Practice Essentials
Lead toxicity has the potential to cause irreversible health effects and can interfere with a number of body functions, primarily affecting the central nervous, hematopoietic, hepatic, and renal systems. Acute toxicity is related to occupational exposure and is quite uncommon; chronic toxicity is much more common. Lead poisoning is probably the most important chronic environmental illness affecting children. Despite efforts to control it and despite apparent success in decreasing incidence, serious cases of lead poisoning still appear in hospital emergency departments, clinics, and private physicians’ offices. [1, 2, 3, 4, 5]
Approximately 590,000 children in the United States between the ages of 1 and 5 years had elevated blood lead levels (≥3.5 μg/dL ) in 2016, and in 2019, 4.3 million children were living in homes with lead paint. Despite improvements, racial and other disparities remain statistically significant. [2]
Lead environmental pollution is a major health hazard throughout the world. The World Health Organization estimated that nearly half of the 2 million lives lost to known chemicals exposure in 2019 were due to lead exposure. [6] The mechanisms of contamination include ingestion, inhalation, prenatal exposure, and dermal exposure, but the most common are ingestion and inhalation. Ingestion is more common in children, while the inhalation route is more frequent in occupationally exposed adults. Causes of lead poisoning include pica, industrial exposure, drinking moonshine liquor, gunshot wounds, retained lead pellets or particles, and a variety of folk remedies and cosmetics. [7, 8, 9, 10, 11]
The Lead-Based Paint Poisoning Prevention Act of 1971 led to the establishment of the Childhood Lead Poisoning Prevention Program (CLPPP) at the Centers for Disease Control and Prevention (CDC). Because of the Flint, Michigan, water crisis in 2014, national interest in lead poisoning increased as a public health problem, and as a result, Congress established the Flint Registry to provide funds for prevention and treatment of lead poisoning. The Early Periodic Screening, Diagnosis, and Treatment (EPSDT) component of Medicaid requires that all children who are receiving Medicaid be provided with a lead screening at 12 and 24 months, as well as children between 3 and 5 years of age who have not been screened. Also, the Occupational Safety and Health Administration (OSHA) requires employers to implement medical surveillance (ie, lead screening) for any employee who may be exposed to lead concentrations exceeding 30 mg/min over 8 hours for more than 30 days a year. [12, 13, 14, 3, 1, 4, 5, 15]
The Advisory Committee on Childhood Lead Poisoning Prevention of the CDC has defined elevated blood lead concentration as 5 μg/dL or greater on the basis of the 97.5 percentile of blood lead concentrations in the National Health and Nutrition Examination Survey (NHANES) dataset. In 2014, there were 444.5 per 100,000 children aged 1 to 4 years in the United States with a blood lead concentration of 5 to 9 μg/dL. [1, 15]
Concerns have been raised about lead-dust on personal protective equipment (PPE) used by healthcare workers to shield themselves from ionizing radiation when performing imaging studies. Single-center studies have reported a range of 23-61% of the external surface of radiation protection apparel contaminated with lead dust. [16, 17]
Imaging modalities
Plain skeletal radiographs have been used extensively in the diagnosis of lead poisoning in children. These images have been found to be reliable in young infants presenting with an unexplained encephalopathy. A radiograph of the knee showing dense metaphyseal bands strongly supports the diagnosis of lead poisoning. Plain radiography of the knee is an inexpensive and widely available investigation that may be rapidly performed. [18]
These bands are not pathognomonic, and the differential diagnosis for cases involving opaque metaphyseal lines is wide and includes poisoning with other heavy metals; hypervitaminosis D; and the healing stages of leukemia, rickets, and scurvy.
A normal skeletal radiograph does not rule out lead poisoning in children. The classic findings of lead lines on radiographs of long bones are rarely seen because most cases of lead poisoning in children are due to exposures to low or moderate amounts of lead.
