Practice Essentials
Although kidney transplantation has become a mainstay in the long-term management of many patients with renal failure, the procedure can be associated with complications such as neurologic, immunologic, infectious, vascular, and urologic. Although a kidney biopsy may be required in some cases, noninvasive imaging should be attempted first, to identify clinical situations that can be corrected by radiologic or surgical intervention. [1, 2] A sonographic survey of a recently transplanted kidney offers a noninvasive means by which to identify postoperative hemorrhage, urinary leaks, and early signs of posttransplant rejection. Color Doppler ultrasound is often the first imaging modality to be applied to identify transplant renal artery stenosis (TRAS). [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14]
Complications include the following after kidney transplantation [15] :
-
Within the first week: hyperacute rejection–accelerated rejection; acute tubular necrosis; calcineurin inhibitors toxicity; infectious complications (acute graft pyelonephritis).
-
Between 1st and 12th week: acute rejection; calcineurin inhibitors toxicity; infectious complications (acute graft pyelonephritis).
-
After 12th week: chronic rejection; calcineurin inhibitors toxicity; infectious complications (acute graft pyelonephritis); nephropathy relapse.
The American College of Radiology (ACR) has noted the following [4, 16] :
-
Duplex Doppler ultrasound is the first-line imaging evaluation to assess renal transplant dysfunction.
-
Renal scintigraphy using MAG3 (mercaptoacetyltriglycine) serves a complementary role by quantitatively assessing the 3 sequential phases of renal transplant function.
-
Slthough ultrasonography, CT angiography, and MR angiography can be used to noninvasively diagnose renal artery stenosis (RAS), angiography remains the diagnostic reference standard and is utilized to guide intervention.
The need for renal transplantation has grown as the number of patients with diabetes has increased. The obesity epidemic represents an expanding threat of chronic kidney disease (CKD). Strategies to delay or prevent progression of diabetic nephropathy have decreased the rate of persons with diabetes who develop end-stage renal disease (ESRD); however, for those with ESRD attributed to diabetes, kidney transplantation affords better survival and rehabilitation than either hemodialysis or peritoneal dialysis. [16, 17, 18]
Imaging modalities
The most common imaging procedure in the renal transplant recipient is ultrasonography. [19] Immediate complications related to surgery can be demonstrated, and US enables suitable localization before biopsy in most patients. Duplex US also provides important information concerning the vascular status of the graft in cases of acute rejection. Delayed complications related to the renal transplant (eg, lymphocele) may occur, which can compromise the drainage from the renal pelvis or may compress the renal transplant. [4, 5, 6, 20, 21, 22, 23]
Ultrasound is the most frequent imaging method used in the postoperative period and for long-term follow-up. It is widely available, and the relatively low cost and high degree of safety of ultrasound allow for serial examinations, which may be necessary during the postoperative period. [24]
Concurrent with sonographic studies, radionuclide studies are frequently performed. The nuclear studies provide valuable information concerning functional status during the immediate postoperative period and during episodes of rejection. Correlation between renal sonographic and nuclear medicine findings helps differentiate between purely functional disease, such as acute tubular necrosis or rejection, and abnormal fluid collections, such as hematomas, abscesses, and lymphoceles.
Ploussard et al studied the use of systematic aortofemoral color Doppler ultrasound (DUS) in recipients of a first renal transplantation and found that of 84 patients presenting with a normal preoperative physical arterial examination, 12 (14.3%) had an abnormal DUS, revealing atherosclerosis but not stenosis. Of the 16 patients who had had an abnormal examination, 10 (62.5%) also had abnormal findings on DUS, including 4 cases of iliac stenosis, 3 of whom required surgical procedure modification because of right iliac artery stenosis. [25]
CT and MRI studies of the abdomen and pelvis offer similar information. [26, 27] In most cases, CT is preferred because CT-guided aspiration and drainage procedures may obviate surgical interventions. Angiographic study of the transplant may involve CT angiography, MR angiography, or intra-arterial catheter angiography if therapeutic procedures are to be performed. In general, the examination with the least risk should be selected for the diagnostic survey of the renal transplant.
The assessment of potential complications may require the application of a variety of imaging techniques. Multislice CT offers a relatively fast examination with high resolution; however, some applications require the use of IV contrast, which may be contraindicated in some renal transplants that are in rejection or have decreased renal function.
MRI offers high resolution with excellent tissue differentiation but is expensive for routine use and is motion sensitive. The use of contrast agents based on gadolinium is contraindicated in cases of renal transplant failure. Advances in time-of-flight imaging have allowed satisfactory imaging of renal vessels without the use of IV contrast agents. [24]
The donor evaluation is most commonly done with MRI(A) (magnetic resonance angiography) and CT(A) (computed tomographic angiography).
(Complications associated with kidney transplantation are shown in the images below.)
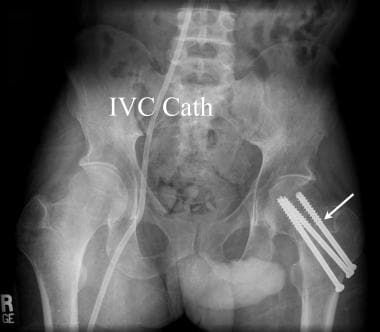 Anteroposterior pelvic radiograph of a renal transplant candidate. Prior to receiving a renal transplant the patient with chronic renal disease must undertake hemodialysis. While most patients are treated using an upper extremity forearm graft, at times indwelling central venous catheters may be necessary (IVC Cath). Metabolic bone disease is very common in the chronically ill pretransplantation patient. A pathological fracture of the left femoral neck was treated in this renal transplant candidate with cannulated femoral neck pins (white arrow).
Anteroposterior pelvic radiograph of a renal transplant candidate. Prior to receiving a renal transplant the patient with chronic renal disease must undertake hemodialysis. While most patients are treated using an upper extremity forearm graft, at times indwelling central venous catheters may be necessary (IVC Cath). Metabolic bone disease is very common in the chronically ill pretransplantation patient. A pathological fracture of the left femoral neck was treated in this renal transplant candidate with cannulated femoral neck pins (white arrow).
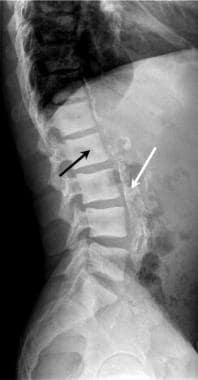 Lateral radiograph of the lumbar spine in renal failure. The effects of chronic renal failure are often seen in renal transplant candidates prior to successful renal transplantation. Note the dense vertebral bodies (black arrow) due to renal osteodystrophy. The aorta and all major arterial branches are densely calcified (white arrow) in this renal transplant candidate.
Lateral radiograph of the lumbar spine in renal failure. The effects of chronic renal failure are often seen in renal transplant candidates prior to successful renal transplantation. Note the dense vertebral bodies (black arrow) due to renal osteodystrophy. The aorta and all major arterial branches are densely calcified (white arrow) in this renal transplant candidate.
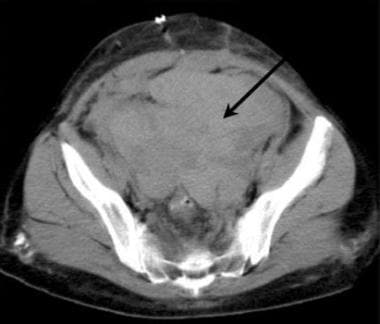 Axial CT of the pelvis in a potential renal transplant patient. A previous CT of the pelvis had demonstrated a large ovarian cyst, which prevented renal transplantation. Immediately after the surgical removal of an ovarian cyst from the left pelvis, a large hematoma (arrow) prevents planned renal transplantation. Planning the surgical preparation of a recipient site long before the scheduled renal transplantation is important.
Axial CT of the pelvis in a potential renal transplant patient. A previous CT of the pelvis had demonstrated a large ovarian cyst, which prevented renal transplantation. Immediately after the surgical removal of an ovarian cyst from the left pelvis, a large hematoma (arrow) prevents planned renal transplantation. Planning the surgical preparation of a recipient site long before the scheduled renal transplantation is important.
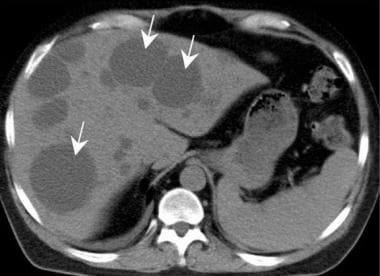 Polycystic liver disease (arrows) may complicate surgical management by enlarging the intra-abdominal contents. Liver failure rarely occurs but the cysts may bleed, causing pain.
Polycystic liver disease (arrows) may complicate surgical management by enlarging the intra-abdominal contents. Liver failure rarely occurs but the cysts may bleed, causing pain.
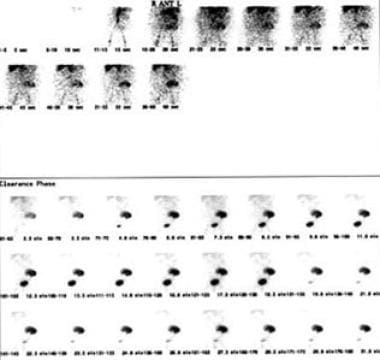 In cases of mild renal failure following renal transplantation, the primary considerations are acute renal rejection versus renal obstruction. The renal scan in this patient demonstrates renal arterial flow to the transplant with no signs of renal obstruction. The delayed and decreased renal function is consistent with mild renal rejection, which was proven by renal biopsy.
In cases of mild renal failure following renal transplantation, the primary considerations are acute renal rejection versus renal obstruction. The renal scan in this patient demonstrates renal arterial flow to the transplant with no signs of renal obstruction. The delayed and decreased renal function is consistent with mild renal rejection, which was proven by renal biopsy.
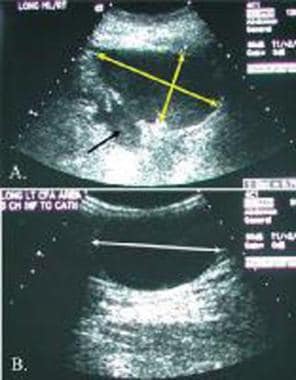 Following a renal transplant, decreased renal function with limited urinary output was noted. The sonography of the transplant site and the pelvis demonstrates a large hypoechoic mass (double yellow arrows) anterior to the transplant (black arrow). B. The hypoechoic mass extended downward into the anterior thigh (double white arrow). The primary diagnostic considerations should include a hematoma versus a urinary leak.
