. MRI is much better at depicting metastases from solid tumors than those from hematologic malignancies.
Myelography and CT myelography have been used to evaluate LC and have the advantage of allowing CSF sampling at the time of the study. However, the test is invasive, has the risk of contrast reaction and downward herniation in patients with CSF obstruction, and has not been shown to be as sensitive as MRI in detecting focal tumor deposits on the cord or nerve roots, although it is more accurate in identifying cord enlargement.
Failure to confirm leptomeningeal enhancement can have serious consequences for the patient, as early diagnosis is important in LC. For that reason, brain MRIs in all patients with a history of previous tumor or in those who have symptoms suggestive of LC should always include contrast, although the disease can be occult even with contrast. Noncontrast MRI has proven to be of little use in detecting leptomeningeal disease and should be avoided in these patients. CT also can easily miss disease, and normal contrast-enhanced CT scan does not exclude the presence of leptomeningeal tumor. The ordering physician must be aware that a normal MRI or CT study does not exclude LC and that the combination of contrast MRI and serial lumbar punctures has the best diagnostic potential.
Radiologists often are asked to place the needle into the lumbar thecal sac prior to intrathecal chemotherapy administration. Confirmation of correct needle placement using intrathecal iodinated contrast is important, as an epidural or split injection results in a poor clinical outcome.
EANO-ESMO guidelines
Guidelines developed by the European Society for Medical Oncology (ESMO) and the European Association of Neuro-Oncology (EANO) include the following [15] :
-
The diagnostic work-up should include cerebrospinal MRI. Brain MRI should include axial T1-weighted, axial FLAIR, axial diffusion, axial T2, post-gadolinium 3D T1, and post-gadolinium 3D FLAIR sequences.
-
Spinal MRI should include post-gadolinium sagittal T1 sequences. Spine sagittal T1W sequences without contrast and sagittal fat suppression T2-weighted sequences, combined with axial T1W images with contrast of regions of interest, may also be considered.
-
CSF flow studies should be considered for patients in whom CSF flow obstruction may be present (eg, patients with hydrocephalus, large nodules potentially reducing the CSF circulation on MRI, unexpected toxicity of intra-CSF treatment) and who are candidates for intra-CSF pharmacotherapy
-
CSF studies with optimized analysis conditions must be carried out as part of the diagnostic work-up. One repeat lumbar puncture with optimized analysis conditions should be carried out in patients with suspected leptomeningeal metastasis and initial negative or equivocal CSF studies.
Computed Tomography
Contrast-enhanced CT (CECT) scans of the brain in leptomeningeal carcinomatosis (LC) are not sensitive in depicting the disease, with a false-negative rate of more than 50%. Common findings include noncommunicating hydrocephalus, intraparenchymal volume loss, and various patterns of meningeal enhancement, as shown in the image below. This enhancement can appear as multiple nodules, diffuse leptomeningeal enhancement, ependymal or subependymal enhancement, dural enhancement, or a combination. In the nodular form of LC, pial enhancement is difficult to distinguish from intraparenchymal enhancement, although recognizing that the nodules follow the course of sulci assists in the diagnosis. [24, 25]
 Postcontrast CT of a patient with metastatic lung cancer and headache demonstrates contrast enhancement along the right parietal sulci with bilateral cortical edema.
Postcontrast CT of a patient with metastatic lung cancer and headache demonstrates contrast enhancement along the right parietal sulci with bilateral cortical edema.
Cranial nerve enhancement is poorly visualized on CECT because of the proximity to osseous structures. Dural enhancement often is missed for the same reason.
In the spine, CECT also has low sensitivity, although CT myelography is approximately equal in sensitivity to MRI in the detection of nerve root thickening and nodularity. The nerve roots appear thickened and beaded; this is best visualized in the cauda equina. Tumor deposits along the surface of the cord lend the cord an irregular border, and the cord may be thickened. In extreme cases, the entire spinal canal can be filled with tumor, causing a complete CSF block.
