Practice Essentials
Gastrointestinal stromal tumors (GISTs) are a subset of GI mesenchymal tumors of varying differentiation. Previously, these tumors were classified as GI leiomyomas, leiomyosarcomas, leiomyoblastomas, or schwannomas, on the basis of histologic findings and the fact that these tumors apparently originate in the muscularis propria layer of the intestinal wall. With the advent of immunohistochemical staining techniques and ultrastructural evaluation, GISTs now are recognized as a distinct group of mesenchymal tumors. In the present classification, GISTs account for approximately 80% of GI mesenchymal tumors. [1, 2, 3]
Grossly, gastrointestinal stromal tumors are well-demarcated spherical masses that appear to arise from the muscularis propria layer of the GI wall. Intramural in origin, they often project exophytically and/or intraluminally, and they may have overlying mucosal ulceration. Larger GISTs nearly always outgrow their vascular supply, leading to extensive areas of necrosis and hemorrhage. [4, 5, 6, 2, 3]
GISTs are most often detected the stomach (60-70%) and small intestine (20-30%); rarely, they occur in the mesentery, omentum, retroperitoneum, and duodenum. [7, 8]
(See the images below.)
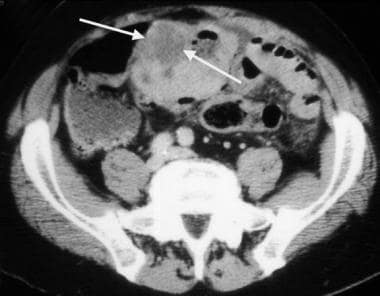 Gastrointestinal stromal tumor. Image obtained in the same patient as in the previous image. A more caudal portion of the tumor has areas of necrosis (arrows), with air present within the necrotic cavity that communicates with the lumen of the small bowel.
Gastrointestinal stromal tumor. Image obtained in the same patient as in the previous image. A more caudal portion of the tumor has areas of necrosis (arrows), with air present within the necrotic cavity that communicates with the lumen of the small bowel.
The diameter of GISTs, as a whole, can range from a few millimeters to more than 30 cm. Although larger tumors have a higher rate of malignancy, size does not predict benignity, and small GISTs have been known to behave in a malignant fashion. [5, 9] Malignancy is characterized by local invasion and metastases, particularly to the liver.
Cytologically, GISTs can be classified into 2 broad categories: spindle cell GISTs and epithelioid GISTs. Spindle cell GISTs are characterized by nuclear palisading or prominent perinuclear vacuolization pattern. Epithelioid GISTs may have either a solid pattern or a myxoid pattern, with a possible compartmental pattern. Although GISTs may differentiate along either or both cell types, some show no significant differentiation at all. [4, 5, 10]
The number of mitotic figures present may be used to histologically grade GISTs. Unfortunately, no standard exists for their classification. In general, GISTs with less than 1 mitotic figure per 50 high-powered fields (HPFs) are correlated with benign behavior. A finding of 1-5 mitoses per 10 HPFs suggests potential malignancy. A finding of more than 5 per 10 HPFs indicates malignancy. A finding of more than 10 per 10 HPFs denotes high-grade malignancy. [4, 5, 11]
Although radiologic or histologic results may suggest GISTs, the diagnosis must be made immunochemically. Independent of location, most GISTs express the CD34 antigen (70-78%) and the CD117 (72-94%) antigen. The CD34 protein is a hematopoietic progenitor cell antigen that occurs in a variety of mesenchymal tumors. CD117 also is known as the c-kit protein; it is a membrane receptor with a tyrosine kinase component. Mutations in the CD117 gene have been linked to malignant behavior in GISTs. [4, 5, 12, 13, 14, 15]
About 10-30% of GISTs have malignant behavior. [1, 4] A benign GIST cannot be conclusively diagnosed, because even small, histologically benign-appearing tumors may later demonstrate clinically aggressive behavior. Factors that are correlated with an improved prognosis include a gastric location, a diameter of less than 2 cm, a low mitotic index, and an absence of tumor spillage with complete gross resection.
(See the images below.)