In select cases, abdominal radiographs may demonstrate paint chips or other objects. The presence of lead foreign bodies in the gastrointestinal tract (caused by pica) may highlight the diagnosis and prompt immediate intervention. Plain abdominal radiographs may also guide therapy (eg, by allowing the prevention of further absorption through gastrointestinal decontamination).
Neuroimaging, as with CT and MRI, plays a minor role in the diagnosis of lead poisoning. However, cerebral edema and microhemorrhages may be seen in patients presenting with encephalopathy. With chronic exposure to lead, patchy calcifications may be seen on CT scans in adults. [19]
Neuroimaging is expensive and the availability may be limited. Young children undergoing CT or MRI may need heavy sedation or general anesthesia. A finding of disruption of brain plasticity, as seen on MRIs, is not a finding unique to lead intoxication. Other clinical disorders, such as metabolic and epileptic encephalopathies and psychosocial deprivation, may be associated with disrupted brain plasticity. Several intellectual disability syndromes and cognitive disorders have been recognized as being secondary to genetic disruption of intracellular signaling cascades.
Anecdotal reports of angiography and ultrasonography used in the diagnosis of lead poisoning have appeared in the literature. [20]
(See the lead poisoning images below.)
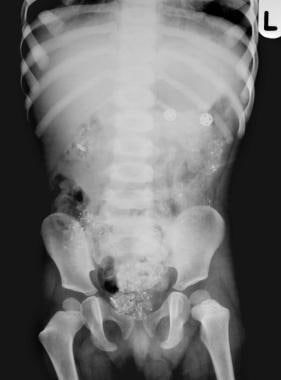 Lead poisoning. Pica. Plain abdominal radiograph in a 3-year-old patient shows multiple metallic particles due to ingested flakes of lead paint.
Lead poisoning. Pica. Plain abdominal radiograph in a 3-year-old patient shows multiple metallic particles due to ingested flakes of lead paint.
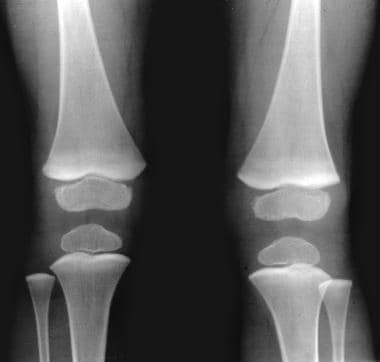 Lead poisoning. Opaque metaphyseal bands in the lower femur, upper tibia, and the upper fibula secondary to lead poisoning in a child.
Lead poisoning. Opaque metaphyseal bands in the lower femur, upper tibia, and the upper fibula secondary to lead poisoning in a child.
With the advent of electrophysiology, it has become obvious that spinal cord involvement is the cause of lead neuropathy. The hypothesis of axonal degeneration in the dying-back variant, starting in the biochemical lesion of perikarya of the anterior horn cells in the spinal cord, is in full agreement with both the electromyographic signs of denervation and the electroneurographic normal range of conduction velocity. [21, 22]
Radiologic differential diagnoses
Albers and Bromberg reported a case of X-linked bulbospinomuscular atrophy, or Kennedy disease, masquerading as lead neuropathy. [23] They described a 43-year-old man who was referred by a veterinary surgeon who evaluated the man's dog for a seizure; the veterinarian suspected a toxic lead exposure in both the dog and its owner. The patient had worked on refurbished houses, removing old paint, and complained of decreased cognition, fatigue, and muscle cramps. He had a depressed affect, postural tremor, right-arm weakness with partial denervation on electromyelography, and borderline-low sensory nerve action potential amplitudes. A mild anemia and elevated serum and urine lead levels supported a diagnosis of lead neuropathy.
Chelation therapy led to an increase in urine lead excretion without symptomatic improvement. The patient's brother worked part-time with him and developed similar findings; in addition, the brother had difficulty chewing and experienced dysphagia, perioral twitching, gynecomastia, and multifocal denervation of extremity and facial muscles. The brother's lead levels were not elevated; an androgen receptor mutation identified on the X chromosome in both brothers confirmed the diagnosis of X-linked bulbospinomuscular atrophy.
