Practice Essentials
Histoplasmosis is the most common endemic mycosis in North America, Central America, and many areas of South America; it also occurs in China, India, Southeast Asia, Africa, Australia, and Europe. Histoplasmosis is a major cause of morbidity and mortality in patients living in endemic areas. [1] Histoplasma capsulatum was first described in 1905 by Samuel Darling, a US Army pathologist stationed in Panama. Darling examined visceral tissues and bone marrow from a young man from Martinique whose death originally was attributed to miliary tuberculosis. The organism initially was described as protozoal. Because it lacked a kinetoplast, Darling assumed that it was not a member of Leishmania. He called the microbe H capsulatum. [2, 3, 4] In 1912, after reviewing tissue specimens, da Rocha-Lima suggested that the organism resembled a yeast rather than a protozoan. H capsulatum has since been found worldwide. In the United States, histoplasmosis is highly prevalent in the Ohio and Mississippi River valleys. [5, 6, 7]
H capsulatum is a soil-based fungus, and when disturbed, the conidia become airborne and are inhaled. The infections are generally asymptomatic, but a granulomatous inflammation results in pulmonary disease that is similar to pulmonary tuberculosis. About 6% of patients develop acute pericarditis. [5, 6]
Severity of disease is determined by host immunity and level of exposure. Symptoms include fever, malaise, cough, headache, chest pain, chills, and myalgias and appear 3-17 days after exposure. Acute pulmonary histoplasmosis is associated with a good outcome and is often self-limiting. [7]
Persons with a history of pulmonary disease can develop chronic pulmonary histoplasmosis. [8] Immunosuppressed persons are at risk for developing chronic disseminated histoplasmosis. Chronic progressive disseminated histoplasmosis has a long-term protracted course, lasting up to years, with long asymptomatic periods. [9] Reports have suggested that chronic pulmonary histoplasmosis can present as nodules, lymphadenopathy, or infiltrates, with cavities being less common. Emphysema is the primary risk factor for cavitary chronic pulmonary histoplasmosis. [6]
Complications of histoplasmosis infection include the following:
-
Mediastinal granuloma
-
Fibrosing mediastinitis
-
Broncholithiasis
-
Cavitary pulmonary histoplasmosis
Imaging modalities
Although chest radiographic findings are normal in most patients, chest radiography is the first radiologic examination performed. Radiographic findings primarily depend on the type of presentations and the immune status of the host. The abnormality that is most frequently found on radiographs consists of a single or multiple poorly defined areas of airspace consolidation, frequently in the lower lobes. With time, these opacities clear and a nodule forms; the nodule may later calcify.
CT scanning is helpful in detecting calcification in a lung nodule (histoplasmoma) and in evaluating patients with fibrosing mediastinitis and broncholithiasis. CT may define the extent of the fibrous mass in the mediastinum/hilum and demonstrate its obstructing effect .
MRI is comparable to CT scanning in its ability to define the extent of hilar or mediastinal lymphadenopathy. The primary advantage of MRI is its ability to diagnose vascular obstruction without the need for intravenous contrast material, especially for patients with renal failure. However, CT is superior to MRI in demonstrating calcification. The adenopathy associated with fibrosing mediastinitis demonstrates relatively low signal intensity on T2-weighted images.
Ultrasonography has a limited role in diagnosing histoplasmosis; however, the modality helps to define hepatomegaly or splenomegaly. Ultrasonography also is useful in assessing pleural and pericardial effusions, cardiac tamponade, calcification of the pericardium, and constrictive pericarditis.
Endobronchial ultrasound (EBUS) has been demonstrated to be useful in the diagnosis of subacute pulmonary histoplasmosis (SPH) with mediastinal lymphadenopathy. EBUS transbronchial needle aspiration (EBUS-TBNA) was found in one study to support a diagnosis of SPH in patients with a degree of clinical suspicion, along with clinical presentation, fungal serologies, and antigen testing. [10]
Angiography may be helpful in demonstrating some conditions associated with histoplasmosis (eg, SVC obstruction) and in determining whether great vessels are involved with fibrosing mediastinitis.