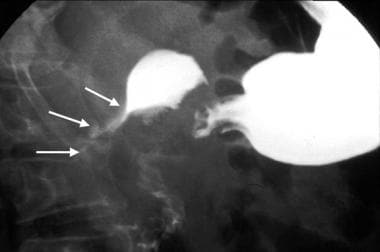
 Gastric gastrointestinal stromal tumor (GIST) en face. Upper GI image obtained during the single contrast enhancement portion shows an incidentally found mass. The smooth borders suggest a submucosal process. At surgery, a GIST was found.
Gastric gastrointestinal stromal tumor (GIST) en face. Upper GI image obtained during the single contrast enhancement portion shows an incidentally found mass. The smooth borders suggest a submucosal process. At surgery, a GIST was found.
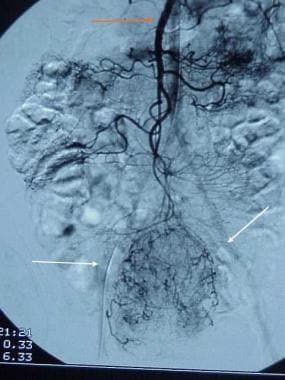 Gastrointestinal stromal tumor. Selective masked injection in the superior mesenteric artery (red arrow) demonstrates a large arterial feeder with tumor staining (white arrows).
Gastrointestinal stromal tumor. Selective masked injection in the superior mesenteric artery (red arrow) demonstrates a large arterial feeder with tumor staining (white arrows).
About 50-70% of GISTs occur in the stomach; 33%, in the small bowel; 5-15%, in the rectocolon; and only 1-5%, in the esophagus. [1, 5, 9, 10, 16, 17] GISTs are multicentric in fewer than 5% of cases (see the images below).
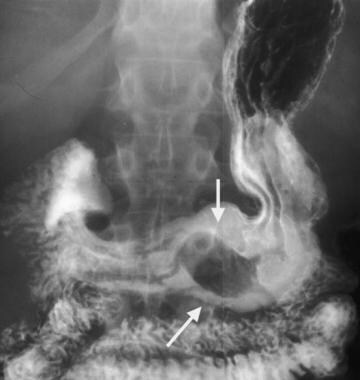
 Multifocal gastrointestinal stromal tumors. Barium examination reveals a smooth mass that causes narrowing in the second portion of the duodenum (arrows).
Multifocal gastrointestinal stromal tumors. Barium examination reveals a smooth mass that causes narrowing in the second portion of the duodenum (arrows).
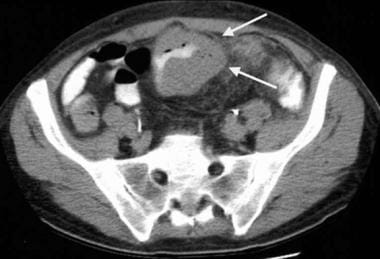 Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows a focal segment of diffusely thickened ileum, which is another GIST.
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows a focal segment of diffusely thickened ileum, which is another GIST.
Imaging modalities
Contrast-enhanced multidetector CT (MDCT) is the most commonly used imaging modality for localizing, characterizing, and staging GISTs. With MDCT, all patterns of enhancement can be seen, including hypoenhancing, isoenhancing, and hyperenhancing neoplasms. [8]
Imaging studies used in the workup of GISTs include chest radiography, abdominal ultrasound, CT scan, magnetic resonance imaging (MRI), and positron emission transverse tomography (PET). According to the National Comprehensive Cancer Network (NCCN) guidelines for evaluation of GISTS, abdominal/pelvic CT scan with contrast, with or without MRI, is indicated and chest imaging should be considered. Very small GISTs (< 2 cm) may be evaluated with endoscopic ultrasound-guided fine-needle aspiration; for GISTs 2 cm or larger, endoscopy with or without ultrasound may also be indicated in select patients. [18, 8, 19, 20, 21, 22]
Characteristics of GIST on CT scans vary depending on the size and whether the tumor is primary or metastatic. A large lesion (>5 cm) is typically exophytic and hypervascular with heterogeneous enhancement on contrast-enhanced CT. Smaller lesions (< 5 cm) are typically submucosal or endoluminal polypoid masses displaying homogeneous contrast enhancement. Metastases show arterial enhancement because of hypervascularity and lack enhancement on portal venous and venous phases. [23]
PET/CT may be useful for differentiating between benign and malignant lesions. PET/CT has shown more accuracy than CT alone for identifying liver metastasis. [24, 25] In a meta-analysis by Kim et al, fluorodeoxyglucose positron emission tomography (FDG-PET) or PET/CT showed good sensitivity and specificity for predicting the malignant potential of GIST. Across 7 studies (188 patients), the pooled sensitivity for FDG PET or PET/CT was 0.88 (95% confidence interval [CI]: 0.80-0.94) without heterogeneity and a pooled specificity of 0.88 (95% CI: 0.75-0.94) with heterogeneity. [25]