Mennel and John reported a case of a 23-month-old boy with osteosclerotic metaphyseal dysplasia (OMD). [24] The patient presented with hypotonia, developmental delay, and complex seizures. Radiographs revealed profound sclerosis of the metaphyses and epiphyses of the long and short bones in the extremities, with a unique pattern of distribution. Sclerosis involved the anterior ribs, iliac crests, talus, and calcaneus. The skull and vertebral bodies appeared unaffected.
The overall appearances were suggestive of lead poisoning. Blood lead levels were normal. OMD is a rare sclerosing bone disorder inherited in an autosomal recessive manner. The syndrome is clinically characterized by developmental delay of a progressive nature, hypotonia, elevated alkaline phosphatase levels, and late-onset spastic paraplegia. Analysis of the metaphyseal bone changes should help distinguish OMD from lead poisoning and other causes of metaphyseal sclerosis.
Radiography
In patients with chronic lead poisoning, opaque transverse metaphyseal bands appear in growing tubular bones. These metaphyseal bands, or lead lines, usually do not occur until blood lead levels (BLLs) reach 70-80 mcg/dL; they are not an early manifestation of lead intoxication. [25] These lines, which are actually growth arrest lines, are not pathognomonic for lead poisoning (see the images below).
 Lead poisoning. Pica. Plain abdominal radiograph in a 3-year-old patient shows multiple metallic particles due to ingested flakes of lead paint.
Lead poisoning. Pica. Plain abdominal radiograph in a 3-year-old patient shows multiple metallic particles due to ingested flakes of lead paint.
 Lead poisoning. Opaque metaphyseal bands in the lower femur, upper tibia, and the upper fibula secondary to lead poisoning in a child.
Lead poisoning. Opaque metaphyseal bands in the lower femur, upper tibia, and the upper fibula secondary to lead poisoning in a child.
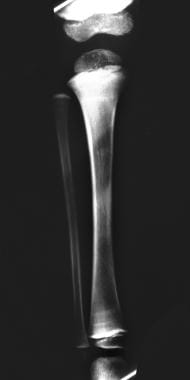 Lead poisoning. Opaque metaphyseal bands in the upper and lower tibia and the upper fibula secondary to lead poisoning in a child.
Lead poisoning. Opaque metaphyseal bands in the upper and lower tibia and the upper fibula secondary to lead poisoning in a child.
The lead lines may persist after the lead exposure ends. Normal bone is laid down on the epiphyseal side of the lead line; with recovery, the lead line becomes broader and less dense and may eventually disappear. The band migrates into the substance of bone at a rate that correlates with bone growth. If normal BLLs are maintained, the lead lines gradually decrease in density and disappear after about 4 years. If the lead exposure is prolonged, there may be lack of bone modeling. In children, the density and width of the lead bands are well correlated with the concentration of lead ingestion or inhalation, as well as with the duration of lead exposure.
Because lead and calcium are used interchangeably by bone, lead deposition occurs in high concentrations in growing bones; the greatest concentration occurs in the metaphyses, particularly those in the distal femora, ends of the tibiae, and distal radii, because these are the parts of the skeleton that grow most rapidly. Thus, lead lines are most prominent at the knees; they can, however, also be seen in the wrist, in any other long bone metaphysis, in the axial skeleton, and in the margins of flat bones.
Single transverse lines predominate, but multiple bands may be seen in cases of episodic lead poisoning. In infants with severe lead poisoning, the bands may be wide and may prevent normal remodeling. In addition, the distal ends of the long bones may disappear and appear clubbed. A bone-within-a-bone appearance has been described.