(See the images of histoplasmosis below.)
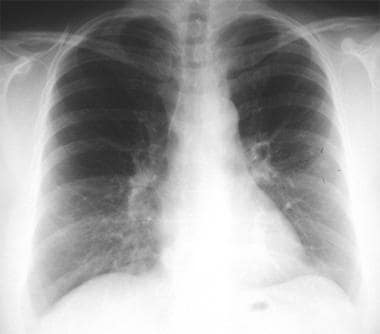
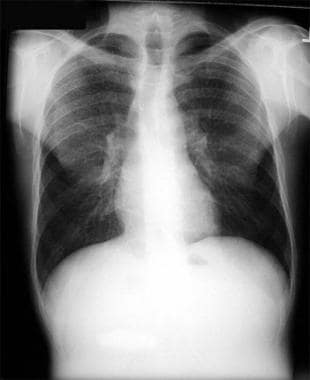 Thoracic histoplasmosis. A 36-year-old man developed progressive cough, fever, and dyspnea. Chest radiograph shows multiple diffuse tiny but discrete pulmonary nodules. This miliary pattern may be seen in miliary tuberculosis, histoplasmosis, blastomycosis, silicosis, berylliosis, miliary sarcoidosis, and metastatic malignancy.
Thoracic histoplasmosis. A 36-year-old man developed progressive cough, fever, and dyspnea. Chest radiograph shows multiple diffuse tiny but discrete pulmonary nodules. This miliary pattern may be seen in miliary tuberculosis, histoplasmosis, blastomycosis, silicosis, berylliosis, miliary sarcoidosis, and metastatic malignancy.
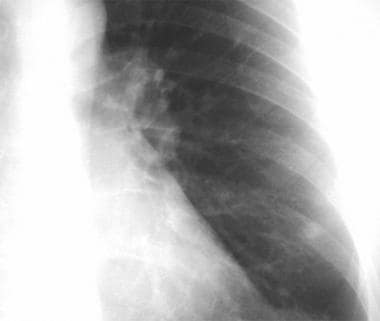
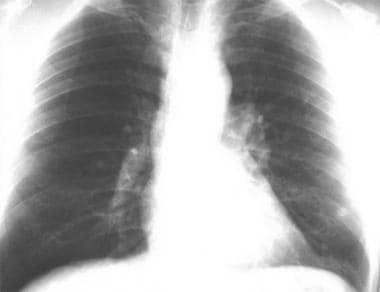 Thoracic histoplasmosis. A chest radiograph in a 44-year-old man originally referred to an oncologist for left lung nodule considered to be a neoplasm. Review of the chest radiograph reveals a well-circumscribed dense calcified nodule in the left lower lobe (arrow) consistent with a healed calcified granuloma. Subsequent history revealed extensive travel to the histoplasma endemic areas. The antibody testing showed H and M precipitins by immunodiffusion test.
Thoracic histoplasmosis. A chest radiograph in a 44-year-old man originally referred to an oncologist for left lung nodule considered to be a neoplasm. Review of the chest radiograph reveals a well-circumscribed dense calcified nodule in the left lower lobe (arrow) consistent with a healed calcified granuloma. Subsequent history revealed extensive travel to the histoplasma endemic areas. The antibody testing showed H and M precipitins by immunodiffusion test.
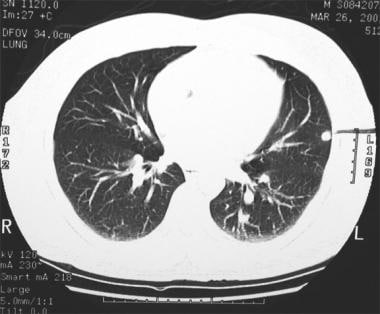 Thoracic histoplasmosis. Lung windows of the chest CT confirms a well-defined granuloma that was calcified on soft tissue windows (not shown). Additionally, there were many other nodules in both lungs that were not seen on the chest radiograph.
Thoracic histoplasmosis. Lung windows of the chest CT confirms a well-defined granuloma that was calcified on soft tissue windows (not shown). Additionally, there were many other nodules in both lungs that were not seen on the chest radiograph.