The European Society for Medical Oncology (ESMO) guidelines recommend CT scanning as the imaging modality of choice for staging most GISTS because it provides comprehensive information regarding size and location of the tumor and its relationship to adjacent structures. CT scanning can also be used to detect multiple tumors and can provide evidence of metastatic spread. MRI is noted as an alternative and may provide better results for staging rectal GISTs. [26]
Limitations of techniques
The aim of radiologic examination is to locate gastrointestinal stromal lesions, evaluate local invasion, and detect distant metastases. The radiographic characteristics of GISTs mirror the gross appearance of these tumors. Small GISTs appear as small, well-circumscribed intramural masses, possibly with overlying ulceration. Larger malignant GISTs appear as complex masses with areas of necrosis, which occur as a result of the tumor's outgrowing its blood supply.
Unfortunately, radiologic findings are nonspecific and may represent several entities. Also, the distinction between benign and malignant GISTs cannot be made with radiologic examinations unless metastatic disease or tumor invasion of adjacent structures is depicted. The definitive diagnosis of GIST is made immunohistochemically. However, the diagnosis may be suggested in the case of a complex bowel mass with liver metastases in the absence of lymphadenopathy. [27]
Nomenclature
Most tumors referred to as leiomyomas and leiomyosarcomas in the older medical literature are actually GISTs. [4] Only with tumors in the esophagus does the term leiomyoma remain accurate. Leiomyomas are the most common mesenchymal tumors in the esophagus; they rarely occur in the stomach and small bowel. In contrast, GISTs are rare in the esophagus, and they are more common in the stomach and small bowel.
The literature about GISTs remains confusing because tumor classification and terminology are being continually refined. Furthermore, the exact definition of GISTs varies among authors. Some use the term to describe any GI submucosal mesenchymal tumor that is not myogenic (eg, leiomyosarcoma) or neurogenic (eg, schwannoma) in origin. Other authors are more restrictive and use the term when specifically referring to GI mesenchymal tumors that express the CD117 and/or CD34 antigen. [28]
Radiography
Plain radiographs usually offer little in the evaluation of gastrointestinal stromal tumors (GISTs). In the chest, esophageal GISTs may appear as a soft tissue mass in the lower two thirds of the mediastinum. In the abdomen, the soft tissue mass may cause deformation of the gastric air shadow, or it may displace loops of bowel. Abdominal films may depict an obstructive bowel pattern. If they are necrotic lesions, collections of air may be visualized within the tumor. [29]
One group reported that double-contrast images show abnormalities in 80% of cases of GISTs. [5]
Regardless of the location of GISTs, barium-enhanced images demonstrate predominantly intramural masses with potential exophytic components. The tumor margins usually are smooth, but with ulceration, some surface irregularity is present. As with other intramural masses, the tumor borders form right or obtuse angles with the adjacent visceral wall. En face, the intraluminal surfaces often have well-defined margins (see the mages below).
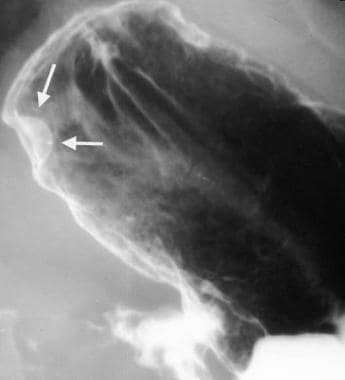 Gastric gastrointestinal stromal tumor in a 49-year-old woman. The mass was found incidentally during an upper GI workup for peptic disease. The smooth appearance suggests a submucosal process.