Following a renal transplant, decreased renal function with limited urinary output was noted. The sonography of the transplant site and the pelvis demonstrates a large hypoechoic mass (double yellow arrows) anterior to the transplant (black arrow). B. The hypoechoic mass extended downward into the anterior thigh (double white arrow). The primary diagnostic considerations should include a hematoma versus a urinary leak.
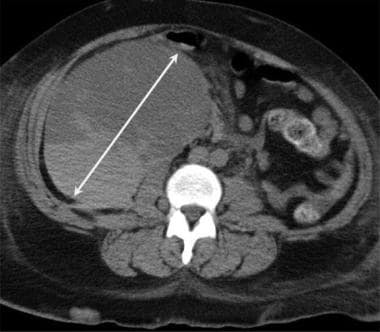 Axial CT of the pelvis demonstrates a large hematoma (double arrow), which developed during the postoperative period following renal transplantation.
Axial CT of the pelvis demonstrates a large hematoma (double arrow), which developed during the postoperative period following renal transplantation.
Perioperative complications occur in 15-20% of renal transplants. Most initial complications can be corrected if detected promptly. The radiologist should help to select the most effective imaging methods for evaluating the many problems encountered in renal transplantation. Ultrasonography is a safe, rapid, and portable means to first detect most surgical emergencies. [3, 4, 5, 6, 28, 29, 30, 31]
Although a full discussion of the potential infections in renal transplant patients is beyond the scope of this discussion, a careful review of chest radiographs, abdominal and pelvic sonograms, and CT scans must include a consideration of unusual fungal, protozoan, and bacterial infections. [32]
After the first few hours, a clinical milestone is the production of urine by the renal transplant. Poor function of the renal transplant may be the result of acute tubular necrosis (ATN). ATN is related to "warm" renal ischemia. Both the severity and duration of ATN is worse in cadaveric transplants. Because of the sensitivity of the injured renal transplant, nuclear medicine examinations are most commonly used to study ATN and to differentiate other causes of poor renal function in the immediate posttransplantation period.
Nuclear medicine scanning and flow studies remain the primary means for evaluating vascular supply to the transplant after surgery. The main advantage of nuclear medicine scans is that they demonstrate the pathophysiology involved. Developments in Doppler US and MRI show promise in improving the quality of renal vascular imaging without the use of potentially nephrotoxic intravenous (IV) contrast materials.
CT scanning and CT-guided interventions remain an important means for examining the preoperative renal donor candidate and for evaluating the complications that develop in patients who undergo renal transplants. The complementary nature of nuclear medicine studies and US in the imaging evaluation of hydronephrosis, renal artery stenosis, flank pain, renal masses, pyelonephritis, and kidney transplant was confirmed in the author's test group.
MRI of the abdomen has evolved into an excellent alternative means for the diagnosis of most renal transplantation complications and for the examination of the living related donor. The contrast agents used for MRI are nontoxic to the transplanted kidney, and MRI often can be used to assess renal function, vascular supply, and postoperative complications. However, MRI remains expensive and may be contraindicated in certain patients. Whenever 2 or more diagnostic techniques are used in the diagnosis of renal transplantation, comparison between the techniques is often very useful. Comparison of recent, current studies with remote, prior examinations is critical. New fluid collections are of greater significance than are slowly resolving fluid collections.
Limitations of techniques
Conventional radiographs provide a limited amount of useful information about the complex clinical presentations of renal transplantation. Often, chest radiographic findings are nonspecific because chronic interstitial pulmonary disease is difficult to differentiate, whereas abdominal radiographs may fail to illustrate fluid collections.
Ultrasound is safe, portable when necessary, and free from radiation. Generally, it should be the first cross-sectional examination in renal transplantation; however, sonograms may fail to differentiate gas from calcifications, and bowel gas may obscure important findings.
CT is not limited by bowel gas; however, the use of IV iodinated contrast material must be limited in many cases. [15]
Radionuclide studies offer important functional information, but the findings are often nonspecific, and the images have relatively poor spatial resolution.
Abdominal and pelvic MRI is valuable. Some patients object to the MRI experience, and others have direct contraindications, such as cardiac pacemakers or cerebral aneurysm clips.
Although Doppler ultrasound and scintigraphy with captopril, when the patient is hypertensive, are helpful, both tests have limitations.
Intervention
Interventional radiology plays an important role in the care and treatment of renal transplant recipients. Historically, catheter angiography has been performed to evaluate the anatomy of the renal arterial and venous systems of potential renal donors. To lower costs and reduce risk, CTA or MRA has largely replaced conventional DSA in the preoperative phase of renal transplantation. However, image-guided interventional techniques play a vital role in the relief of mechanical urinary obstruction, drainage of abscess and cystic fluid collections, and aspiration biopsy of neoplastic and infectious processes.
Radiography
Traditionally, the potential donor was examined with intravenous urography (IVU) and angiography. That no longer occurs. Although a number of pretransplantation urologic evaluations have been recommended in the literature, no one specific universally accepted protocol exists. The expense and potential risk of using IV contrast material should limit duplication of tests. In general, CT of the abdomen and kidneys can be performed at the time of the evaluation of the renal vasculature. If magnetic resonance angiography (MRA) is selected for the renal vascular evaluation, an IVU (as an MR urogram) can follow with sufficient spatial resolution to obviate the need for a radiopaque injection to perform a standard IVU.
(Radiographic images pertaining to kidney transplantation are shown below.)
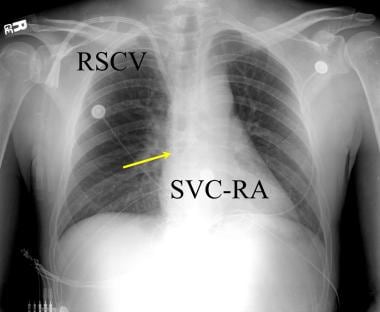 Anteroposterior chest radiograph in a renal transplantation candidate. Chronic central venous line access is common during the long clinical treatment period prior to renal transplantation. Implanted double lumen subclavian vein access lines (RSCV) are most common. Central venous complications should be a clinical consideration in such patients. The catheter (yellow arrow) should extend to the juncture of the superior vena cava-right atrium (SVC-RA).
Anteroposterior chest radiograph in a renal transplantation candidate. Chronic central venous line access is common during the long clinical treatment period prior to renal transplantation. Implanted double lumen subclavian vein access lines (RSCV) are most common. Central venous complications should be a clinical consideration in such patients. The catheter (yellow arrow) should extend to the juncture of the superior vena cava-right atrium (SVC-RA).
 Anteroposterior pelvic radiograph of a renal transplant candidate. Prior to receiving a renal transplant the patient with chronic renal disease must undertake hemodialysis. While most patients are treated using an upper extremity forearm graft, at times indwelling central venous catheters may be necessary (IVC Cath). Metabolic bone disease is very common in the chronically ill pretransplantation patient. A pathological fracture of the left femoral neck was treated in this renal transplant candidate with cannulated femoral neck pins (white arrow).
Anteroposterior pelvic radiograph of a renal transplant candidate. Prior to receiving a renal transplant the patient with chronic renal disease must undertake hemodialysis. While most patients are treated using an upper extremity forearm graft, at times indwelling central venous catheters may be necessary (IVC Cath). Metabolic bone disease is very common in the chronically ill pretransplantation patient. A pathological fracture of the left femoral neck was treated in this renal transplant candidate with cannulated femoral neck pins (white arrow).
 Lateral radiograph of the lumbar spine in renal failure. The effects of chronic renal failure are often seen in renal transplant candidates prior to successful renal transplantation. Note the dense vertebral bodies (black arrow) due to renal osteodystrophy. The aorta and all major arterial branches are densely calcified (white arrow) in this renal transplant candidate.
Lateral radiograph of the lumbar spine in renal failure. The effects of chronic renal failure are often seen in renal transplant candidates prior to successful renal transplantation. Note the dense vertebral bodies (black arrow) due to renal osteodystrophy. The aorta and all major arterial branches are densely calcified (white arrow) in this renal transplant candidate.
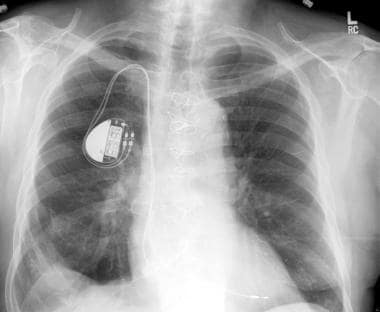 Chest radiograph. Increasing numbers of patients are receiving multiple organ transplants. Primary renal failure or renal failure due to drug toxicity may require a cardiac transplantation patient to require renal transplantation. Following cardiac transplantation chest radiographs are routinely obtained at frequent intervals. This chest radiograph demonstrates a cardiac pacemaker and postoperative findings consistent with heart transplantation. This was a renal transplantation candidate at the time of this chest radiograph.
Chest radiograph. Increasing numbers of patients are receiving multiple organ transplants. Primary renal failure or renal failure due to drug toxicity may require a cardiac transplantation patient to require renal transplantation. Following cardiac transplantation chest radiographs are routinely obtained at frequent intervals. This chest radiograph demonstrates a cardiac pacemaker and postoperative findings consistent with heart transplantation. This was a renal transplantation candidate at the time of this chest radiograph.
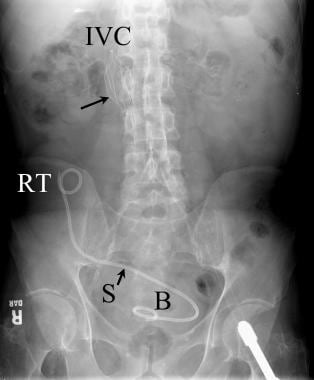 Abdominal radiograph following renal transplantation with ureteral stent placement. The double pigtail stent (S) extends from the renal transplant (RT) to the urinary bladder (B). A filter (long black arrow) is noted in the inferior vena cava (IVC).