Radiography
Radiographic findings depend primarily on the type of presentation and on the immune status of the host. (See the images below). [11] Chest radiographic findings are normal in most patients. A solitary pulmonary nodule is a frequent finding on chest radiographs of the patient with asymptomatic primary infection. These nodules vary from a few millimeters to several centimeters. Most of these nodules have well-defined margins and central, laminar, or diffuse calcification patterns. Interestingly, some nodules slowly enlarge because of continued elaboration of collagen at the periphery of the lesion; in such cases, histoplasmosis may be difficult to distinguish from malignancy.
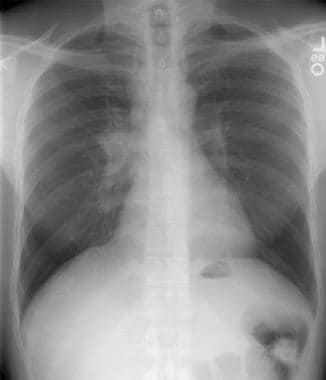 Thoracic histoplasmosis. Chest radiograph demonstrates a lobulated soft tissue mass in the right paratracheal region, with an enlarged and dense right hilum, suggesting lymphadenopathy. The radiographic differential diagnosis for these findings includes lung cancer, primary tuberculosis, histoplasmosis in endemic areas, lymphoma, and (less likely) sarcoidosis (owing to the asymmetric nature of nodal enlargement). Further workup revealed that this patient had a sarcoid-type reaction to histoplasmosis.
Thoracic histoplasmosis. Chest radiograph demonstrates a lobulated soft tissue mass in the right paratracheal region, with an enlarged and dense right hilum, suggesting lymphadenopathy. The radiographic differential diagnosis for these findings includes lung cancer, primary tuberculosis, histoplasmosis in endemic areas, lymphoma, and (less likely) sarcoidosis (owing to the asymmetric nature of nodal enlargement). Further workup revealed that this patient had a sarcoid-type reaction to histoplasmosis.
 Thoracic histoplasmosis. A chest radiograph in a 44-year-old man originally referred to an oncologist for left lung nodule considered to be a neoplasm. Review of the chest radiograph reveals a well-circumscribed dense calcified nodule in the left lower lobe (arrow) consistent with a healed calcified granuloma. Subsequent history revealed extensive travel to the histoplasma endemic areas. The antibody testing showed H and M precipitins by immunodiffusion test.
Thoracic histoplasmosis. A chest radiograph in a 44-year-old man originally referred to an oncologist for left lung nodule considered to be a neoplasm. Review of the chest radiograph reveals a well-circumscribed dense calcified nodule in the left lower lobe (arrow) consistent with a healed calcified granuloma. Subsequent history revealed extensive travel to the histoplasma endemic areas. The antibody testing showed H and M precipitins by immunodiffusion test.
 Thoracic histoplasmosis. A case of histoplasmoma showing 2 well-defined left upper lobe nodules confirmed by needle biopsy. In cases with noncalcified nodules, resection or percutaneous needle biopsy is occasionally required to establish the benign etiology of the lesion, as in this case.
Thoracic histoplasmosis. A case of histoplasmoma showing 2 well-defined left upper lobe nodules confirmed by needle biopsy. In cases with noncalcified nodules, resection or percutaneous needle biopsy is occasionally required to establish the benign etiology of the lesion, as in this case.
As many as 10-25% of patients with asymptomatic infection develop single or multiple poorly defined areas of airspace consolidation and/or nodules, with or without hilar lymph node enlargement. Enlarged lymph nodes often are seen after an asymptomatic infection. Adenopathy is frequently seen with lung parenchymal abnormalities. Calcification is often present with adenopathy; in some cases, such calcification may be seen only on computed tomography (CT) scans. Cases of noncalcified mediastinal adenopathy need to be distinguished from cases of sarcoidosis, lymphoma, and metastases. Enlarged lymph nodes may cause significant bronchial or tracheal compression or obstruction and may cause esophageal obstruction. Atelectasis, collapse, and obstructive pneumonitis may develop as a consequence of airway obstruction.