Gastric gastrointestinal stromal tumor in a 49-year-old woman. The mass was found incidentally during an upper GI workup for peptic disease. The smooth appearance suggests a submucosal process.
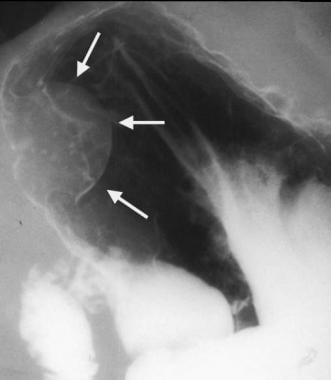 Gastrointestinal stromal tumor (GIST). Image obtained 1 year later in the same patient as in the previous image. The mass has increased in size. A GIST was found at surgery.
Gastrointestinal stromal tumor (GIST). Image obtained 1 year later in the same patient as in the previous image. The mass has increased in size. A GIST was found at surgery.
 Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows the same GIST. It appears as an intramural mass with both exophytic and endophytic components.
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows the same GIST. It appears as an intramural mass with both exophytic and endophytic components.
 Gastric gastrointestinal stromal tumor (GIST) en face. Upper GI image obtained during the single contrast enhancement portion shows an incidentally found mass. The smooth borders suggest a submucosal process. At surgery, a GIST was found.
Gastric gastrointestinal stromal tumor (GIST) en face. Upper GI image obtained during the single contrast enhancement portion shows an incidentally found mass. The smooth borders suggest a submucosal process. At surgery, a GIST was found.
Because the tumors are intramural but extramucosal, the overlying mucosa may be intact. With tumors of the stomach, areae gastricae is preserved over the tumor mass. However, overlying mucosal ulcerations are often present; they are more common in malignant GISTs. These ulcerations fill with barium, causing a bull's-eye or target-lesion appearance (see the image below).
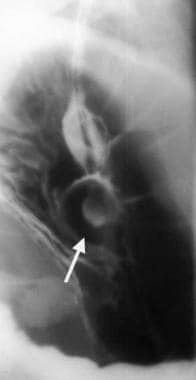 Gastrointestinal stromal tumor with central bull's eye appearance, which is compatible with contrast material collection in an ulceration.
Gastrointestinal stromal tumor with central bull's eye appearance, which is compatible with contrast material collection in an ulceration.
If necrosis and cavitation are present, barium may fill the inner parts of the tumor mass (see the images below). [5, 9, 29, 30, 31]
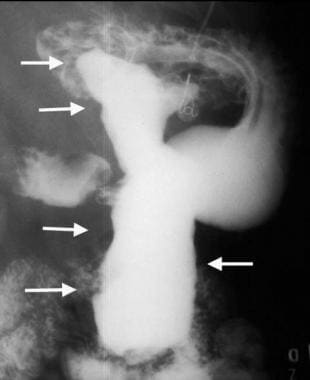 Gastric gastrointestinal stromal tumor with huge exophytic component, which has become ulcerated. Barium collects in the exophytic ulcer crater (arrows).
Gastric gastrointestinal stromal tumor with huge exophytic component, which has become ulcerated. Barium collects in the exophytic ulcer crater (arrows).
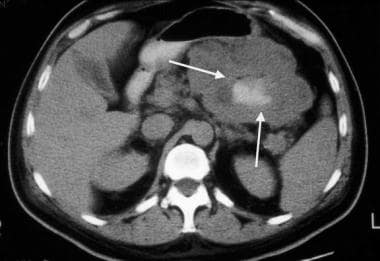 Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image demonstrates the GIST with large exophytic ulceration (arrows).
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image demonstrates the GIST with large exophytic ulceration (arrows).
At times, the mass is entirely exophytic and, thus, is not appreciated during contrast-enhanced examination. Barium images outline the intraluminal portion of this tumor; frequently, a substantial exophytic extension is present (see the images below).
 Proximal jejunal gastrointestinal stromal tumor that is completely exophytic and not visible at small-bowel barium examination.
Proximal jejunal gastrointestinal stromal tumor that is completely exophytic and not visible at small-bowel barium examination.