Abdominal radiograph following renal transplantation with ureteral stent placement. The double pigtail stent (S) extends from the renal transplant (RT) to the urinary bladder (B). A filter (long black arrow) is noted in the inferior vena cava (IVC).
Degree of confidence
Donor anomalies related to the upper collecting system and duplicated ureters may make the kidney from a living related donor unacceptable. This is readily illustrated on CT and MRI examinations, replacing IVU. To confirm 2 kidneys with no gross abnormality, US can be used as a screening study. Conventional angiography was long considered the criterion standard for the evaluation of the renal arteries and veins of the living related donor candidate. Today, as noted before, CTA (with spiral multidetector and multiplanar reconstruction) and MRA have become the imaging techniques of choice.
Prior to transplantation, if there is the question of recipient kidney size or gross abnormality, US can be performed. If vascular status, severe arteriosclerosis, or venous abnormalities of the pelvic arteries is suspected that might complicate the creation of the renal artery anastomosis, MRA or CTA can be performed. Angioplasty or stent placement may be required with a subsequent interventional procedure.
As with all imaging techniques, anomalies and misleading images can imitate pathologic situations. The radiologist must be aware of absent or duplicated vessels, benign masses (cortical invagination), and physiologic phenomenon (diuresis) that can simulate hydronephrosis.
Computed Tomography
An increased interest in CT imaging has paralleled the improvement in the image quality and speed of the CT examination. With the introduction of spiral CT imaging, both the resolution and speed of the available examinations have improved. [33, 34] Concurrent with improved imaging, the expanded role of interventional radiologic procedures has created an important role for CT imaging in transplant recipients. [35]
Because of its availability, cost-effectiveness, and safety, color Doppler imaging is the first-line imaging modality for graft dysfunction, particularly for hematomas in the immediate postoperative period; the overall incidence of postoperative hematomas is 4-8%. [10] It is also considered a good way to screen for TRAS.
(CT scans associated with kidney transplantation are shown below.)
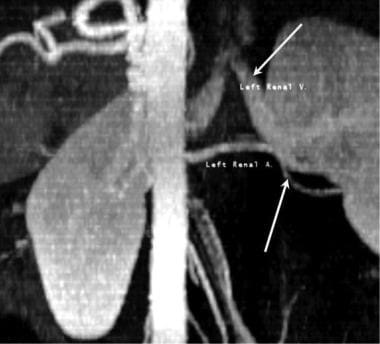 Coronal multiplanar reconstructed image from a renal transplantation donor CT angiography. This coronal reconstructed CT angiogram was obtained in a living related donor. The findings from the preoperative evaluation of the kidneys of a potential donor who is living and related to the recipient must clearly define the anatomic structures of the donor kidney, especially the renal artery (white arrow) and the renal vein (yellow arrow). Underlying diseases of the renal donor can include conditions that might place the potential donor at risk or may make the donor's kidneys unsuitable for transplantation.
Coronal multiplanar reconstructed image from a renal transplantation donor CT angiography. This coronal reconstructed CT angiogram was obtained in a living related donor. The findings from the preoperative evaluation of the kidneys of a potential donor who is living and related to the recipient must clearly define the anatomic structures of the donor kidney, especially the renal artery (white arrow) and the renal vein (yellow arrow). Underlying diseases of the renal donor can include conditions that might place the potential donor at risk or may make the donor's kidneys unsuitable for transplantation.
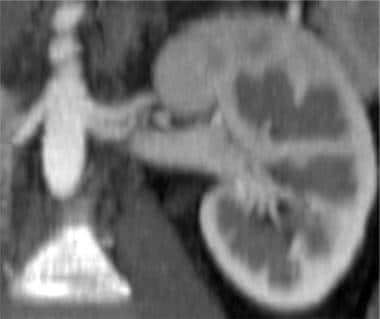
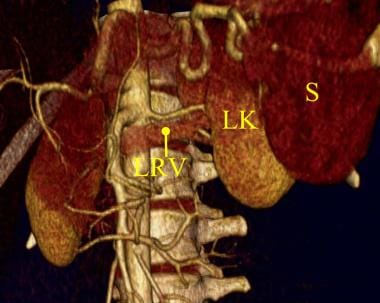 Three-dimensional color-shaded CT angiography of the left kidney. The lower HU density in the left renal vein (LRV) is demonstrated as a color similar to the left kidney (LK) and distinct from the spleen (S). Unfortunately, in most 3-dimensional CT models, there is enough density in the left renal vein to obscure the clear visualization of the left renal artery.
Three-dimensional color-shaded CT angiography of the left kidney. The lower HU density in the left renal vein (LRV) is demonstrated as a color similar to the left kidney (LK) and distinct from the spleen (S). Unfortunately, in most 3-dimensional CT models, there is enough density in the left renal vein to obscure the clear visualization of the left renal artery.
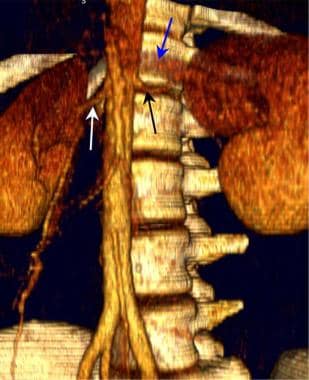 Three-dimensional computed tomographic angiographic (CTA) volume in a renal donor demonstrates single right and left renal arteries. The left renal vein is also depicted.
Three-dimensional computed tomographic angiographic (CTA) volume in a renal donor demonstrates single right and left renal arteries. The left renal vein is also depicted.
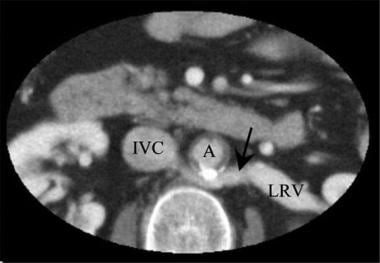 Axial CT scan in a renal transplant donor: Note that the left renal vein (LRV) passes posterior (arrow) to the aorta (A). Inferior vena cava (IVC).
Axial CT scan in a renal transplant donor: Note that the left renal vein (LRV) passes posterior (arrow) to the aorta (A). Inferior vena cava (IVC).
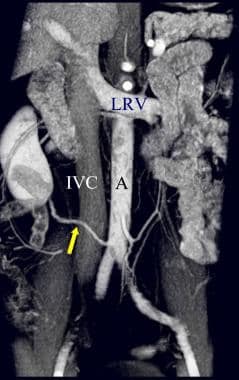
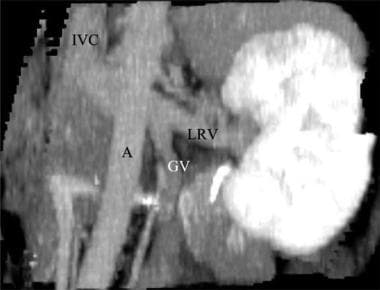 Coronal CT angiogram (CTA) in a potential renal donor: The maximum intensity projection (MIP) image shows an enlarged gonadal vein (GV) related to the left renal vein (LRV).
Coronal CT angiogram (CTA) in a potential renal donor: The maximum intensity projection (MIP) image shows an enlarged gonadal vein (GV) related to the left renal vein (LRV).
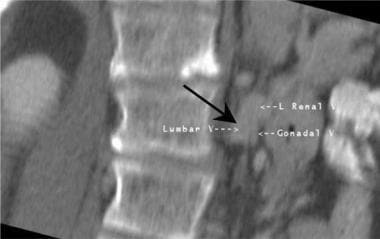 Oblique coronal multiplanar reconstructed image from a CT of the abdomen in kidney transplantation. A large anomalous left lumbar vein is closely positioned near the left renal vein and left ovarian vein in this potential renal donor. The discovery of vascular anomalies prior to nephrectomy helps to avoid surgical complications.
Oblique coronal multiplanar reconstructed image from a CT of the abdomen in kidney transplantation. A large anomalous left lumbar vein is closely positioned near the left renal vein and left ovarian vein in this potential renal donor. The discovery of vascular anomalies prior to nephrectomy helps to avoid surgical complications.
 Coronal multiplanar reconstructed image from a computed tomographic angiogram (CTA) of the kidneys demonstrates two left renal arteries and the left renal vein.
Coronal multiplanar reconstructed image from a computed tomographic angiogram (CTA) of the kidneys demonstrates two left renal arteries and the left renal vein.
 Three-dimensional grayscale volume CT angiography of a potential renal donor. There is one main right renal artery (yellow arrow). Less than two centimeters from the origin of the right renal artery there is a large early branch to the upper pole of the right kidney. A large early branch from the renal artery can result in complications during the donor nephrectomy and during the renal transplantation.
Three-dimensional grayscale volume CT angiography of a potential renal donor. There is one main right renal artery (yellow arrow). Less than two centimeters from the origin of the right renal artery there is a large early branch to the upper pole of the right kidney. A large early branch from the renal artery can result in complications during the donor nephrectomy and during the renal transplantation.
 Three-dimensional (3D) volume CT angiography of a potential renal donor. The use of the entire 3D CT model provides accurate evaluation of this early renal artery branching. The 3D model presents important anatomic correlations with related structures such as renal veins and GI structures.
Three-dimensional (3D) volume CT angiography of a potential renal donor. The use of the entire 3D CT model provides accurate evaluation of this early renal artery branching. The 3D model presents important anatomic correlations with related structures such as renal veins and GI structures.
 Three-dimensional model of CT angiography of a potential renal donor. The examination demonstrates a large accessory renal artery to the right kidney (yellow arrow) that arises from the aorta (A) below the superior mesenteric artery. IVC = inferior vena cava, LRV = left renal vein. Preoperative renal transplant donor evaluation must include angiographic studies to identify low accessory renal arteries.
Three-dimensional model of CT angiography of a potential renal donor. The examination demonstrates a large accessory renal artery to the right kidney (yellow arrow) that arises from the aorta (A) below the superior mesenteric artery. IVC = inferior vena cava, LRV = left renal vein. Preoperative renal transplant donor evaluation must include angiographic studies to identify low accessory renal arteries.