In acute symptomatic pulmonary histoplasmosis, radiographic findings include areas of airspace parenchymal consolidation that involve more than one segment or lobe, simulating acute bacterial pneumonia.
Pleural effusions are seen in a minority of patients with acute pulmonary histoplasmosis. Organisms are rarely isolated from pleural fluid.
After heavy exposure, radiographs may show widely disseminated, diffuse, fairly discrete nodular shadows throughout the lungs, with individual lesions measuring 1-10 mm in diameter. This form of disease is termed miliary histoplasmosis (see the image below); it is similar to miliary tuberculosis. The infiltrates clear within 2-8 months; however, lesions may fibrose and calcify or persist for many years.
 Thoracic histoplasmosis. A 36-year-old man developed progressive cough, fever, and dyspnea. Chest radiograph shows multiple diffuse tiny but discrete pulmonary nodules. This miliary pattern may be seen in miliary tuberculosis, histoplasmosis, blastomycosis, silicosis, berylliosis, miliary sarcoidosis, and metastatic malignancy.
Thoracic histoplasmosis. A 36-year-old man developed progressive cough, fever, and dyspnea. Chest radiograph shows multiple diffuse tiny but discrete pulmonary nodules. This miliary pattern may be seen in miliary tuberculosis, histoplasmosis, blastomycosis, silicosis, berylliosis, miliary sarcoidosis, and metastatic malignancy.
Lung cavitation usually is noted in persons with underlying obstructive lung disease; it is similar to chronic active tuberculosis, with predominantly upper lobe disease characterized by fibrosis, necrosis, cavitation, and granulomatous inflammation.
Failure to consider histoplasmosis because the chest radiograph is normal may delay diagnosis.
Computed Tomography
CT scanning is helpful in detecting calcification in a lung nodule (histoplasmoma) and in evaluating patients with fibrosing mediastinitis and broncholithiasis. (Soft-tissue masses are not associated with broncholithiasis; if found, consideration should be given to other diseases, such as lung cancer engulfing a calcified node.) [12, 13] This modality is more sensitive in detecting subtle calcification in a nodule and may identify other, smaller nodules that are not seen on chest radiographs.
(See the images below.)
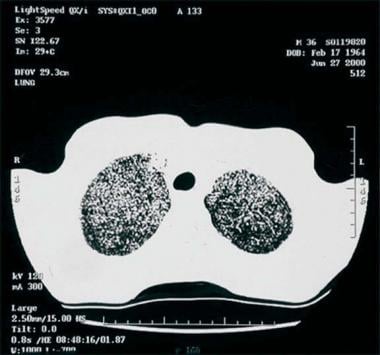 Thoracic histoplasmosis. High-resolution CT of the chest confirms chest radiographic findings and nicely shows the tightly formed micronodules in both upper lobes.
Thoracic histoplasmosis. High-resolution CT of the chest confirms chest radiographic findings and nicely shows the tightly formed micronodules in both upper lobes.
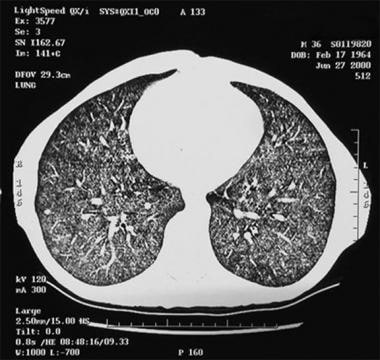 Thoracic histoplasmosis. CT image at the lung bases confirms the diffuse nature of the disease (a miliary form of disseminated pulmonary histoplasmosis [DPH]).
Thoracic histoplasmosis. CT image at the lung bases confirms the diffuse nature of the disease (a miliary form of disseminated pulmonary histoplasmosis [DPH]).
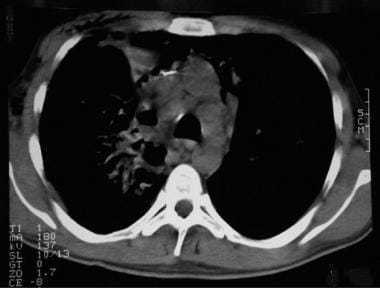 Thoracic histoplasmosis. Noncontrast chest CT image shows multiple enlarged lower paratracheal lymph nodes (arrows). There is a vague airspace opacity in the right upper lobe, as well.
