Practice Essentials
The term thyrotoxicosis refers to the hypermetabolic clinical syndrome resulting from serum elevations in thyroid hormone levels, specifically free thyroxine (T4) and/or triiodothyronine (T3). It is often confused with hyperthyroidism, which is caused by excessive endogenous thyroid hormone production. In the United States, prevalence of thyrotoxicosis is 1.2%, and the disease usually occurs between the ages of 20 and 50 years. [1, 2]
According to the American Thyroid Association (ATA), thyrotoxicosis can occur in the following situations [3] :
-
If the thyroid is excessively stimulated by trophic factors.
-
If constitutive activation of thyroid hormone synthesis and secretion occurs, leading to autonomous release of excess thyroid hormone.
-
If thyroid stores of preformed hormone are passively released in excessive amounts because of autoimmune, infectious, chemical, or mechanical insult.
-
If there is exposure to extrathyroidal sources of thyroid hormone, which may be either endogenous (struma ovarii, metastatic differentiated thyroid cancer) or exogenous (factitious thyrotoxicosis).
(Thyroid hormone homeostasis is illustrated in the image below.)
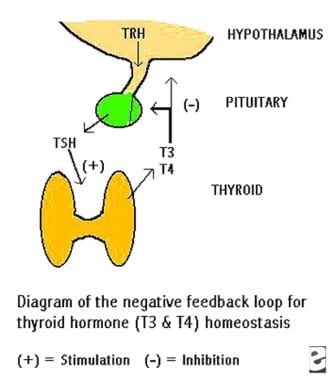 Illustration of the negative feedback loop causing homeostasis of thyroid hormone levels. A decrease in blood thyroid hormone triiodothyronine (T3)/thyroxine (T4) levels results in the inhibition of thyrotropin-releasing hormone and thyrotropin production. The released thyrotropin stimulates synthesis and release of T3/T4 by the thyroid, which in turn tends to inhibit further thyrotropin release. THS is thyrotropin. TRH is thyrotropin-releasing hormone.
Illustration of the negative feedback loop causing homeostasis of thyroid hormone levels. A decrease in blood thyroid hormone triiodothyronine (T3)/thyroxine (T4) levels results in the inhibition of thyrotropin-releasing hormone and thyrotropin production. The released thyrotropin stimulates synthesis and release of T3/T4 by the thyroid, which in turn tends to inhibit further thyrotropin release. THS is thyrotropin. TRH is thyrotropin-releasing hormone.
Neoplasms leading to thyrotoxicosis include autonomously functioning toxic nodules and toxic, multinodular goiters (TMNGs).
Infections that can produce thyrotoxicosis include subacute thyroiditis (SAT) and, very rarely, acute suppurative thyroiditis. Some studies have shown that COVID-19 infection can cause thyroid-related illness. [4, 5, 6]
Hyperthyroidism is a type of thyrotoxicosis in which accelerated thyroid hormone biosynthesis and secretion by the thyroid gland produce thyrotoxicosis. However, hyperthyroidism and thyrotoxicosis are not synonymous. [7] This is because, although many patients have thyrotoxicosis caused by hyperthyroidism, other patients may have thyrotoxicosis resulting from inflammation of the thyroid gland, which causes the release of stored thyroid hormone but not accelerated synthesis, or they may have thyrotoxicosis, which is caused by ingestion of exogenous thyroid hormone.
Differentiating between thyrotoxicosis caused by hyperthyroidism and thyrotoxicosis not caused by hyperthyroidism is important, because disease management and therapy differ for each form. Thyroid imaging and radiotracer thyroid uptake measurements, combined with serologic data, enable specific diagnosis and appropriate patient treatment. [8, 9, 10, 11]
The common causes of thyrotoxicosis have different pathophysiologic features and include autoimmune diseases, functioning thyroid adenomas, and infections.