CT of the renal donor may reveal small renal cell cancers, cysts, [29] upper collecting system duplications, and partial renal collecting system obstructions. Particularly important, the CT examination should be performed prior to the IV administration of contrast agent to look for renal stones, as well as immediately after administration of the bolus.
Multidetector CT with 3-dimension reconstruction is the technique of choice in evaluating living kidney donors to identify renal parenchymal disease, arterial and venous anomalies, and collecting system abnormalities. It has been shown to be 93-99% accurate in identifiying accessory renal arteries, prehilar branching, and renal venous anomalies. [10]
MDCT at 1 month postoperatively can provide early detection of asymptomatic lymphocele, wound problems, and hydroureter. Lymphocele usually occurs 2-6 weeks after surgery. [36]
Early postinjection-phase images best demonstrate the renal cortex, while images obtained approximately 40 seconds after injection of the contrast agent bolus best show the combined enhancement within the renal medullary space and the renal pyramids. Nephrogram-phase images have been shown to be most sensitive in the overall detection of renal masses. By reexamining the kidneys, ureters, and bladder after a 2-3-minute delay, accurate evaluation of the renal collecting system, ureters, and urinary bladder is possible.
By carefully reviewing the axial CT images obtained during the first 20-30 seconds after the IV bolus, most renal artery duplications and many renal vein anomalies can be detected. Image collimation of 2-3 mm with an image index of 1-2 mm is necessary to separate small vessels, which are closely positioned. The patient must hold his or her breath during the arterial phase of CT scanning. Special imaging reformatting and reconstruction methods improve the general diagnostic accuracy of renal CT angiography and, in selected cases, the demonstration of small accessory arteries and venous anomalies that otherwise might not be detected. Such 3-dimensional (3D) imaging also provides the surgical team with an excellent overall review of the surgical anatomy.
CT scanning is useful in the evaluation of both immediate and delayed complications of renal transplantation. MDCT urography has been shown to be superior to conventional IVP in the detection of urinary leaks and ureteral obstructions. In the immediate posttransplantation period, perigraft hemorrhage and urinary leaks can be detected with CT. In most cases, either US or functional nuclear medicine imaging should be performed first. CT scanning is the best means for surveying the most common intermediate and delayed surgical complications. CT findings are related to density differences between areas of hemorrhage, lymphoceles, and abscesses. [37, 38]
Degree of confidence
Regarding the donor, after a careful review of the axial CT images with a cine technique on a workstation or picture archiving and communication system (PACS), the evaluation of maximum intensity projection images in multiple projections and variable slab thicknesses is useful. Coronal, curved, multiplanar, reformatted images best outline the full length of each renal artery.
Measurements of the renal arteries are obtained; these include the diameter of the renal artery origins and distance of each renal artery to a major branching point. The presence of fibromuscular hyperplasia or renal artery stenosis due to ASVD is assessed. Volume image sets that are displayed with 3D image rendering is useful in complex cases and in explaining surgical options to the renal transplantation team.
Immediate complications related to recipient renal transplantation is best evaluated with combined or independent renal US, functional renal nuclear medicine study, CT, and occasionally MRI. Acute perirenal collections with a high CT attenuation of 28 Hounsfield units (HU) or greater is most likely perinephric hemorrhage. Perirenal or pararenal fluid collections of 18-24 HU are most likely lymphoceles. Complex fluid collections anywhere in the abdomen or pelvis of more than 28 HU on a delayed basis may be abscess cavities or chronic (liquefying) hematoma. In general, the findings of perirenal transplant complications have a pattern similar to that of diseases of the abdomen in other immunocompromised patients. Multidetector CT urography is more complete and precise compared to more conventional diagnostic techniques. The overall diagnostic accuracy of CT urography has been reported to be 90%.
False positives/negatives
The process of organ transplantation begins with the evaluation of patients with end-stage kidney disease, which may require CT and/or MRI imaging depending on the clinical presentation. Chronic renal failure makes the potential transplant recipient more susceptible to a wide range of neoplasms, including carcinoma of the kidney. The significance of an atypical cyst or a small renal deformity is much greater in the face of chronic renal insufficiency. In a patient with a polycystic kidney, bleeding into a cyst may mimic a small cystic renal cell carcinoma. The bleeding associated with polycystic renal disease is not necessarily related to renal cell carcinoma.
A detailed surgical history is useful in the evaluation of the transplanted kidney. In cases in which native vessels are thrombosed, unusual alternate venous outflow patterns may result. In cases of IVC thrombosis, the transplanted kidney renal vein may drain into the mesenteric veins. This should not be mistaken for a pathologic AVF or other primary venous anomaly.
Surgical complications after pancreas transplantation include difficulties in the management of organ rejection, as well as the potential for pancreatitis with or without ischemic necrosis.
Magnetic Resonance Imaging
MRI is being increasingly used as a second-line imaging modality for the evaluation of kidney transplants. MRA has several advantages compared to conventional digital subtraction angiography (DSA). MRI and MRA are noninvasive and use no ionizing radiation. MRA provides true anatomic images and permits multiplanar reformatting. High-performance MR gradient systems allow the acquisition of 3D volumes in a single breath hold of shorter than 30 seconds. Imaging of the renal arteries is important in the examination of potential renal donors and of transplant patients with suspected renal artery stenosis. [39, 4, 10]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness. The use of MR angiographic techniques that do not require the administration of gadolinium-based contrast agents may allow pretransplantation evaluation and postoperative reevaluation of the renal transplant without the need for gadolinium-based agents. [40, 41, 42]
(See the images below.)
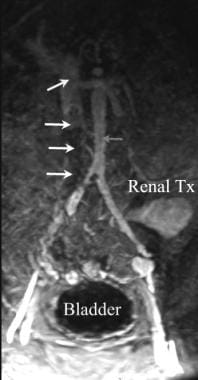 Magnetic resonance angiogram (MRA) in a renal transplant patient with rapidly worsening renal function. Thrombosis of the inferior vena cava (IVC) is present (white arrows) distal to an IVC filter. The proximal IVC remains patent (yellow arrow). The aorta (red arrow) and transplanted renal artery are widely patent.
Magnetic resonance angiogram (MRA) in a renal transplant patient with rapidly worsening renal function. Thrombosis of the inferior vena cava (IVC) is present (white arrows) distal to an IVC filter. The proximal IVC remains patent (yellow arrow). The aorta (red arrow) and transplanted renal artery are widely patent.
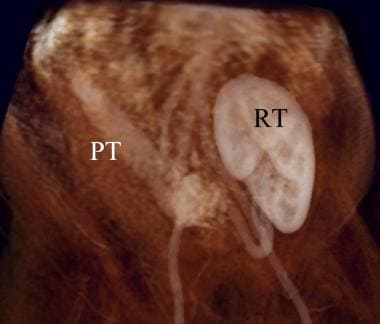 Volume model gadolinium enhanced MR angiography of pancreas-renal transplantation. The pancreas transplant (PT) is noted in the right lower quadrant of the lower abdomen. The relationship with the urinary bladder is not demonstrated on this exam. The renal transplant lies in the left upper pelvis anterior to the iliac artery.
Volume model gadolinium enhanced MR angiography of pancreas-renal transplantation. The pancreas transplant (PT) is noted in the right lower quadrant of the lower abdomen. The relationship with the urinary bladder is not demonstrated on this exam. The renal transplant lies in the left upper pelvis anterior to the iliac artery.
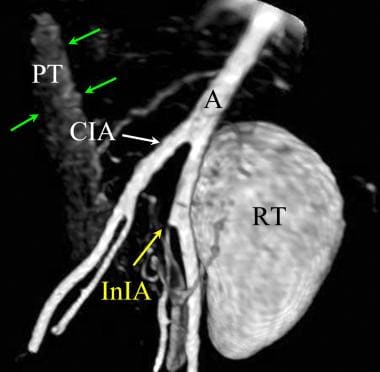 Volume model of a MIP post gadolinium enhanced MR angiography of a pancreas renal transplantation. The renal transplant (RT) is lateral and anterior to the left iliac artery. The pancreatic transplant (PT, green arrows) demonstrates less enhancement on this early arterial phase gadolinium enhanced MR angiography. The origin of the left internal iliac artery is stenotic (white arrow).
Volume model of a MIP post gadolinium enhanced MR angiography of a pancreas renal transplantation. The renal transplant (RT) is lateral and anterior to the left iliac artery. The pancreatic transplant (PT, green arrows) demonstrates less enhancement on this early arterial phase gadolinium enhanced MR angiography. The origin of the left internal iliac artery is stenotic (white arrow).
MRI offers several advantages in the pretransplantation donor and recipient evaluation. MRI offers good spatial resolution with excellent tissue differentiation. MRA with gadolinium enhancement has been improved by the development of fast 3D gradient-echo sequences. The T1 shortening effect of the IV injection of paramagnetic contrast material significantly improves vessel imaging by eliminating signal loss from saturation effects, minimizing dependence of the inflow phenomenon, and possibly decreasing intravoxel dephasing effects.
MRA demonstrates accessory renal arteries using gadolinium agents with the same degree of accuracy as CTA. MRA has been successfully applied to the diagnosis of most of the complications related to acute renal transplant vascular thrombosis. Gadopentetate dimeglumine (DTPA), when administered as an IV bolus during MRA, provides qualitative functional information concerning the renal transplant. It demonstrates the morphology in a manner similar to CT and is generally superior to diagnostic US.
Degree of confidence
MRI has several limitations that should be considered in the choice of MRI, CT, US, or radionuclide-based imaging. The spatial resolution of MRI is generally less than that of CT, while the functional information available is generally qualitative compared to the quantitative data provided by nuclear renal scans. Patients with pacemakers, cochlear implants, most intracranial aneurysm clips, or severe claustrophobia cannot be safely examined using MRI. The use of conscious sedation in mildly claustrophobic patients and young children can be considered in MRI, but this adds to the cost.
The layering of excreted gadolinium into the urinary bladder and ureters generates artifacts that are less easily managed at MRI than at CT. During MRI, changing the patient's position requires additional time in the scanner. In comparison, prone or decubitus positioning at CT allows the mixing of contrast layers, which enables a more complete evaluation of the urinary bladder, including the anterior bladder surfaces. In general, CT of the kidneys and urinary bladder is better in the detection of renal stones and of renal, ureter, and bladder anomalies. CT with CTA generally costs less than MRI. In most applications, MRI is reserved for examination of the transplant recipient, whereas CT and CTA are used in the potential donors.