A negative radiograph does not rule out lead poisoning in children. In healthy infants 3 years of age or younger, the finding of increased metaphyseal opacity is not unusual. This may sometimes be pronounced and may lead to a false-positive diagnosis of lead intoxication. This increased metaphyseal opacity is a normal variant and represents exuberant calcification of the zone of provisional calcification. It is often seen in healthy children, especially after exposure to sunlight after winter. Opaque metaphyseal bands are also seen after poisoning with other heavy metals; with hypervitaminosis D; and during the healing stages of leukemia, rickets, and scurvy.
Woolf and associates examined 15 Omani infants (2-4 mo of age) who had acute lead encephalopathy and concluded that any young infant presenting with unexplained encephalopathy should undergo radiography of the knee and that, in such cases, the presence of opaque metaphyseal bands strongly supports the diagnosis of lead poisoning. [26] Plain skeletal radiography revealed opaque metaphyseal bands in all 15 infants; these were best seen around the knee joint. In 6 infants, there was evidence of multiple lead lines, indicating previous episodes of exposure to lead. In 4 infants, lead lines were also seen in the axial skeleton.
In select cases, abdominal radiographs may demonstrate lead-containing paint chips (resulting from pica) or other lead-containing objects. The presence of lead foreign bodies in the gastrointestinal tract may highlight the diagnosis and prompt immediate intervention. Plain abdominal radiographs may also guide therapy in preventing further absorption through gastrointestinal decontamination.
K-shell x-ray fluorescence (KXRF) techniques have been used in epidemiologic studies to establish rates of environmental lead exposure in high-risk communities. [27] In addition, this modality has been utilized to identify health effects resulting from lead exposure, but the equipment is bulky and requires significant maintenance and licensing procedures. [28] A portable x-ray fluorescence (XRF) device has been developed to overcome these disadvantages but introduced a measurement dependency on soft tissue thickness. [29]
Hu et al measured bone lead levels in 34 adults with no known history of excessive lead exposure and found that bone lead levels were greater in older persons; they concluded that the KXRF technique may help distinguish low levels of lead burden, as shown in epidemiologic studies. [30] The investigators used a questionnaire to gather information about the participants' occupational and environmental lead exposure. A 30-minute measurement with an average estimated uncertainty of 6 μg lead/g of bone mineral was obtained at the midtibial diaphysis.
In the study by Hu et al, 18 patients had bone lead levels below the measurement uncertainty. The remainder had BLLs up to 21 μg/g. Among young adults, bone lead levels greater than the degree of measurement uncertainty were confined entirely to individuals who had grown up in housing that was estimated to have been built before 1955. Such a childhood environment fosters exposure to biologically absorbable lead through the ingestion of dust contaminated with lead paint and water contaminated from lead pipe.
Ahlgren et al used x-ray fluorescence analysis to determine the lead concentration in vivo of 5 former workers in a metal industry and found that their lead levels were about 3-9 times higher than levels found in people from southern Sweden. The mean lead concentration in the skeletons of the 5 metal workers was estimated to be 62 μg/g; the standard error was ± 5 μg/g. [31]
Computed Tomography
CT plays a minor role in the diagnosis of lead poisoning. However, cerebral edema and microhemorrhages may be seen in patients presenting with encephalopathy. With chronic exposure to lead, patchy calcifications may be seen on CT scans in adults. [19]
Reyes et al described the CT findings of cerebral and cerebellar calcification in 3 adults with known exposure to lead of 30 years or longer, and on imaging, they found that the patterns of calcification varied and were punctiform, curvilinear, specklike, or diffuse. At admission, the patients were found to have increased serum lead levels (54-72 mcg/dL; normal range, 0-30 mcg/dL). All 3 patients had nonspecific neurologic symptoms of dementia, loss of visual acuity, peripheral neuropathy, syncope, dizziness, nystagmus, easy fatigue, or back pain. These patterns were found in the subcortical area, basal ganglia, vermis, and cerebellum. Two patients developed chronic renal disease and hypertension; in both, serum parathormone levels were elevated; serum calcium and phosphorus levels were normal in all 3. No other abnormality was depicted on CT. [32]
Benson and Price evaluated elderly persons who grew up in a high-lead environment in Queensland, Australia, and experimental and neuropathologic studies demonstrated an association between exposure to lead and perivascular cerebellar calcification [33] The subjects' childhood residence and occupational status provided circumstantial evidence of a relationship between excessive lead intake and cerebellar calcification, as seen on CT scans.