Autoimmune diseases resulting in thyrotoxicosis include the following:
-
Graves disease (the most common cause of hyperthyroidism; see the images below)
-
Lymphocytic thyroiditis with hyperthyroidism (ie, silent thyroiditis)
-
Postpartum thyrotoxicosis (PPT)
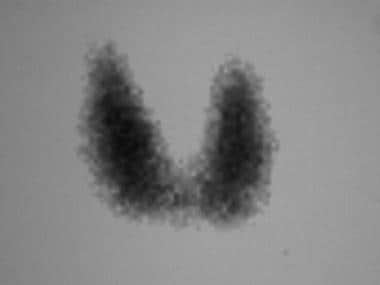 Iodine-123 thyroid scan in a patient with Graves disease: Tracer uptake is uniform throughout the gland. The 5-hour iodine uptake was high at 53%.
Iodine-123 thyroid scan in a patient with Graves disease: Tracer uptake is uniform throughout the gland. The 5-hour iodine uptake was high at 53%.
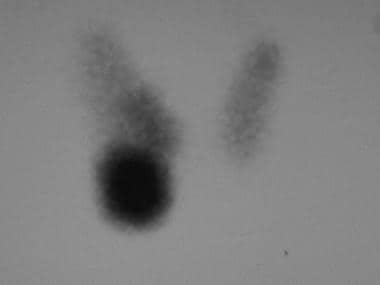
 Iodine-123 thyroid scan in a patient with Graves disease. The 5-hour iodine uptake was elevated at 29%. Note the high level of iodine concentration near the thyroid. Also note the pyramidal lobe, which often is visualized in a hyperstimulated gland. The cold nodule in the right lobe must be addressed in the same way that a solitary cold nodule in a patient without Graves disease is evaluated. Fine-needle aspiration of the nodule prior to iodine-131 treatment did not reveal a carcinoma.
Iodine-123 thyroid scan in a patient with Graves disease. The 5-hour iodine uptake was elevated at 29%. Note the high level of iodine concentration near the thyroid. Also note the pyramidal lobe, which often is visualized in a hyperstimulated gland. The cold nodule in the right lobe must be addressed in the same way that a solitary cold nodule in a patient without Graves disease is evaluated. Fine-needle aspiration of the nodule prior to iodine-131 treatment did not reveal a carcinoma.
Neoplasms that cause thyrotoxicosis include autonomously functioning toxic nodules (AFTN) and toxic, multinodular goiters (TMNGs) (see the images below); infections that lead to the condition include subacute thyroiditis (SAT) and, very rarely, acute suppurative thyroiditis.
 Iodine-123 scan in a patient with a palpable nodule in the right neck, a low serum level for thyrotropin, and a slightly elevated serum level of free triiodothyronine. The autonomously functioning nodule only partially suppresses uptake in the remainder of the gland. The 5-hour iodine uptake was mildly elevated at 22%.
Iodine-123 scan in a patient with a palpable nodule in the right neck, a low serum level for thyrotropin, and a slightly elevated serum level of free triiodothyronine. The autonomously functioning nodule only partially suppresses uptake in the remainder of the gland. The 5-hour iodine uptake was mildly elevated at 22%.
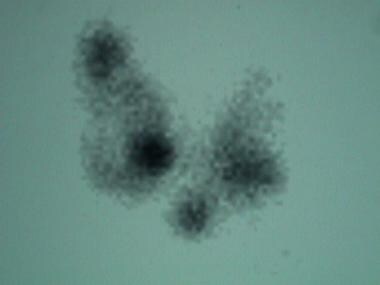 Scan in a patient with a toxic, multinodular goiter: The 5-hour iodine uptake was elevated at 28%. Note the multiple foci of variably increased tracer uptake.
Scan in a patient with a toxic, multinodular goiter: The 5-hour iodine uptake was elevated at 28%. Note the multiple foci of variably increased tracer uptake.