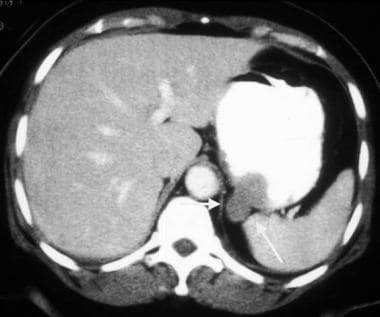
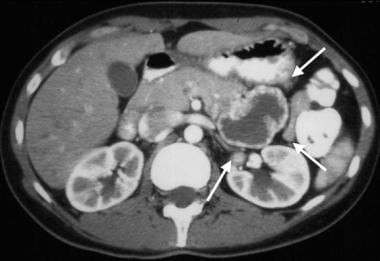 Gastrointestinal stromal tumor (GIST). Contrast-enhanced CT obtained in the same patient as in the previous image demonstrates a peripherally enhancing mass immediately adjacent to the pancreas that was thought to be a pancreatic neoplasm. However, at surgery, this proved to be a proximal exophytic jejunal GIST.
Gastrointestinal stromal tumor (GIST). Contrast-enhanced CT obtained in the same patient as in the previous image demonstrates a peripherally enhancing mass immediately adjacent to the pancreas that was thought to be a pancreatic neoplasm. However, at surgery, this proved to be a proximal exophytic jejunal GIST.
Computed Tomography
Contrast-enhanced multidetector CT (MDCT) is the most commonly used imaging modality for localizing, characterizing, and staging gastrointestinal stromal tumors (GISTs). With MDCT, all patterns of enhancement can be seen, including hypoenhancing, isoenhancing, and hyperenhancing neoplasms. [8]
Overall, CT scanning has good sensitivity for the detection of GISTs, with abnormalities seen in 87% of cases. [5, 32, 33]
Characteristics of GIST on CT scans vary depending on the size and whether the tumor is primary or metastatic. A large lesion (>5 cm) is typically exophytic and hypervascular with heterogeneous enhancement on contrast-enhanced CT. Smaller lesions (< 5 cm) are typically submucosal or endoluminal polypoid masses displaying homogeneous contrast enhancement. Metastases show arterial enhancement because of hypervascularity and lack enhancement on portal venous and venous phases. [23]
CT for GISTs should be performed with both oral and intravenous contrast materials. CT is ideal in defining the endoluminal and exophytic extent of tumor. Smaller gastrointestinal stromal tumors appear as smooth, sharply defined intramural masses with homogeneous attenuation (see the images below).
 Gastric gastrointestinal stromal tumor in a 49-year-old woman. The mass was found incidentally during an upper GI workup for peptic disease. The smooth appearance suggests a submucosal process.
Gastric gastrointestinal stromal tumor in a 49-year-old woman. The mass was found incidentally during an upper GI workup for peptic disease. The smooth appearance suggests a submucosal process.
 Gastrointestinal stromal tumor (GIST). Image obtained 1 year later in the same patient as in the previous image. The mass has increased in size. A GIST was found at surgery.
Gastrointestinal stromal tumor (GIST). Image obtained 1 year later in the same patient as in the previous image. The mass has increased in size. A GIST was found at surgery.
 Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows the same GIST. It appears as an intramural mass with both exophytic and endophytic components.
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows the same GIST. It appears as an intramural mass with both exophytic and endophytic components.
With contrast enhancement, the tumor may appear to have a rim, or it may be uniform in appearance (see the images below).
 Proximal jejunal gastrointestinal stromal tumor that is completely exophytic and not visible at small-bowel barium examination.
Proximal jejunal gastrointestinal stromal tumor that is completely exophytic and not visible at small-bowel barium examination.
 Gastrointestinal stromal tumor (GIST). Contrast-enhanced CT obtained in the same patient as in the previous image demonstrates a peripherally enhancing mass immediately adjacent to the pancreas that was thought to be a pancreatic neoplasm. However, at surgery, this proved to be a proximal exophytic jejunal GIST.
Gastrointestinal stromal tumor (GIST). Contrast-enhanced CT obtained in the same patient as in the previous image demonstrates a peripherally enhancing mass immediately adjacent to the pancreas that was thought to be a pancreatic neoplasm. However, at surgery, this proved to be a proximal exophytic jejunal GIST.