Previous autopsy studies have shown that cerebellar calcification is more common in Queensland than elsewhere. Results of cranial CT, which is more sensitive than skull radiography, has confirmed that cerebellar and basal ganglia calcification occurs more commonly in patients examined in Queensland than in patients in North America. Although no distinctive neurologic syndrome could be demonstrated, the incidence of hypertension and hyperuricemia was increased in the affected patients, and serum creatinine levels were increased.
Evidence suggests that cerebellar calcification is a marker of previous lead intoxication. The common occurrence of renal impairment in these patients may be the result of associated lead nephropathy. Subclinical lead exposure is associated with hyperuricemia.
Schroter and associates reported a case of a 59-year-old potter who presented with lead polyneuropathy after 37 years of occupational exposure and noted that cranial CT showed extensive, bilateral, symmetrical calcification in the cerebellar hemispheres and minor calcification in the subcortical area of the cerebral hemispheres and basal ganglia. In addition, T2-weighted MRI showed high signal intensity in the periventricular white matter, basal ganglia, insula, posterior thalamus, and pons. [34]
The patient in the study by Schroter et al had a 25-year history of normochromic normocytic anemia with moderate basophilic stippling, mild renal failure, hyperuricemia, and abnormal porphyrins. The patient also reported having experienced 3 short psychotic episodes. [34]
Although the pathophysiologic mechanism of these findings remains poorly understood, it has been suggested that chronic lead exposure be included in the differential diagnosis of unexplained intracranial calcifications in adults.
Magnetic Resonance Imaging
As with CT, MRI plays a minor role in the diagnosis of lead poisoning. However, cerebral edema and microhemorrhages may be seen in patients presenting with encephalopathy. With chronic exposure to lead, patchy calcifications may be seen on MRI scans (though these are better seen on CT scans).
The symptoms of lead encephalopathy are mainly those associated with cerebral edema. Focal signs may predominate, mimicking a brain tumor in an area of generally diffuse brain edema.
Perelman et al reported a case in which MRI results excluded a focal mass, and early treatment of edema prevented neurosurgical exploration. Since Biemond and Van Creveld first described the condition in 1939, a total of 7 cases of lead encephalopathy with a clinical presentation of a cerebellar tumor have been reported. [35]
The developing brain is subject to major changes during fetal life, as well as for at least the first decade of childhood. Initially, the brain develops more neurons and synaptic connections than are needed for function in later life. These excess neurologic units are "sculpted" away during early brain development before the mature brain finally develops. This process is thought to be the basis for the plasticity of the developing brain or its capacity to adapt in behavior and circuitry in response to stimulation from the external environment.
Functional MRI now offers the means to noninvasively study this plastic reorganization of the brain in children and adults. MRI may demonstrate how the brain's functional maps undergo major reorganization in response to early environmental changes. The neurobiology of brain reorganization during development is also being studied in light of new insights into the molecular mechanisms for activity-dependent neuronal plasticity. Clinical disorders such as lead poisoning may arise from disrupted brain plasticity. [36]
Trope et al found that that magnetic resonance spectroscopy (MRS) may have a role as a noninvasive technique for the in vivo examination of the brain of children exposed to lead. The authors examined 2 male cousins who were living in the same household. [37] One, a 10-year-old boy, had elevated blood lead levels (BLLs). His cousin, a 9-year-old boy, had not been exposed to lead. Both underwent a comprehensive neuropsychological evaluation. High-resolution MRI and MRS were performed in both children by use of a 3-inch surface coil.