Diagnosis
The assessment of thyroid blood flow by color-flow Doppler ultrasonography is valuable in the differentiation of destructive thyrotoxicosis from Graves disease, according to a study by Kumar et al. The study was carried out in 65 patients. Destructive thyrotoxicosis was present in 31 patients; the remaining patients had Graves disease. Color-flow Doppler ultrasonography parameters correlated significantly with pertechnetate scan results, demonstrating a comparable sensitivity of 96% and a specificity of 95%. [12]
The ATA notes in its guidelines that ultrasonography with color flow Doppler can distinguish thyroid hyperactivity (increased flow) from destructive thyroiditis and may be particularly useful when radioactive iodine is contraindicated, such as during pregnancy or breastfeeding. [3]
Phillips and Hennessey noted that, although radioactive iodine is often useful in the diagnosis and treatment of thyrotoxicosis, such tests cannot be performed in many patients because of recent use of iodinated contrast for other diagnostic studies, such as CT scanning. In their study, the investigators found that 45% of patients with newly diagnosed thyrotoxicosis had received iodinated contrast within 2 weeks before endocrinology evaluation; 43 had received iodine for CT and the other 2 for angiography. Only 1 patient required emergent treatment of a condition diagnosed by CT before further diagnostic studies could have been performed. [13]
Donkol et al concluded that color-flow Doppler of the inferior thyroid artery was useful in the differential diagnosis of thyrotoxicosis, particularly when a patient has a contraindication of thyroid scintigraphy by radioactive material. [14]
The diagnosis of thyrotoxicosis is predominantly based on laboratory results, including an elevated free T3/T4 level and suppressed thyrotropin level; however, the clinical examination may reveal the etiology. If the thyroid gland is normal or diffusely enlarged on physical examination, the most likely diagnosis is Graves disease.
If one or more thyroid nodules are palpated, the patient probably has an autonomously functioning thyroid nodule (AFTN) or a TMNG. If the thyroid gland is markedly tender, subacute thyroiditis is likely. However, silent thyroiditis is almost always in the differential diagnosis with Graves disease. In addition, some patients with silent thyroiditis may have a tender thyroid gland, and some patients with subacute thyroiditis have only mild thyroid tenderness.
As a result of the clinical overlap, knowledge of thyroid iodine uptake is necessary for specific diagnosis and appropriate therapy in most patients. Also, thyroid radionuclide scintigraphy can help to distinguish Graves disease from a toxic nodule and a TMNG.
Thyroid uptake testing, thyroid scintigraphy, and thyroid ultrasonography are not the primary testing modalities for the diagnosis of thyrotoxicosis, but their findings can be critical in the differential diagnosis of the disease and in selecting treatment once thyrotoxicosis is established with serologic test results. [12, 14, 15, 16, 17, 18]
In Graves disease, radioactive iodine uptake is diffuse, unless nodules or fibrosis is present. Patients with single toxic adenoma have focal uptake in the adenoma and suppressed uptake in surrounding thyroid tissue. A toxic multinodular goiter displays multiple areas of focal increased uptake of radioactive iodine. In patients with painless, postpartum, or subacute thyroiditis, the uptake of radioactive iodine will be near zero. [1, 2]
Radioiodine uptake testing with iodine-123 or iodine-131 allows accurate evaluation and quantification of iodine uptake in thyroid cells. Scintigraphy with iodine-123 or technetium-99m-pertechnetate can identify topographic distribution and detect and localize ectopic thyroid tissue. [19]
ICD-10 codes
The ICD-10 codes for thyrotoxicosis include the following:
-
E05 Thyrotoxicosis (hyperthyroidism)
-
E05.0 Thyrotoxicosis with diffuse goiter
-
E05.1 Thyrotoxicosis with toxic single thyroid nodule
-
E05.2 Thyrotoxicosis with toxic multinodular goiter
-
E05.3 Thyrotoxicosis from ectopic thyroid tissue
-
E05.4 Thyrotoxicosis factitia
-
E05.5 Thyroid crisis or storm
-
E05.8 Other thyrotoxicosis
-
E05.9 Thyrotoxicosis, unspecified
Ultrasonography
Thyroid ultrasonography is not necessary for the differential diagnosis of thyrotoxicosis, although certain findings are important. [3, 12, 14, 16, 20, 18, 21]
In Graves disease, the thyroid appears normal or moderately enlarged. Color-flow Doppler imaging demonstrates a general mild to marked increase in blood flow through the parenchyma, as seen in the image below. With autonomously functioning toxic nodules (AFTN) and toxic, multinodular goiters (TMNGs) AFTN, ultrasonograms demonstrate 1 or more nodules, but they do not indicate the functional status of any nodule. [20, 22]
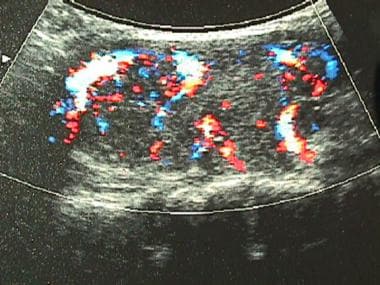 Color-flow ultrasonogram in a patient with Graves disease. Generalized hypervascularity is visible throughout the gland, which often can be heard as a hum or bruit with a stethoscope.