False positives/negatives
False-positive MRI findings in the transplanted kidney and/or pancreas are commonly related to movement or susceptibility artifacts. Breathing or vascular pulsatile movement may cause a misregistration of the walls of a cyst; this may be mistaken for enhancement related to renal cell carcinoma in the wall of a cyst. Benign fat-containing tissue near the surface of a transplanted kidney or pancreas may have a suspicious appearance if movement occurs during the examination. MRI is insensitive to calcified renal stones or calcifications in the walls of cysts.
False-negative MRI results related to venous anomalies and the detection of incomplete venous thrombosis is more common in MRA than in CTA.
Ultrasonography
According to the American College of Radiology Appropriateness Criteria for Renal Transplant Dysfunction, duplex Doppler ultrasound is the first-line imaging evaluation to assess renal transplant dysfunction. [4] Ultrasound is the most frequent imaging method used in the postoperative period and for long-term follow-up. It is widely available, and the relatively low cost and high degree of safety of ultrasound allow for serial examinations, which may be necessary during the postoperative period. [3, 24, 43, 44]
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) recommends using contrast-enhanced ultrasound (CEUS) to rule out vascular complications, inflammatory complications, and different posttransplantation parenchymal abnormalities, including renal lesions. Grayscale ultrasound with color Doppler analysis is considered the first-line imaging modality for assessing complications, and color Doppler analysis has become the preferred imaging tool to evaluate the graft status within the first 24 hours after transplantation. [45, 46]
Development of lymphoceles is a frequent complication after kidney transplantation, with an incidence of 0.6-33.9%. Ultrasound-guided percutaneous drainage is recommended as the first line of treatment, with a success rate exceeding 50%. [10]
Typical renal transplant sonograms demonstrate the following: arcuate arteries, echo-poor medullary pyramids, hyperechoic renal sinus, absence of hydronephrosis, stable renal volume (formula: 0.5 x length x transverse x anteroposterior dimensions), normal position of renal transplant anterior to the external iliac vessels, and otherwise normal echogenicity in the parenchyma, collecting system, and surrounding tissues.
(See the images below.)
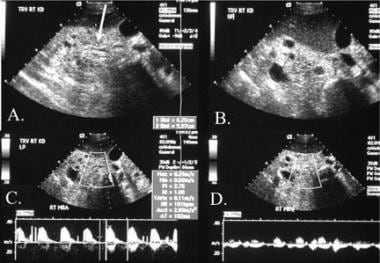
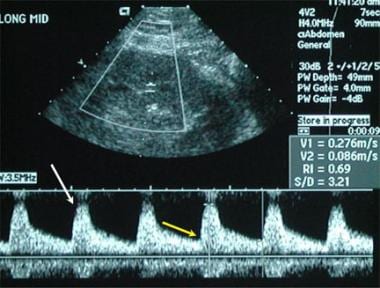 The primary means of imaging a renal transplant is sonography with vascular duplex imaging. A normal resistive index (0.6) in this case is associated with a normal systolic arterial waveform (white arrow) and a normal diastolic flow pattern (yellow arrow).
The primary means of imaging a renal transplant is sonography with vascular duplex imaging. A normal resistive index (0.6) in this case is associated with a normal systolic arterial waveform (white arrow) and a normal diastolic flow pattern (yellow arrow).
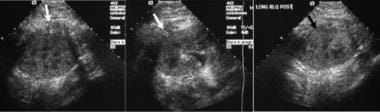 Renal sonography is helpful in localization of a safe pathway to perform renal transplant biopsy (white arrow).
Renal sonography is helpful in localization of a safe pathway to perform renal transplant biopsy (white arrow).
 Following a renal transplant, decreased renal function with limited urinary output was noted. The sonography of the transplant site and the pelvis demonstrates a large hypoechoic mass (double yellow arrows) anterior to the transplant (black arrow). B. The hypoechoic mass extended downward into the anterior thigh (double white arrow). The primary diagnostic considerations should include a hematoma versus a urinary leak.
Following a renal transplant, decreased renal function with limited urinary output was noted. The sonography of the transplant site and the pelvis demonstrates a large hypoechoic mass (double yellow arrows) anterior to the transplant (black arrow). B. The hypoechoic mass extended downward into the anterior thigh (double white arrow). The primary diagnostic considerations should include a hematoma versus a urinary leak.
 Renal sonography of a patient with a renal transplant with failing renal function. The renal transplant has become smaller. The arterial waveform is abnormal with a flat, broad peak while the diastolic waveform is prolonged. The resistive index was elevated (1.00) in this patient. Chronic renal rejection was the diagnosis made by renal biopsy.
Renal sonography of a patient with a renal transplant with failing renal function. The renal transplant has become smaller. The arterial waveform is abnormal with a flat, broad peak while the diastolic waveform is prolonged. The resistive index was elevated (1.00) in this patient. Chronic renal rejection was the diagnosis made by renal biopsy.
The measurement of the vascular resistive index (RI) is a part of the evaluation. The RI measures resistance to arterial flow within the renal vascular bed. RI is calculated from the peak diastolic arterial waveform. The normal RI is less than 0.7-0.8. An RI of greater than 0.9 indicates transplant dysfunction but not the cause. In some instances, a nonfunctioning renal allograft can have "normal" RI and pulsatility index (PI). [47]
In uneventful transplantations, ultrasound is often performed immediately after transfer to the ICU, to exclude early vessel occlusion of the artery and vein, using color and pulse-wave Doppler ultrasonography. The next routine ultrasound is performed on the second postoperative day and then once a week during hospitalization if the clinical course is uneventful. Posttransplantation arterial hypertension is relatively common in children because ofl underlying factors such as primary kidney disease, side effects of immunosuppressive medication, hormonal disturbances, familial disposition, and kidney artery stenosis. [48]
Peritransplant fluid collections
Sonographic evaluation of renal transplant complications is often successful because many of the potential peritransplant complications are associated with fluid collections. The superficial nature of a typical renal transplant allows good resolution with relatively few reverberation echoes. During the immediate postoperative transplant period, most fluid collections in and around the bed of the transplant are hematomas or urinomas. A hyperacute hematoma often presents as a hypoechoic fluid-filled mass near the surgical site. Hematomas may be stable in size but can enlarge rapidly. The enlarging hematoma may compress the kidney, resulting in a reduction of urine output. Later, a hematoma becomes complex with both hypoechoic and echoic areas.
A second possible cause for fluid around the transplant during the first 24 hours following transplantation is a urinoma. A urinoma results from a leak between the renal transplant ureter and the recipient's bladder. Such a leak may be the result of surgical failure or from necrosis of the distal portion of the transplant ureter. A urinoma enlarges more slowly and remains hypoechoic. If the urinoma becomes infected the quality of the fluid may become more complex as an abscess forms. After several weeks, a spontaneous hematoma or a urinoma becomes less likely.
A lymphocele may form anywhere in the general area of the renal transplant. While lymphoceles are more likely to occur soon after the transplant, they may develop long after the skin wound has healed. An uncomplicated lymphocele is hypoechoic and remains hypoechoic unless it becomes infected. While lymphoceles are slow to develop, a lymphocele can create significant pressure resulting in urinary or venous obstruction.
An abscess may develop at any time during the life history of a renal transplant recipient. Abscesses may be around the kidney, within the transplant, or in the abdominal cavity remote from the transplant. Most abscesses have complex sonographic qualities. An abscess can be diagnosed best by comparing the sonographic texture of a fluid collection to the white blood cell count and the patient's fever curve. In cases in which an abscess is considered likely, sonographically guided aspiration is recommended. Diagnostic ultrasound may both initially diagnose the fluid mass and guide the surgical or radiologically based drainage. If percutaneous drainage results in a recurrence of a lymphocele, laparoscopic drainage is indicated.
Acute kidney transplant rejection
Sonographic findings include globular enlargement of the kidney, swelling and hypoechogenicity of the medullary pyramids, an indistinct cortical-medullary junction, and hypoechoic foci in the renal cortex. [49] Among the early findings of acute renal transplant rejection is renal enlargement. The length of a renal transplant should not increase from the stabilized posttransplantation measurements.
Although acute transplant rejection must be considered in all cases of declining renal function after initially successful renal transplantation, other acute events may occur in the immediate postoperative period or later clinical follow-up. Perinephric hematoma and acute hydronephrosis are among the complications than can result in the acute loss of kidney function.
Perinephric hematoma appears as a peripheral, mixed, hypoechoic rim or ring surrounding the kidney. The bleeding may proceed away from the kidney. The extent of the bleeding may be difficult to evaluate at US because of overlying bowel gas. Acute or chronic hydronephrosis has a presentation that is generally similar to hydronephrosis in a native kidney. The central collecting system may be dilated and filled with hypoechoic fluid (urine), and a variable degree of dilatation is generally preset in the renal collecting system. Several diseases may affect the transplanted kidney simultaneously. Mild tissue rejection may cause transplant edema, and a mechanical obstruction may cause hydronephrosis.
Comparison with prior sonographic studies and communication between the radiologist and other members of the transplantation team are critical. Correlation with findings from functional examinations, including nuclear medicine study and dynamic MRA, should be performed whenever US cannot confirm the cause of the decreased renal function. Changes in native kidneys of renal transplant patients should include a survey to detect masses. Cysts frequently develop in the kidneys of patients with ESRD. New patterns of internal echoes or rapid enlargement should be noted. Correlation with CT, MRI, or aspiration biopsy results may be necessary to exclude carcinoma or other pathology. [50]
Degree of confidence
If prior US results are compared and correlated with the clinical findings and if appropriate renal transplantation team members are consulted, US evaluation of renal transplants is highly accurate and clinically useful. The physical limitations of diagnostic US apply in transplantation as well.
Fluid within the peritoneal space is not a specific finding. Hypoechoic abdominal fluid may remain after peritoneal dialysis. Urine, bowel content, sepsis, and hemorrhage may have variable sonographic appearances. Recent collections should be aspirated for diagnosis by US guidance. Large fluid collections may be drained for symptomatic relief.