False-negative CECT scans occur in more than 50% of patients with LC. In some of these, the disease is not detectable on any imaging study, but in others, the limitations of CT imaging result in a missed diagnosis. Potential errors include lower contrast resolution than MRI, adjacent dense osseous structures, and beam-hardening artifact, particularly in the posterior fossa.
False positives can be caused by benign meningeal enhancement, such as in patients with dural enhancement following lumbar puncture or surgery and in those with intracranial hypotension. Diffuse benign parenchymal loss can mimic the volume loss and hydrocephalus associated with LC.
Magnetic Resonance Imaging
A protocol for evaluation of intracranial leptomeningeal tumor should include postcontrast T1-weighted images in more than one plane. The coronal plane is helpful in the detection of disease over the convexities and in the cerebellum. Thin-section sequences such as magnetization prepared-rapid acquisition gradient echo (MP-RAGE) or spoiled gradient-recalled acquisition in a steady state (SPGR) following contrast are of use when evaluating the cranial nerves, as are precontrast, high-resolution, bright-CSF sequences such as constructive interference in a steady state (CISS). [26, 27, 28, 29, 9, 2, 14, 19, 15, 16, 17]
Postcontrast fast FLAIR images have been reported to be even more sensitive to leptomeningeal enhancement than postcontrast T1 sequences, as shown in the images below. However, noncontrast FLAIR, which was thought to be a good imaging sequence for leptomeningeal carcinomatosis (LC), is less sensitive than T1 contrast-enhanced images. In addition, patients who have previously received gadolinium chelate may demonstrate increased signal intensity in the subarachnoid space on FLAIR imaging. [30, 31, 32]
 Postcontrast fluid attenuated inversion recovery image demonstrates the use of this sequence in leptomeningeal carcinomatosis. Enhancement of the subarachnoid space is present scattered over both hemispheres, but most pronounced along the right parietal lobe in this patient with metastatic lung carcinoma.
Postcontrast fluid attenuated inversion recovery image demonstrates the use of this sequence in leptomeningeal carcinomatosis. Enhancement of the subarachnoid space is present scattered over both hemispheres, but most pronounced along the right parietal lobe in this patient with metastatic lung carcinoma.
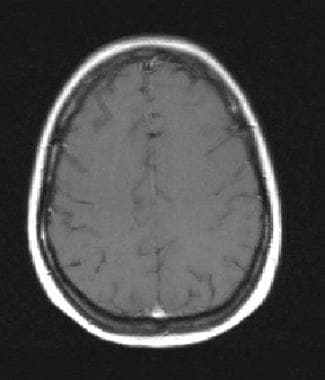 Axial T1 postgadolinium in does not show the leptomeningeal tumor. Postcontrast fluid attenuated inversion recovery has been shown to be more sensitive than routine postcontrast T1 sequences in the detection of leptomeningeal carcinomatosis.
Axial T1 postgadolinium in does not show the leptomeningeal tumor. Postcontrast fluid attenuated inversion recovery has been shown to be more sensitive than routine postcontrast T1 sequences in the detection of leptomeningeal carcinomatosis.
Leptomeningeal tumor has a spectrum of appearances on MRI ranging from diffuse leptomeningeal enhancement to bulky extra-axial tumor foci. The most common appearance is diffuse nonnodular enhancement of the basilar cisterns and/or supratentorial cortex, although this MRI appearance is not specific for LC. Hydrocephalus without discernible enhancement also is commonly seen, but the appearance is nonspecific.
Multiple enhancing leptomeningeal nodules also can be visualized in patients with LC, with scans typically demonstrating larger tumor deposits in the basilar cisterns than in the supratentorial sulci. These nodules may form large clumps of tumor that cause a mass effect on the adjacent brain. [33] Even small amounts of tumor may result in obstructive hydrocephalus if they are located in narrow portions of the ventricular system, such as the fourth ventricle and aqueduct of Sylvius. Tumor deposits in the supratentorial sulci may be difficult to distinguish from intraparenchymal metastases, although recognizing that the enhancing nodules follow the course of the deep sulci suggests the correct diagnosis of leptomeningeal disease. Another pattern that has been described as mimicking intraparenchymal spread is nodules within the Virchow-Robin (perivascular) spaces.