Thoracic histoplasmosis. Noncontrast chest CT image shows multiple enlarged lower paratracheal lymph nodes (arrows). There is a vague airspace opacity in the right upper lobe, as well.
 Thoracic histoplasmosis. Lung windows of the chest CT confirms a well-defined granuloma that was calcified on soft tissue windows (not shown). Additionally, there were many other nodules in both lungs that were not seen on the chest radiograph.
Thoracic histoplasmosis. Lung windows of the chest CT confirms a well-defined granuloma that was calcified on soft tissue windows (not shown). Additionally, there were many other nodules in both lungs that were not seen on the chest radiograph.
CT scanning is very useful in the evaluation of patients with suspected fibrosing mediastinitis. CT may define the extent of the fibrous mass in the mediastinum/hilum and demonstrate its obstructing effect on the superior vena cava (SVC), pulmonary vessels, esophagus, trachea, or bronchi. Venous collaterals may be seen; they indicate long-standing venous occlusion. Stippled or dense calcification within the mass is present in most patients with fibrosing mediastinitis and may easily be seen on CT (see the image below).
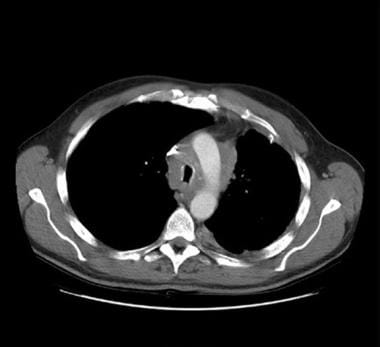 Thoracic histoplasmosis. Fibrosing mediastinitis is a rare but well-known clinical manifestation of histoplasmosis. Contrast enhanced chest CT at the level of the aortic arch in a patient with histoplasmosis and fibrosing mediastinitis shows an ill-defined soft tissue mass encasing and narrowing the trachea. The superior vena cava is also significantly narrowed (arrowhead).
Thoracic histoplasmosis. Fibrosing mediastinitis is a rare but well-known clinical manifestation of histoplasmosis. Contrast enhanced chest CT at the level of the aortic arch in a patient with histoplasmosis and fibrosing mediastinitis shows an ill-defined soft tissue mass encasing and narrowing the trachea. The superior vena cava is also significantly narrowed (arrowhead).
Serial CT scans may identify progression of the fibrosis and may be useful in assessing indications for surgery. CT is superior to magnetic resonance imaging (MRI) in demonstrating calcification.
In a retrospective review of the radiographic findings of fibrosing mediastinitis in 33 patients on various imaging studies, including chest radiographs, CT scans, MRI examinations, esophagrams, ventilation/perfusion scans, angiograms, and venograms, Sherrick et al reported the following [11] :
-
Bronchial narrowing in 11 patients (33%)
-
Pulmonary artery obstruction/narrowing in 6 patients (18%)
-
Esophageal narrowing in 3 patients (9%)
-
SVC obstruction/narrowing in 13 patients (39%)
Two distinctly different radiographic patterns were identified in the study by Sherrick et al: (1) a localized pattern, observed in 27 patients (82%), that often contained calcification, and (2) a diffuse pattern, found in 6 patients (18%), that had no calcification. The localized pattern most likely was a result of histoplasmosis in which there was no radiographic evidence of improvement with steroid therapy. The diffuse pattern was most likely truly idiopathic or had a noninfectious etiology. In several patients with the diffuse pattern, there was radiographic evidence of improvement with steroid therapy. For patients without calcification or with progressive radiographic findings, it is advisable to obtain tissue specimens for definitive diagnosis.