Neuropsychological evaluation in the 2 cousins demonstrated areas of impairment in the lead-exposed child; these included difficulties in the academic skills of reading, writing, and arithmetic. The affected child was also deficient in linguistic skills and attention mechanisms. His cousin, who was not exposed to lead, had normal neuropsychological function. Although in both children the MRI scans were normal, the lead-exposed boy had significant alterations in brain metabolites, with a reduced ratio of N -acetylaspartate to creatine in both gray matter and white matter. [37]
In another study, Trope et al concluded that lead has an effect on brain metabolites, as detected by MRS in vivo. More specifically, they found significant reductions in the levels of brain metabolites in gray matter but not white matter in individuals exposed to lead. Their results imply that MRS may depict metabolic abnormalities in individuals with lead poisoning. [38]
Disrupted brain plasticity is not confined to cases of lead intoxication; other clinical disorders, such as metabolic and epileptic encephalopathies, as well as psychosocial deprivation, may be associated with disrupted brain plasticity.
Several intellectual disability syndromes and cognitive disorders have been recognized as being secondary to genetic disruption of intracellular signaling cascades. Understanding how the brain's circuitry is sculpted during development provides an important perspective for thinking about neurodevelopmental disorders. [36]
Ultrasonography
Ultrasonography is not routinely used in the diagnosis of lead intoxication, though transcranial sonography may be used to investigate congenital lead poisoning and lead intoxication in infant patients presenting with lead encephalopathy.
Zou et al studied the effects on systolic and diastolic cardiac function in 54 individuals exposed to lead and in 24 workers not exposed, and the Doppler echocardiographic results suggested that cardiac systolic function increased in the exposed group as a compensatory response to the effect of lead on myocardium. Pulsed Doppler imaging showed that time-related parameters were comparable in all groups but that blood flow velocity through the mitral valve and Doppler area fractions changed significantly in lead-exposed groups. This change was evidenced by increased A values and decreased E values and E/A ratios. [39]
The decrease in diastolic cardiac function was more significant in the lead-intoxication group than in the nonexposed group. In addition, the serum activity of the MB isoenzymes of creatine phosphokinase (CPK-MB), one of the indices of myocardial damage, was significantly higher in the exposed group than that in control subjects; a positive correlation was found between CPK-MB activity and Pb-B. These findings suggest that an increase in lead burden leads to an increase in the release of CPK-MB from the myocardial cells and that slight myocardial damage occurs, which might conceivably impair diastolic cardiac function.
Tutar and associates described a case of a 12-year-old girl who presented with recurrent pericardial effusion caused by a firearm pellet injury to the left ventricle. [40] A slightly elevated blood lead level (BLL) of the patient was alarming because of the possibility of subsequent lead poisoning associated with retained pellets. The pellet was localized by means of 2-dimensional echocardiography of the left ventricular apical wall. Because the patient was asymptomatic, left ventriculotomy was not used to extract the pellet; only pericardial tube drainage was carried out. Hollerman et al described the substantial contribution radiologists can make to the evaluation and treatment of the patient with a gunshot wound. [41, 42, 43] Plain radiographs, CT, angiography, and sometimes MRI may all be used to localize the missile, to determine the path it followed in the body, to assess missile and bone fragmentation, and to identify missile emboli. Certain locations of missile fragments predispose the patient to lead poisoning or lead arthropathy.
-
Lead poisoning. Pica. Plain abdominal radiograph in a 3-year-old patient shows multiple metallic particles due to ingested flakes of lead paint.
-
Lead poisoning. Opaque metaphyseal bands in the lower femur, upper tibia, and the upper fibula secondary to lead poisoning in a child.
-
Lead poisoning. Opaque metaphyseal bands in the upper and lower tibia and the upper fibula secondary to lead poisoning in a child.