Cranial nerves often are involved clinically in LC, although many of these cases are radiologically occult. High-resolution contrast-enhanced techniques with fat saturation aid in the detection of cranial nerve tumor deposits, but close attention to the nerves is often necessary to detect the subtle abnormalities in these cases. Although multiplanar imaging increases the sensitivity for detecting LC, coronal images best demonstrate the cranial nerves in most patients. Involved cranial nerves may have mild peripheral enhancement, which may be easily missed, as the only MRI abnormality. More grossly involved nerves may be enlarged and irregular, with nodular enhancement, making detection easier. However, without a high index of suspicion, many cases can be missed easily because of the small size of the nerves and their course through the skull base.
Spinal cord involvement mimics that of the brain stem, with peripheral linear or nodular enhancement of the pia mater. In addition, enhancement of the nerve roots within the cauda equina is often seen, ranging from tiny nodules to large masses. When spinal leptomeningeal disease is caused by direct extension from vertebral body metastases, fat-saturated enhanced T1-weighted images can be used to optimally demonstrate the vertebral body and epidural enhancement.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). [34, 35, 36] NSF/NFD has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MR angiography scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Degree of confidence
Although MRI is the most sensitive and specific technique for visualizing leptomeningeal tumor, a negative MRI scan does not exclude the possibility of disease. Gomori et al reported that 12% of patients with LC diagnosed by positive CSF cytology on lumbar puncture had a negative MRI spine examination but also found that MRI was positive in 60% of LC patients with negative CSF cytology. [37] This suggests that the two techniques are complementary; if one test is negative, the other should be performed.
MRI findings in LC are nonspecific, and may be similar to those in patients with bacterial or fungal meningitis, leptomeningeal sarcoidosis, recent surgery, and even, on occasion, cerebral infarction. The estimated sensitivity of MRI in the detection of LC is 34-71%, although this includes all tumor types. Separating LC into two subtypes, Freilich et al found MRI abnormalities in 90% of LC from solid tumors and in 55% of LC that originated from hematologic malignancies. [38] Another study found even lower sensitivity of MRI in hematologic LC, reporting only 6% sensitivity, [39] although this was performed on earlier MRI machines.
Although radioisotope CSF flow studies are unsurpassed for the evaluation of possible CSF flow obstruction, MRI may also be used for this purpose. Flow studies using cardiac-gated phase contrast techniques can be used to display and quantitate CSF flow in the head and spine, and to evaluate CSF flow obstruction. In addition, spinal cord motion also may be observed to evaluate tethering or compression by metastatic disease. [40]
False positives/negatives
As previously stated, recent surgery can mimic LC because of postoperative leptomeningeal enhancement. Any procedure involving CSF access, including lumbar puncture, can result in long-term dural enhancement that can mimic neoplasm.
Normally enhancing vessels on the surface of the cord can be mistaken for leptomeningeal tumor spread if the linearity of the vessel is not appreciated. These are usually venous structures, as the anterior and posterior spinal arteries usually run in the same location relative to the cord. Patients who have undergone radiation therapy to the spine may have dilated vessels that can mimic LC.
Meningitis may also mimic LC, and oncology patients are often predisposed to this infection secondary to immunosuppression and hospitalization.