Computed tomography was used in one study to observe a calcified primary complex pulmonary histoplasmosis and predict which patients with N2 disease would benefit from surgery. The data obtained showed a predictable pattern of segmental and lobar lymphatic drainage to the mediastinum and suggested that skip involvement could represent the initial mediastinal node involvement via direct lymphatic drainage. [14]
-
Thoracic histoplasmosis. A 36-year-old man developed progressive cough, fever, and dyspnea. Chest radiograph shows multiple diffuse tiny but discrete pulmonary nodules. This miliary pattern may be seen in miliary tuberculosis, histoplasmosis, blastomycosis, silicosis, berylliosis, miliary sarcoidosis, and metastatic malignancy.
-
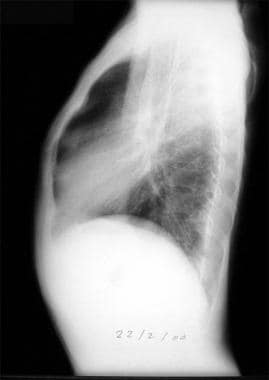
Thoracic histoplasmosis: lateral view.
-
Thoracic histoplasmosis. Gomori methenamine silver staining performed on lung tissue shows the yeast phase of histoplasmosis.
-
Thoracic histoplasmosis. Another image of the yeast phase of histoplasmosis.
-
Thoracic histoplasmosis. A transbronchial biopsy was performed. Pathologic examination of the specimen revealed multiple noncaseating granulomas present diffusely.
-
Thoracic histoplasmosis. High-resolution CT of the chest confirms chest radiographic findings and nicely shows the tightly formed micronodules in both upper lobes.
-
Thoracic histoplasmosis. CT image at the lung bases confirms the diffuse nature of the disease (a miliary form of disseminated pulmonary histoplasmosis [DPH]).
-
Thoracic histoplasmosis. Chest radiograph demonstrates a lobulated soft tissue mass in the right paratracheal region, with an enlarged and dense right hilum, suggesting lymphadenopathy. The radiographic differential diagnosis for these findings includes lung cancer, primary tuberculosis, histoplasmosis in endemic areas, lymphoma, and (less likely) sarcoidosis (owing to the asymmetric nature of nodal enlargement). Further workup revealed that this patient had a sarcoid-type reaction to histoplasmosis.
-
Thoracic histoplasmosis. Noncontrast chest CT image shows multiple enlarged lower paratracheal lymph nodes (arrows). There is a vague airspace opacity in the right upper lobe, as well.
-
Thoracic histoplasmosis. Fibrosing mediastinitis is a rare but well-known clinical manifestation of histoplasmosis. Contrast enhanced chest CT at the level of the aortic arch in a patient with histoplasmosis and fibrosing mediastinitis shows an ill-defined soft tissue mass encasing and narrowing the trachea. The superior vena cava is also significantly narrowed (arrowhead).
-
Thoracic histoplasmosis. A chest radiograph in a 44-year-old man originally referred to an oncologist for left lung nodule considered to be a neoplasm. Review of the chest radiograph reveals a well-circumscribed dense calcified nodule in the left lower lobe (arrow) consistent with a healed calcified granuloma. Subsequent history revealed extensive travel to the histoplasma endemic areas. The antibody testing showed H and M precipitins by immunodiffusion test.
-
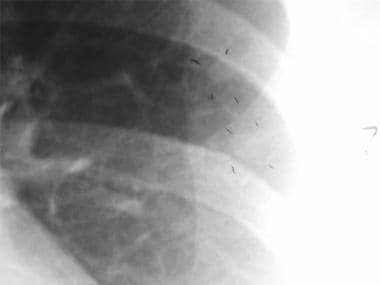
Thoracic histoplasmosis. A close-up of the left lower lobe nodule is shown here.
-
Thoracic histoplasmosis. Lung windows of the chest CT confirms a well-defined granuloma that was calcified on soft tissue windows (not shown). Additionally, there were many other nodules in both lungs that were not seen on the chest radiograph.
-
Thoracic histoplasmosis. A case of histoplasmoma showing 2 well-defined left upper lobe nodules confirmed by needle biopsy. In cases with noncalcified nodules, resection or percutaneous needle biopsy is occasionally required to establish the benign etiology of the lesion, as in this case.
-
Thoracic histoplasmosis. A close-up view of the histoplasmoma.