Promising results have been reported for contrast-enhanced ultrasound (CEUS) to diagnose vascular rejection in kidney transplant patients. In a study of 57 renal transplant patients, CEUS had a sensitivity of 85.7%, a specificity of 100%, a positive predictive value (PPV) of 100%, and a negative predictive value (NPV) of 98.0%. [51]
False positives/negatives
False-positive US results may occur if a full understanding of the surgical history of the patient is not available. In double (pediatric) renal transplantation, one of the kidneys may be much smaller than the other. The smaller kidney may be mistaken for a mass. Patients may undergo more than one renal transplant. The contralateral, or prior, transplant is generally smaller and can be mistaken for a mass.
Other false-positive results relate to the interpretation of duplex findings of renal artery stenosis and elevated RI. Tortuous renal arteries may suggest renal artery stenosis in the absence of hemodynamically significant narrowing. Increases in the RI should be interpreted carefully. Variations in the location of the sampled arterial signal may suggest elevation of the RI. Whenever possible, examinations should be performed in a standard way using a departmental protocol.
False-negative US results are related to the limitations of the technique. Gas within the vascular or ductal systems may be interpreted as calcifications. Aneurysms and venous varices may be mistaken for lymphoceles unless duplex signals are routinely assessed. The degree of hydronephrosis may be underestimated in a patient with acute transplant rejection. Comparison with prior findings and correlation with the results of other examinations help to prevent most false-negative errors.
Nuclear Imaging
Nuclear medicine studies have a role in all stages of postrenal transplantation evaluation. Immediately after surgery, nuclear medicine studies can be used to evaluate diminished renal function. The most immediate cause of renal transplant failure may be renal artery occlusion.
During the period immediately after the bolus IV injection of the technetium-99m mercaptotriglycylglycine (MAG3) agent, both images and activity counts are collected from the area of the abdominal aorta and the renal transplant site. Visualization of the renal artery and the renal transplant should occur within 20-30 seconds, with no significant difference between the time of aortic and renal visualization (see the images below).
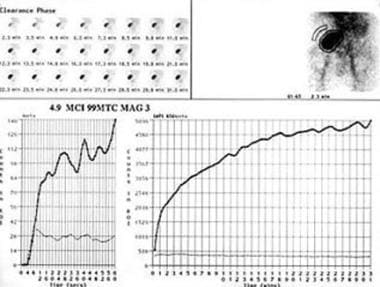 The nuclear renal scan of a hypoechoic perirenal mass demonstrates delayed function with preserved perfusion. There is no evidence of a urinary leak. This confirms that the perirenal mass is a hematoma or a lymphocele. The mass effect was confirmed to be a hematoma, which compressed the kidney resulting in decreased renal function.
The nuclear renal scan of a hypoechoic perirenal mass demonstrates delayed function with preserved perfusion. There is no evidence of a urinary leak. This confirms that the perirenal mass is a hematoma or a lymphocele. The mass effect was confirmed to be a hematoma, which compressed the kidney resulting in decreased renal function.
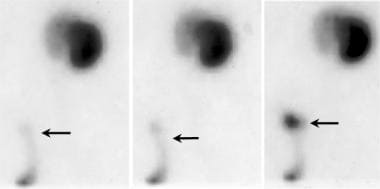 Posterior view Tc 99m MAG3 nuclear scan demonstrates an accumulation of activity outside the distal ureter or bladder (arrow).
Posterior view Tc 99m MAG3 nuclear scan demonstrates an accumulation of activity outside the distal ureter or bladder (arrow).
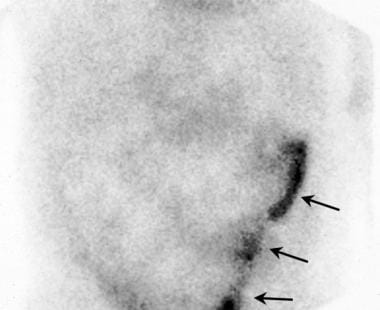 After a 2-hour delay, this anterior scan demonstrates persistent radionuclide activity in the lateral aspect of the lower pelvis. The distal ureter of the transplanted kidney was found to be infarcted, and it was leaking urine (arrows). After a repair of the ureter-bladder anastomosis, the renal graft functioned normally.
After a 2-hour delay, this anterior scan demonstrates persistent radionuclide activity in the lateral aspect of the lower pelvis. The distal ureter of the transplanted kidney was found to be infarcted, and it was leaking urine (arrows). After a repair of the ureter-bladder anastomosis, the renal graft functioned normally.
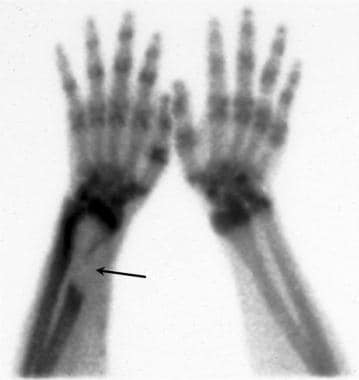 Technetium Tc 99m bone scan demonstrates a large area of decreased uptake consistent with a purely lytic bone tumor in the distal radius (arrow) in a patient with a long-standing vascular access graft in the same forearm. The lesion was shown to be sarcoma.
Technetium Tc 99m bone scan demonstrates a large area of decreased uptake consistent with a purely lytic bone tumor in the distal radius (arrow) in a patient with a long-standing vascular access graft in the same forearm. The lesion was shown to be sarcoma.
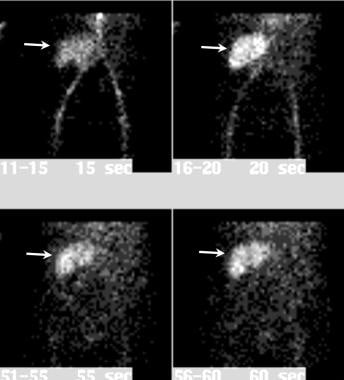 Dynamic nuclear renal flow study. Selected images from a dynamic arterial phase nuclear renal flow study demonstrates adequate arterial flow to the renal transplant (arrows).
Dynamic nuclear renal flow study. Selected images from a dynamic arterial phase nuclear renal flow study demonstrates adequate arterial flow to the renal transplant (arrows).
The causes of diminished renal function include ATN, rejection, and drug nephrotoxicity. ATN is the most common cause of delayed graft function. ATN is associated with prolonged ischemia and reperfusion injury. ATN occurs after placement of most cadaveric grafts. Recovery is usually spontaneous within 2 weeks. ATN is less common in patients whose transplants are from living related donors.
Functional activity of the renal transplant is assessed using activity curves obtained from the renal transplant, aorta, and background. Orthoiodohippurate-131 and technetium-99m MAG3 studies demonstrate a delayed transit with a delayed time to maximum activity and a high ratio of findings at 20 minutes versus those at 3 minutes. Mechanical leaks from the renal pelvis, ureter, or urinary bladder can be detected as extrarenal nuclide collections.
Renal scintigraphy with either technetium-99 m diethylene-triamine-pentaacetate (Tc-99m DTPA) or technetium-99 m mercaptoacetyltriglycine (Tc-99m MAG3) have both been used to evaluate early allograft function after kidney transplantation. A study by Theerakulpisut et al found that Tc-99m MAG3 had superior predictiveness over Tc-99 m DTPA in time to peak, 20-min to peak ratio, and 20-min to 3-min ratio. In addition, acute tubular necrosis was better predicted by Tc-99m MAG3. [52]
Degree of confidence
Although complete thrombosis and renal graft infarction are easily determined with nuclide studies, moderately severe transplant renal artery stenosis may not have a clear pattern on a renal scan. In dual (pediatric) renal transplants, the delay in the visualization of one of the kidneys may indicate partial thrombosis or stenosis of the arterial supply to that kidney. However, in most cases, only a single renal transplant is present, and comparing its activity with that of the other kidney is not possible. The degree of renal dysfunction must be compared with the known surgical history and the current laboratory value.
Renal scans and renograms of transplanted kidneys are subject to artifacts similar to those of other nuclear medicine tests of the abdomen and pelvis. The presence of barium used for other imaging tests may cause artifacts on frontal views.
Angiography
Conventional angiography has been replaced by CTA or MRA in many diagnostic applications both in the pretransplantation and posttransplantation periods. Cost, potential morbidity, and possible nephrotoxic effects of iodinated contrast must be considered. In most cases, digital subtraction angiography (DSA) provides the most fully diagnostic evaluation of the renal artery, the recipient's vascular status, and the patency of the renal veins. CTA can show detailed anatomic structures before percutaneous angiography. [4]
In select cases, renal transplant angiography may be preferred to CTA or MRA. In all cases requiring vascular intervention, DSA angiography immediately precedes the interventional procedure. The potential diagnoses best made with angiography include renal artery stenosis, renal vein thrombosis, aneurysms, and arteriovenous fistula. Except for a pelvic location, the diagnosis of renal transplant circulation disease is similar to the diagnosis of renal artery disease in the native kidney.
Angiography is used for treatment of complications such as renal artery stenosis (RAS), pseudoaneurysm (PSA), and arteriovenous fistula (AVF). [4]
Degree of confidence
Direct visualization of the lumen of a blood vessel is considered the most accurate means to determine the presence of arterial stenosis, venous thrombosis, and arteriovenous malformation (AVM) or arteriovenous fistula (AVF). Because angiography is generally performed just before therapy, other testing typically is not performed to confirm a catheter-proven lesion. Vascular flow gradients can be assessed at the time of the procedure to confirm a hemodynamically important lesion prior to treatment and to confirm the absence of a gradient after angioplasty with or without stent placement.
False-positive findings related to renal artery or iliac artery stenosis involve an overestimation of the degree of stenosis. Measurements made from 2 or more projections help to reduce errors. Direct gradient determination remains an important procedure.
Both false-positive and false-negative results can occur as a result of movement (breathing) during the angiography. DSA is particularly sensitive to misregistration artifacts. Barium in the GI tract from other tests must be avoided.
-
Anteroposterior chest radiograph in a renal transplantation candidate. Chronic central venous line access is common during the long clinical treatment period prior to renal transplantation. Implanted double lumen subclavian vein access lines (RSCV) are most common. Central venous complications should be a clinical consideration in such patients. The catheter (yellow arrow) should extend to the juncture of the superior vena cava-right atrium (SVC-RA).