Occasionally, dense focal calcifications are present. Larger GISTs with necrosis appear as heterogeneous masses with enhancing borders of variable thickness and irregular central areas of fluid, air, or oral contrast attenuation that reflect necrosis (see the images below). Overlying mucosal ulcerations may be present, and the tumor may extend into nearby structures.
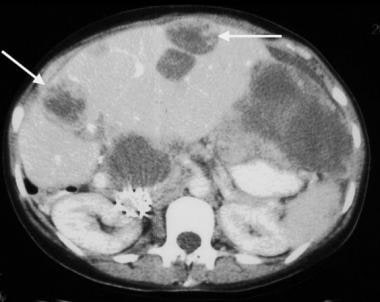 Gastrointestinal stromal tumor. Image obtained in the same patient as in the previous image after imatinib mesylate (Gleevec) administration. Note the decreased size of the liver metastases.
Gastrointestinal stromal tumor. Image obtained in the same patient as in the previous image after imatinib mesylate (Gleevec) administration. Note the decreased size of the liver metastases.
 Gastric gastrointestinal stromal tumor with huge exophytic component, which has become ulcerated. Barium collects in the exophytic ulcer crater (arrows).
Gastric gastrointestinal stromal tumor with huge exophytic component, which has become ulcerated. Barium collects in the exophytic ulcer crater (arrows).
 Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image demonstrates the GIST with large exophytic ulceration (arrows).
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image demonstrates the GIST with large exophytic ulceration (arrows).
CT is sensitive for the detection of metastatic liver, peritoneal, lung, and bone lesions. The diagnosis of GIST may be suggested by the presence of a large, complex, intestinal mass with liver lesions but without significant lymphadenopathy. Liver lesions can be hypervascular, or they may appear as cystic multilocular lesions with fluid-fluid levels (see the image below). [4, 9, 12, 29, 30, 34]
Magnetic Resonance Imaging
Among imaging studies, MRI has the best tissue contrast, which aids in the identification of masses within the GI tract. Furthermore, the ability to image in multiple planes facilitates localization and diagnosis. Intravenously administered contrast material is needed to evaluate lesion vascularity.
Gastrointestinal stromal tumors (GISTs) appear as sharply delineated, heterogeneous masses with cystic and necrotic areas. The masses tend to be isointense relative to skeletal muscle on T1-weighted images and hyperintense on T2-weighted images. Signal-intensity voids are present if gas is present within areas of necrotic tumor. [29, 30, 32, 35, 36, 37]
Ultrasonography
Ultrasonography is only moderately sensitive for the detection and evaluation of gastrointestinal stromal tumors (GISTs) . Bowel gas and acoustic shadowing obscure portions of the bowel and mesentery. Ultrasonography is ideal for guided-needle biopsy of known lesions, if such a procedure is indicated. With immunohistochemical staining methods, the diagnosis may be made before surgery. [38] However, aspiration (eg, fine-needle aspiration) and biopsy should be used selectively because of the risk of tumor seeding or peritoneal spillage. [5, 39] Both are associated with a worse prognosis.
On sonograms, larger gastrointestinal stromal tumors appear as complex masses with cystic and solid components, which are consistent with their tendency to undergo necrosis. [29, 35]
Endoscopic ultrasonography may be valuable in the evaluation of GISTs. The tumors appear as hypoechoic masses that are contiguous with the fourth hypoechoic layer of the GI wall, which corresponds to the muscularis propria. Characteristics associated with malignancy include tumor size greater than 4 cm, an irregular extraluminal border, echogenic foci, and the presence of cystic spaces. [5]
Angiography
On angiography, gastrointestinal stromal tumors (GISTs) appear as relatively well-circumscribed, hypervascular lesions with central avascularity. They have large feeding arteries and draining veins, and they show intense tumor staining (see the images below). [12]
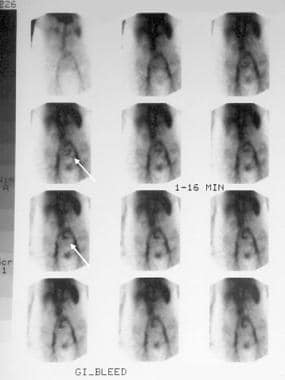 Gastrointestinal stromal tumor. A 62-year-old women presented with 2 episodes of massive GI bleeding. Nuclear medicine GI bleed scanning demonstrates increased tracer activity in the mid abdomen (arrows) consistent with a small bowel active bleed. Courtesy of Dr Jon Wood and Dr John McDermott, University of Wisconsin Hospitals and Clinics.