Color-flow ultrasonogram in a patient with Graves disease. Generalized hypervascularity is visible throughout the gland, which often can be heard as a hum or bruit with a stethoscope.
In silent thyroiditis and postpartum thyrotoxicosis (PPT), the gland may be normal, or it may be generally large or plump. The pyramidal lobe may be prominent. The parenchyma may be heterogeneously hyperechoic. With subacute thyroiditis (SAT), the gland is edematous; the edema is reflected as hypoechogenicity. This finding can be regional, because the gland may not be affected uniformly.
According to a study of color flow Doppler ultrasound compared with radioiodine scintigraphy in patients with thyrotoxicosis, Doppler ultrasound can be used instead of scintigraphy in situations in which the latter is contraindicated or is of limited value to define the etiology of thyrotoxicosis. Color flow Doppler ultrasound had 100% specificity, and in 10 cases in which scintigraphy and Doppler ultrasound provided discordant results, the diagnosis suggested by the latter was correct. [18]
Nuclear Imaging
Nuclear medicine examinations are used to differentiate the causes of thyrotoxicosis after the diagnosis is made clinically and confirmed by using appropriate laboratory tests. At that point, measurements of thyroid radiotracer uptake with iodine-123 (123I) or iodine-131 (131I) and findings on a thyroid scan obtained with 123I or technetium-99m (99mTc) confirm the diagnosis, and treatment can be initiated. Iodine uptake is demonstrated in the images below. [17, 23]
 Iodine-123 thyroid scan in a patient with Graves disease: Tracer uptake is uniform throughout the gland. The 5-hour iodine uptake was high at 53%.
Iodine-123 thyroid scan in a patient with Graves disease: Tracer uptake is uniform throughout the gland. The 5-hour iodine uptake was high at 53%.
 Iodine-123 thyroid scan in a patient with Graves disease. The 5-hour iodine uptake was elevated at 29%. Note the high level of iodine concentration near the thyroid. Also note the pyramidal lobe, which often is visualized in a hyperstimulated gland. The cold nodule in the right lobe must be addressed in the same way that a solitary cold nodule in a patient without Graves disease is evaluated. Fine-needle aspiration of the nodule prior to iodine-131 treatment did not reveal a carcinoma.
Iodine-123 thyroid scan in a patient with Graves disease. The 5-hour iodine uptake was elevated at 29%. Note the high level of iodine concentration near the thyroid. Also note the pyramidal lobe, which often is visualized in a hyperstimulated gland. The cold nodule in the right lobe must be addressed in the same way that a solitary cold nodule in a patient without Graves disease is evaluated. Fine-needle aspiration of the nodule prior to iodine-131 treatment did not reveal a carcinoma.
 Iodine-123 scan in a patient with a palpable nodule in the right neck, a low serum level for thyrotropin, and a slightly elevated serum level of free triiodothyronine. The autonomously functioning nodule only partially suppresses uptake in the remainder of the gland. The 5-hour iodine uptake was mildly elevated at 22%.
Iodine-123 scan in a patient with a palpable nodule in the right neck, a low serum level for thyrotropin, and a slightly elevated serum level of free triiodothyronine. The autonomously functioning nodule only partially suppresses uptake in the remainder of the gland. The 5-hour iodine uptake was mildly elevated at 22%.
 Scan in a patient with a toxic, multinodular goiter: The 5-hour iodine uptake was elevated at 28%. Note the multiple foci of variably increased tracer uptake.