-
Anteroposterior pelvic radiograph of a renal transplant candidate. Prior to receiving a renal transplant the patient with chronic renal disease must undertake hemodialysis. While most patients are treated using an upper extremity forearm graft, at times indwelling central venous catheters may be necessary (IVC Cath). Metabolic bone disease is very common in the chronically ill pretransplantation patient. A pathological fracture of the left femoral neck was treated in this renal transplant candidate with cannulated femoral neck pins (white arrow).
-
Lateral radiograph of the lumbar spine in renal failure. The effects of chronic renal failure are often seen in renal transplant candidates prior to successful renal transplantation. Note the dense vertebral bodies (black arrow) due to renal osteodystrophy. The aorta and all major arterial branches are densely calcified (white arrow) in this renal transplant candidate.
-
Chest radiograph. Increasing numbers of patients are receiving multiple organ transplants. Primary renal failure or renal failure due to drug toxicity may require a cardiac transplantation patient to require renal transplantation. Following cardiac transplantation chest radiographs are routinely obtained at frequent intervals. This chest radiograph demonstrates a cardiac pacemaker and postoperative findings consistent with heart transplantation. This was a renal transplantation candidate at the time of this chest radiograph.
-
Drawing of typical renal transplantation anatomy. This drawing illustrates the anatomical position of a typical renal transplantation. Blue arrow indicates allograft renal vein to the recipient iliac vein; red arrow, hypogastric artery to allograft renal artery; black arrow, ureter.
-
Coronal multiplanar reconstructed image from a renal transplantation donor CT angiography. This coronal reconstructed CT angiogram was obtained in a living related donor. The findings from the preoperative evaluation of the kidneys of a potential donor who is living and related to the recipient must clearly define the anatomic structures of the donor kidney, especially the renal artery (white arrow) and the renal vein (yellow arrow). Underlying diseases of the renal donor can include conditions that might place the potential donor at risk or may make the donor's kidneys unsuitable for transplantation.
-
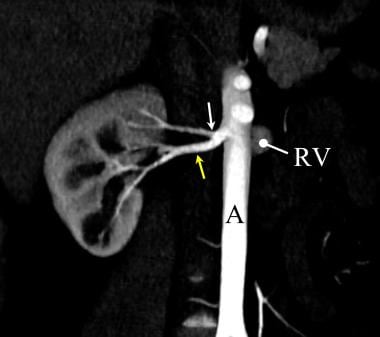
Coronal multiplanar reconstructed (MPR) image of a potential renal donor. This coronal maximum intensity projection (MIP) image from a renal donor computed tomographic angiogram (CTA) demonstrates single right and the single left renal arteries (black arrows). The left renal vein is also well seen (RV). The use of thin section MPR image more clearly demonstrates underlying structures in renal CT angiography.
-
Three-dimensional color-shaded CT angiography of the left kidney. The lower HU density in the left renal vein (LRV) is demonstrated as a color similar to the left kidney (LK) and distinct from the spleen (S). Unfortunately, in most 3-dimensional CT models, there is enough density in the left renal vein to obscure the clear visualization of the left renal artery.
-
Three-dimensional computed tomographic angiographic (CTA) volume in a renal donor demonstrates single right and left renal arteries. The left renal vein is also depicted.
-
Spoiled gradient (SPGR) contrast-enhanced magnetic resonance angiogram (MRA) of the renal arteries demonstrates single main renal arteries on the right and left.
-
Volume-rendered magnetic resonance angiogram (MRA) of renal arteries. One of the principal advantages of studying a volume image is the ability to rotate the image into any projection to evaluate small-branch renal arteries. Note the small branch of the superior mesenteric artery (SMA), with minimal opacification that might otherwise be mistaken for an accessory renal artery.
-
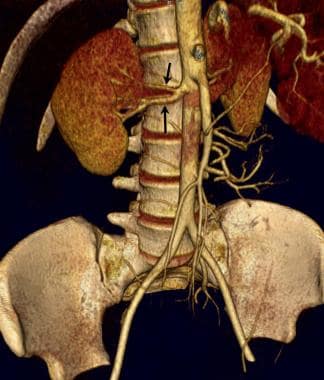
The left kidney is preferred for renal transplantation primarily because of the longer length of its vascular pedicle. Note the full length of the left renal vein compared with the right renal vein. (A, aorta, IVC, inferior vena cava.)
-
Axial CT scan in a renal transplant donor: Note that the left renal vein (LRV) passes posterior (arrow) to the aorta (A). Inferior vena cava (IVC).
-
Coronal CT angiogram (CTA) in a potential renal donor: The maximum intensity projection (MIP) image shows an enlarged gonadal vein (GV) related to the left renal vein (LRV).
-
Oblique coronal multiplanar reconstructed image from a CT of the abdomen in kidney transplantation. A large anomalous left lumbar vein is closely positioned near the left renal vein and left ovarian vein in this potential renal donor. The discovery of vascular anomalies prior to nephrectomy helps to avoid surgical complications.
-
Coronal multiplanar reconstructed image from a computed tomographic angiogram (CTA) of the kidneys demonstrates two left renal arteries and the left renal vein.
-
Computed tomographic angiogram (CTA) in a renal donor demonstrates two left renal arteries with the origins within a few millimeters.
-
Three-dimensional grayscale volume CT angiography of a potential renal donor. There is one main right renal artery (yellow arrow). Less than two centimeters from the origin of the right renal artery there is a large early branch to the upper pole of the right kidney. A large early branch from the renal artery can result in complications during the donor nephrectomy and during the renal transplantation.
-
Three-dimensional (3D) volume CT angiography of a potential renal donor. The use of the entire 3D CT model provides accurate evaluation of this early renal artery branching. The 3D model presents important anatomic correlations with related structures such as renal veins and GI structures.
-
Coronal volume section of a 3-dimensional (3D) CT angiography of a potential renal donor. By examination of the entire 3D model in sections that are 10 mm in thickness, the 2 left renal arteries (white arrows) are clearly seen from the aorta (A) to the left kidney (LK).
-
Three-dimensional volume image of a computed tomographic angiogram (CTA) in a renal donor demonstrates two left renal arteries with nearly common origins. The left renal vein (LRV) is noted in relationship to the left renal arteries (arrows).
-
Three-dimensional model of CT angiography of a potential renal donor. The examination demonstrates a large accessory renal artery to the right kidney (yellow arrow) that arises from the aorta (A) below the superior mesenteric artery. IVC = inferior vena cava, LRV = left renal vein. Preoperative renal transplant donor evaluation must include angiographic studies to identify low accessory renal arteries.
-
Coronal slab through a 3-dimensional maximum intensity projection model of the CT angiography of a potential renal donor. Three renal arteries are demonstrated toward the right kidney with two left renal arteries noted (yellow arrows). A third artery on the left, was shown to be the inferior mesenteric artery (IMA).
-
Axial CT of the pelvis in a potential renal transplant patient. A previous CT of the pelvis had demonstrated a large ovarian cyst, which prevented renal transplantation. Immediately after the surgical removal of an ovarian cyst from the left pelvis, a large hematoma (arrow) prevents planned renal transplantation. Planning the surgical preparation of a recipient site long before the scheduled renal transplantation is important.
-
Polycystic liver disease (arrows) may complicate surgical management by enlarging the intra-abdominal contents. Liver failure rarely occurs but the cysts may bleed, causing pain.
-
Coronal CT scans of the abdomen and pelvis in a renal transplant patient. Note the large native kidneys due to polycystic renal disease (white arrows). The mass in the liver was not cystic. A biopsy of the hepatic mass was lymphoma.
-
Chest CT angiography anteroposterior oblique view of the anterior chest wall. Varices of the anterior chest wall are noted immediately following the injection of IV contrast in the left cephalic vein.
-
Coronal multiplanar reconstructed view of CT angiography of the chest in a renal transplantation candidate. The left subclavian vein (LSCV) is dilated. The superior vena cava is stenotic just above the level of the right atrium.
-
CT image of unusual venous collaterals (white arrows) due to chronic inferior vena cava (IVC) thrombosis (black arrow) in a patient before renal transplantation.
-
Large subcutaneous collaterals (arrows) are noted on this coronal 3-dimensional renal computed tomographic angiogram (CTA) in a renal transplant candidate prior to transplantation. The patient had long-standing inferior vena cava (IVC) thrombosis.
-
Magnetic resonance angiogram (MRA) in a renal transplant patient with rapidly worsening renal function. Thrombosis of the inferior vena cava (IVC) is present (white arrows) distal to an IVC filter. The proximal IVC remains patent (yellow arrow). The aorta (red arrow) and transplanted renal artery are widely patent.
-
The primary means of imaging a renal transplant is sonography with vascular duplex imaging. A normal resistive index (0.6) in this case is associated with a normal systolic arterial waveform (white arrow) and a normal diastolic flow pattern (yellow arrow).
-
In cases of mild renal failure following renal transplantation, the primary considerations are acute renal rejection versus renal obstruction. The renal scan in this patient demonstrates renal arterial flow to the transplant with no signs of renal obstruction. The delayed and decreased renal function is consistent with mild renal rejection, which was proven by renal biopsy.
-
Renal sonography is helpful in localization of a safe pathway to perform renal transplant biopsy (white arrow).
-
Following a renal transplant, decreased renal function with limited urinary output was noted. The sonography of the transplant site and the pelvis demonstrates a large hypoechoic mass (double yellow arrows) anterior to the transplant (black arrow). B. The hypoechoic mass extended downward into the anterior thigh (double white arrow). The primary diagnostic considerations should include a hematoma versus a urinary leak.
-
The nuclear renal scan of a hypoechoic perirenal mass demonstrates delayed function with preserved perfusion. There is no evidence of a urinary leak. This confirms that the perirenal mass is a hematoma or a lymphocele. The mass effect was confirmed to be a hematoma, which compressed the kidney resulting in decreased renal function.
-
Axial CT of the pelvis demonstrates a large hematoma (double arrow), which developed during the postoperative period following renal transplantation.