Gastrointestinal stromal tumor. A 62-year-old women presented with 2 episodes of massive GI bleeding. Nuclear medicine GI bleed scanning demonstrates increased tracer activity in the mid abdomen (arrows) consistent with a small bowel active bleed. Courtesy of Dr Jon Wood and Dr John McDermott, University of Wisconsin Hospitals and Clinics.
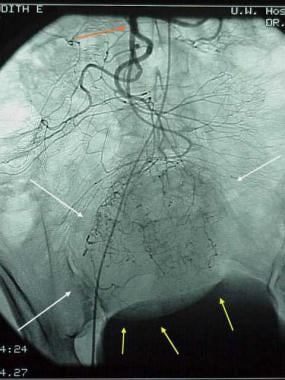 Gastrointestinal stromal tumor (GIST). Patient in the previous image then underwent angiography, which demonstrates a mass (white arrows) in the same location as the small-bowel bleed. The blood supply is from the superior mesenteric artery (red arrow). Minimal mass effect on the bladder is noted (yellow arrows). Findings are consistent with a small bowel mass, which stained positive for CD117; this result is consistent with a GIST.
Gastrointestinal stromal tumor (GIST). Patient in the previous image then underwent angiography, which demonstrates a mass (white arrows) in the same location as the small-bowel bleed. The blood supply is from the superior mesenteric artery (red arrow). Minimal mass effect on the bladder is noted (yellow arrows). Findings are consistent with a small bowel mass, which stained positive for CD117; this result is consistent with a GIST.
 Gastrointestinal stromal tumor. Selective masked injection in the superior mesenteric artery (red arrow) demonstrates a large arterial feeder with tumor staining (white arrows).
Gastrointestinal stromal tumor. Selective masked injection in the superior mesenteric artery (red arrow) demonstrates a large arterial feeder with tumor staining (white arrows).
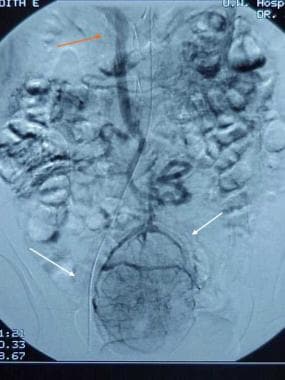 Gastrointestinal stromal tumor. Delayed images demonstrate large draining vein, which empties into the superior mesenteric vein (red arrow).
Gastrointestinal stromal tumor. Delayed images demonstrate large draining vein, which empties into the superior mesenteric vein (red arrow).
-
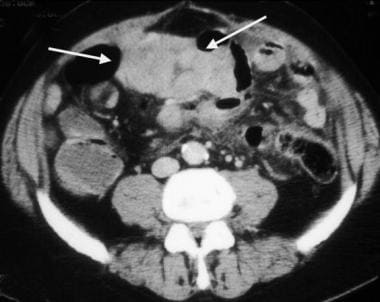
Small-bowel gastrointestinal stromal tumor with a diffusely thickened bowel wall.
-
Gastrointestinal stromal tumor. Image obtained in the same patient as in the previous image. A more caudal portion of the tumor has areas of necrosis (arrows), with air present within the necrotic cavity that communicates with the lumen of the small bowel.
-
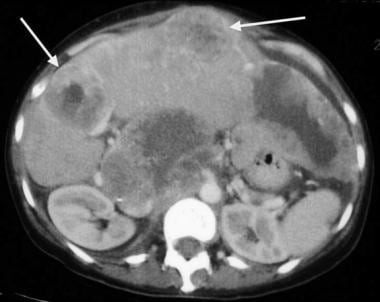
Small-bowel gastrointestinal stromal tumor with multiple liver metastases (arrows).
-
Gastrointestinal stromal tumor. Image obtained in the same patient as in the previous image after imatinib mesylate (Gleevec) administration. Note the decreased size of the liver metastases.
-
Multifocal gastrointestinal stromal tumors. Barium examination reveals a smooth mass that causes narrowing in the second portion of the duodenum (arrows).