Scan in a patient with a toxic, multinodular goiter: The 5-hour iodine uptake was elevated at 28%. Note the multiple foci of variably increased tracer uptake.
Establishing the cause of thyrotoxicosis
In the thyroid radioiodine tracer uptake test, a measured dose of radiotracer, usually123I or 131I, is administered to the patient. [13]
After 4-24 hours, activity in the thyroid (ie, neck activity corrected for background levels) is imaged, and the percentage of the administered dose within the thyroid is calculated. Each laboratory should establish their normal values, but generally, normal values are in the range of 5 to 25%. Thyrotoxicosis (ie, Graves disease, AFTN, TMNG) caused by hyperfunctional thyroid tissue is associated with normal to markedly increased uptake.
Thyrotoxicosis (ie, SAT, silent thyroiditis) caused by inflammation of the thyroid gland has low to absent uptake. Thyroid scintigraphy after the oral administration of 123I or intravenous administration of 99mTc is helpful in demonstrating diffuse tracer uptake (Graves disease) versus nodular tracer concentration (AFTN, TMNG). The image can also be used distinguish low thyroid uptake from a high thyroid uptake, but the findings are not as quantitative as those of the thyroid uptake test.
Normal results on 4- to 24-hour thyroid uptake scans do not preclude a diagnosis of hyperthyroidism. Many multivitamins and other food supplements contain large amounts of iodine, and the extra iodine competes with radioiodine for thyroid clearance. Other sources of iodine ingestion also may be present.
In cases studies of 5 patients by Elshimy et al, scintigraphic uptake of technetium-99m-sestamibi (MIBI), also known as Tc-99m-methoxy isobutyl isonitrile, was found to be a valuable method for the diagnosis and classification of amiodarone-induced thyrotoxicosis. [24]
In a study by Perdomo et al regarding the differential diagnosis of thyrotoxicosis, analysis of 37 histologic specimens indicated that thyroid radionuclide scintigraphy had greater accuracy than thyrotropin receptor antibodies (81% vs 75.7%) and higher specificity (79.2% vs 57.1%) but that sensitivity was lower (84.6% vs 92.3%). [25]
-
Illustration of the negative feedback loop causing homeostasis of thyroid hormone levels. A decrease in blood thyroid hormone triiodothyronine (T3)/thyroxine (T4) levels results in the inhibition of thyrotropin-releasing hormone and thyrotropin production. The released thyrotropin stimulates synthesis and release of T3/T4 by the thyroid, which in turn tends to inhibit further thyrotropin release. THS is thyrotropin. TRH is thyrotropin-releasing hormone.
-
Iodine-123 thyroid scan in a patient with Graves disease: Tracer uptake is uniform throughout the gland. The 5-hour iodine uptake was high at 53%.
-
Iodine-123 thyroid scan in a patient with Graves disease. The 5-hour iodine uptake was elevated at 29%. Note the high level of iodine concentration near the thyroid. Also note the pyramidal lobe, which often is visualized in a hyperstimulated gland. The cold nodule in the right lobe must be addressed in the same way that a solitary cold nodule in a patient without Graves disease is evaluated. Fine-needle aspiration of the nodule prior to iodine-131 treatment did not reveal a carcinoma.
-
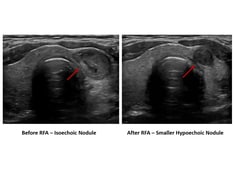
Ultrasonogram of the right lobe of the thyroid in the same patient as in the previous image. Fine-needle aspiration of the nodule prior to iodine-131 treatment did not reveal a carcinoma.
-
Iodine-123 scan in a patient with a palpable nodule in the right neck, a low serum level for thyrotropin, and a slightly elevated serum level of free triiodothyronine. The autonomously functioning nodule only partially suppresses uptake in the remainder of the gland. The 5-hour iodine uptake was mildly elevated at 22%.
-
Scan in a patient with a toxic, multinodular goiter: The 5-hour iodine uptake was elevated at 28%. Note the multiple foci of variably increased tracer uptake.
-
Color-flow ultrasonogram in a patient with Graves disease. Generalized hypervascularity is visible throughout the gland, which often can be heard as a hum or bruit with a stethoscope.