Nuclear Imaging
The nuclear medicine study most commonly ordered in leptomeningeal carcinomatosis (LC) is a radioisotope CSF flow study to document the presence or absence of an obstruction to CSF flow in the spine or skull base. Although much less sensitive than MRI to the presence of LC, CSF flow studies are considered almost 100% sensitive to the presence of obstructive CSF space disease. [13, 22, 15]
Obstructed flow of CSF will be present in 30-70% of patients with leptomeningeal carcinomatosis. CSF flow studies utilize indium-111 DTPA or technetium-99m and are more sensitive than conventional MRI studies. [19, 15, 41, 42]
The procedure is performed by injecting indium-111–labeled diethylenetriamine pentaacetic acid (DTPA) into the CSF through either a lumbar puncture or an indwelling catheter such as an Ommaya reservoir. Images generally are obtained for 60-90 minutes, and a 24-hour delayed image is frequently obtained. Chamberlain has described the normal time for radioisotope to be seen in the different compartments following both lumbar and intraventricular injections and provided normal values for intraventricular injections in adult and pediatric patients. [43]
CSF flow studies are important when considering intrathecal chemotherapy, as up to 70% of patients with LC have a CSF flow obstruction that will affect the spread of the chemotherapeutic agent, and thus its toxic effects and efficacy. Areas that demonstrate obstruction on a CSF flow study can be treated with external beam radiation prior to intrathecal chemotherapy.
In CSF flow studies performed through a lumbar puncture, ensure that the injection is within the subarachnoid space, as a subdural or epidural injection mimics a complete obstruction. Confirmation of needle placement using a small amount of contrast avoids this potential error.
-
Axial T1-weighted postcontrast image demonstrates diffuse enhancement of the basal cisterns and leptomeninges from metastatic pilocytic astrocytoma. This appearance has been referred to as "zuckerguss" or sugar icing. In children, primitive neuroectodermal tumors are the most common cause of leptomeningeal carcinomatosis, but pilocytic astrocytomas (which are generally low grade) occasionally can demonstrate this type of aggressive behavior.
-
Sagittal T1-weighted postcontrast MR through the cervical spine. Note the diffuse enhancement of the subarachnoid space from extensive tumor deposits. Enhancing material surrounds the cord with no cerebrospinal fluid visible, giving the appearance of a T2 image. Marrow changes are consistent with prior radiation and/or chemotherapy.
-
Axial T1-weighted image postcontrast at the level of the inferior cerebellar peduncles demonstrates a more subtle case of leptomeningeal carcinomatosis, with faint thin enhancing tumor covering the peduncles and medulla (arrows). Note enhancement of cranial nerves IX and X on each side (arrowheads). This was a patient with leptomeningeal spread of a glioblastoma multiforme.
-
Sagittal T1-weighted postcontrast image of the lumbar spine with fat saturation reveals diffuse tumor seeding of the cauda equina from metastatic squamous cell carcinoma. Tiny tumor foci give a "string of beads" appearance to some of the nerve roots.
-
Sagittal T1 postcontrast image through the lumbar spine in this patient with small cell lung carcinoma demonstrates enhancing foci in the cauda equina and adjacent to the conus (arrows). In addition, notice the finely enhancing nerve root at the S1-2 level (arrowhead). This patient was experiencing lower extremity weakness.
-
Postcontrast CT of a patient with metastatic lung cancer and headache demonstrates contrast enhancement along the right parietal sulci with bilateral cortical edema.
-
Postcontrast fluid attenuated inversion recovery image demonstrates the use of this sequence in leptomeningeal carcinomatosis. Enhancement of the subarachnoid space is present scattered over both hemispheres, but most pronounced along the right parietal lobe in this patient with metastatic lung carcinoma.
-
Axial T1 postgadolinium in does not show the leptomeningeal tumor. Postcontrast fluid attenuated inversion recovery has been shown to be more sensitive than routine postcontrast T1 sequences in the detection of leptomeningeal carcinomatosis.
-
Coronal T1 postcontrast image demonstrates metastatic disease in this patient whose breast cancer was thought to be cured. Metastatic spread can occur decades after the initial tumor has been treated. Leptomeningeal carcinomatosis from a second primary is another possibility, and central nervous system spread of melanoma or lung cancer could have this appearance. Sarcoid also can demonstrate leptomeningeal spread.