-
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows a focal segment of diffusely thickened ileum, which is another GIST.
-
Gastric gastrointestinal stromal tumor in a 49-year-old woman. The mass was found incidentally during an upper GI workup for peptic disease. The smooth appearance suggests a submucosal process.
-
Gastrointestinal stromal tumor (GIST). Image obtained 1 year later in the same patient as in the previous image. The mass has increased in size. A GIST was found at surgery.
-
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image shows the same GIST. It appears as an intramural mass with both exophytic and endophytic components.
-
Gastric gastrointestinal stromal tumor (GIST) en face. Upper GI image obtained during the single contrast enhancement portion shows an incidentally found mass. The smooth borders suggest a submucosal process. At surgery, a GIST was found.
-
Gastrointestinal stromal tumor with central bull's eye appearance, which is compatible with contrast material collection in an ulceration.
-
Gastric gastrointestinal stromal tumor with huge exophytic component, which has become ulcerated. Barium collects in the exophytic ulcer crater (arrows).
-
Gastrointestinal stromal tumor (GIST). CT scan obtained in the same patient as in the previous image demonstrates the GIST with large exophytic ulceration (arrows).
-
Proximal jejunal gastrointestinal stromal tumor that is completely exophytic and not visible at small-bowel barium examination.
-
Gastrointestinal stromal tumor (GIST). Contrast-enhanced CT obtained in the same patient as in the previous image demonstrates a peripherally enhancing mass immediately adjacent to the pancreas that was thought to be a pancreatic neoplasm. However, at surgery, this proved to be a proximal exophytic jejunal GIST.
-
Gastrointestinal stromal tumor. A 62-year-old women presented with 2 episodes of massive GI bleeding. Nuclear medicine GI bleed scanning demonstrates increased tracer activity in the mid abdomen (arrows) consistent with a small bowel active bleed. Courtesy of Dr Jon Wood and Dr John McDermott, University of Wisconsin Hospitals and Clinics.
-
Gastrointestinal stromal tumor (GIST). Patient in the previous image then underwent angiography, which demonstrates a mass (white arrows) in the same location as the small-bowel bleed. The blood supply is from the superior mesenteric artery (red arrow). Minimal mass effect on the bladder is noted (yellow arrows). Findings are consistent with a small bowel mass, which stained positive for CD117; this result is consistent with a GIST.
-
Gastrointestinal stromal tumor. Selective masked injection in the superior mesenteric artery (red arrow) demonstrates a large arterial feeder with tumor staining (white arrows).
-
Gastrointestinal stromal tumor. Delayed images demonstrate large draining vein, which empties into the superior mesenteric vein (red arrow).
-
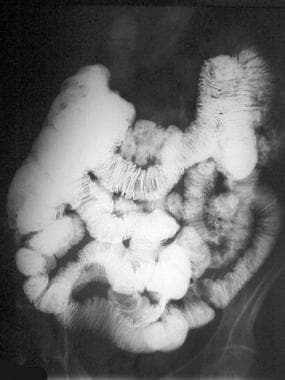
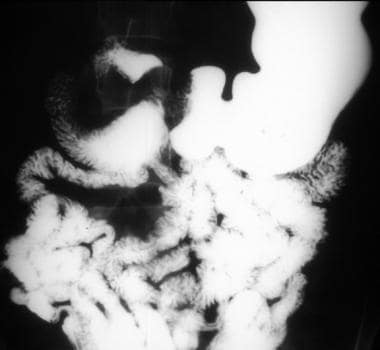
Gastrointestinal stromal tumor. Enteroclysis results were surprisingly normal. No abnormalities are seen, and no significant mass effect suggests the presence of the mass.
-
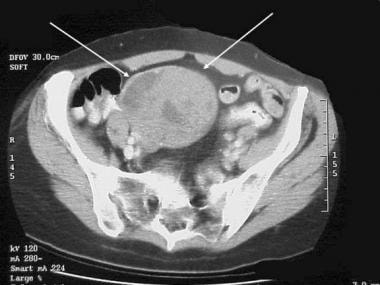
Gastrointestinal stromal tumor (GIST). CT demonstrates a complex mass originating from the small bowel with characteristics of a GIST.