-
A large hematoma (double arrow) has tracked downward into the pelvis during the postoperative period after renal transplantation. Such collections may obstruct the ureter near the bladder.
-
This renal sonogram demonstrates a mild pattern of hydronephrosis (arrow). Progression of hydronephrotic obstruction can be a cause of renal transplant failure and may require surgical revision or placement of a urinary stent.
-
Posterior view Tc 99m MAG3 nuclear scan demonstrates an accumulation of activity outside the distal ureter or bladder (arrow).
-
After a 2-hour delay, this anterior scan demonstrates persistent radionuclide activity in the lateral aspect of the lower pelvis. The distal ureter of the transplanted kidney was found to be infarcted, and it was leaking urine (arrows). After a repair of the ureter-bladder anastomosis, the renal graft functioned normally.
-
Cystogram in renal-pancreas transplantation. Contrast has been introduced into the urinary bladder (B) via a Foley catheter. Reflux of the contrast outlines the renal transplant (RT). The position of the pancreatic transplant is noted (PT) in relationship to the donor duodenum (Duo). Extravasation of contrast (white arrow) indicates a fistula related to the pancreatic transplant.
-
Post drainage cystogram in renal-pancreas transplantation. Contrast has been drained from the urinary bladder (B) via a Foley catheter. The stent within the renal transplant ureter is seen. The position of the pancreatic transplant is noted (Panc) in relationship to the donor duodenum. Extravasation of contrast (black arrow) indicates a fistula related to the pancreatic transplant.
-
Renal sonography of a patient with a renal transplant with failing renal function. The renal transplant has become smaller. The arterial waveform is abnormal with a flat, broad peak while the diastolic waveform is prolonged. The resistive index was elevated (1.00) in this patient. Chronic renal rejection was the diagnosis made by renal biopsy.
-
Axial CT image in a patient with a double kidney (pediatric) renal transplant (arrows) with a recent obstruction in both of the transplanted kidneys due to a fluid collection located medial to both kidneys near the urinary bladder.
-
Coronal T1-weighted MRI of a renal transplant within the right pelvis. The normal pelvic fat (arrows) helps to outline this healthy renal transplant.
-
CT scan of a renal transplant obtained after the oral and intravenous administration of contrast material. Note the uniform enhancement of the renal cortex of the transplant (white arrow). Metal clips (yellow arrow) indicate the vascular anastomosis.
-
Delayed axial CT scan in a renal transplantation case demonstrates mild hydronephrosis (arrows).
-
Coronal T1-weighted, fat-suppressed magnetic resonance angiogram (MRA) obtained after a 2-minute delay shows uniform enhancement of the renal transplant (yellow arrow). Both the iliac artery and the iliac veins are enhanced (white arrow).
-
Axial CT images show small kidneys in end-stage renal disease (left image, arrows). The transplanted kidney (right, arrow) has a poor nephrogram, which is consistent with chronic renal rejection.
-
Axial abdominal CT of a patient with a childhood renal transplant. An abscess (A) is noted in the left psoas muscle. Fluid is noted within the abdominal cavity along the liver edge (white arrows). The abscess cavity contained histoplasmosis.
-
Axial CT of the upper abdomen in a patient with a long history of renal transplantation. Calcified mesenteric lymph nodes are noted (white arrows) anterior to the liver (L) and near the pancreas. The patient has developed cirrhosis complicated by ascites (A) and splenomegaly (S).
-
CT of the chest in a patient with a long history of renal transplantation. Large masses are noted within the mediastinum (LN). The partially calcified lymph nodes represent stable, calcified adenopathy due to histoplasmosis.
-
Axial CT image of the transplanted kidney. The irregular margins (arrows) of the solid mass (RC) are consistent with a tumor. The mass was removed and was determined to be a renal cell carcinoma within the renal transplant.
-
Oblique coronal 3D CT reconstruction of the transplanted kidney. The irregular margins (arrows) of the solid mass (RC) are consistent with a tumor. The mass was removed and was determined to be a renal cell carcinoma within the renal transplant.
-
Axial CT of renal transplant. Following surgical resection of carcinoma that developed over time within the renal transplant, a defect is noted within the anterior medial aspect of the transplant (arrow). A Spigelian hernia (SH) is noted in the surgical site. A Spigelian herniation represents a defect in a weak point along the lateral boarder of the abdominal musculature
-
Because renal cysts related to chronic renal failure (arrow) may evolve into carcinoma, nephrectomy of one or both native kidneys may be necessary before or after renal transplantation.
-
Precontrast CT attenuation (left image, arrow) increased to more than 70 Hounsfield units after the intravenous administration of contrast material (right image, arrow). Biopsy findings in the mass indicated carcinoma of the native kidney.
-
Aspiration biopsy findings in the calcified renal cyst resulted in a diagnosis of papillary form renal cell carcinoma. Radiographic changes in renal cysts should be carefully followed up in transplantation patients.
-
Right renal transplant (yellow arrow) in a patient with lymphoma of the small intestine (white arrow). Note the free fluid in the left lateral abdominal cavity (black arrow); this finding demonstrates a perforation.
-
Among the causes of pelvic pain after a renal transplant, neoplasms must be considered. Several years after renal transplantation, this patient noticed a mass in the lower right abdominal and pelvic area.
-
Biopsy findings in the mass (arrows) revealed anaplastic carcinoma of the bladder. Malignant neoplasms of the genitourinary (GU) tract are more common in renal transplant recipients than in others.
-
Image shows the distal radius in a transplant patient with a long prior history of renal dialysis. Lytic defects within the distal radius are consistent with a neoplasm.
-
Technetium Tc 99m bone scan demonstrates a large area of decreased uptake consistent with a purely lytic bone tumor in the distal radius (arrow) in a patient with a long-standing vascular access graft in the same forearm. The lesion was shown to be sarcoma.
-
CT scan of the chest demonstrates a small peripheral lung nodule (ring) consistent with metastatic angioblastic sarcoma from the distal radius.
-
Volume model gadolinium enhanced MR angiography of pancreas-renal transplantation. The pancreas transplant (PT) is noted in the right lower quadrant of the lower abdomen. The relationship with the urinary bladder is not demonstrated on this exam. The renal transplant lies in the left upper pelvis anterior to the iliac artery.
-
Longitudinal projection of a sonography of a pancreatic transplant. The pancreatic transplant (PT) is hypoechoic with surrounding edema consistent with pancreatitis. The position of a sonographically guided biopsy of the pancreatic transplant has been marked (yellow).
-
Recent renal transplant (short white arrow) and pancreatic transplant (long white arrow) both demonstrate enhancement after the intravenous administration of contrast material.
-
Pelvic CT of pancreas renal transplantation. A small volume of free fluid is noted anterior to the pancreatic transplant (yellow arrow). Oral contrast was given to identify the distal small bowel (TI). IV contrast was given due to decreased renal function of the kidney transplant (KT). The kidney transplant is mildly hydronephrotic (white arrow).
-
Axial CT of the pelvis in a patient with a pancreas renal transplant. The pancreatic transplant (PT) contains a small pseudocyst (white arrow) following an episode of pancreatitis. Note the relationship of the pancreas transplant to the urinary bladder (UB).
-
After resolution of pancreatitis, the hydronephrosis of the renal transplant has resolved.
-
Sonogram and duplex image of an arteriovenous fistula (AVF) caused by biopsy of a renal transplant. Note the high-velocity flow pattern.
-
Duplex renal sonography of renal transplant kidney. The high-velocity flow pattern of an arteriovenous fistula (AVF) is demonstrated which resulted from a biopsy of the renal transplant.
-
Volume model of a MIP post gadolinium enhanced MR angiography of a pancreas renal transplantation. The renal transplant (RT) is lateral and anterior to the left iliac artery. The pancreatic transplant (PT, green arrows) demonstrates less enhancement on this early arterial phase gadolinium enhanced MR angiography. The origin of the left internal iliac artery is stenotic (white arrow).
-
Loculated fluid collection (yellow arrow) results in hydronephrotic obstruction in a patient with a double (pediatric) renal transplant (white arrows).
-
Image shows CT-guided aspiration of a loculated fluid collection in a patient with a double renal transplant. With CT localization, fluid was aspirated without incident.
-
Image shows CT-guided drainage of a lymphocele. The obstruction of the double renal transplant was relieved with the placement of a pigtail catheter. Clear yellowish fluid consistent with a lymphocele was recovered.
-
Delayed image demonstrates the small bowel and colon contained within an abdominal wall hernia. The interposition of colon or small bowel is a consideration in renal transplant biopsy.
-
CT of the upper pelvis in a renal transplant. The renal transplant (RT) is in a normal position in the upper right pelvis. Omental fat has herniated through a weakness in the anterior abdominal wall (arrow). An incarcerated loop of small intestine (SI) is noted anterior to the abdominal wall.
-
Axial CT of renal transplant. Following surgical resection of carcinoma that developed over time within the renal transplant, a defect in the lateral wall of the lower abdominal wall (white arrow) has resulted in a Spigelian hernia. The Spigelian hernia represents a defect in a weak point along the lateral boarder of the rectus muscle.
-
Abdominal radiograph following renal transplantation with ureteral stent placement. The double pigtail stent (S) extends from the renal transplant (RT) to the urinary bladder (B). A filter (long black arrow) is noted in the inferior vena cava (IVC).
-
Dynamic nuclear renal flow study. Selected images from a dynamic arterial phase nuclear renal flow study demonstrates adequate arterial flow to the renal transplant (arrows).
-
CT of the upper pelvis in a case of renal transplantation. The renal transplant (RT) demonstrates moderate hydronephrosis (double arrows). The patient was known to have a double pigtail stent from the transplant to the urinary bladder, which was thought to be in satisfactory position.
-
Axial CT of the midpelvis in a case of renal transplantation. A low attenuation fluid collection is noted anterior and lateral to the pigtail stent (white arrow). The collection (U) was proven to be a urinoma. The stent was successfully revised with relief from the hydronephrosis, which was related to the urinoma.
-
Patients with ESRD have a significant risk for renal cell carcinoma in the cortical cysts that develop in the native kidneys.
-
A recent renal transplant may fail because of a vascular or urologic complication. However, most transient transplant failures are related to ATN.








