Practice Essentials
Paragangliomas arise from paraganglion cells, which serve varied regulatory tasks in the body. When these cells demonstrate neoplasia within the head and neck, they typically present in characteristic locations, including the carotid space, the jugular foramen, the middle ear, and along the course of the vagus nerve. [1] Imaging is the primary investigative modality for glomus tumors of the head and neck (paragangliomas).
Glomus tumors (paragangliomas) represent 0.6% of neoplasms of the head and neck and 0.03% of all neoplasms; they are the most common tumors of the inner ear and the second most common tumors of the temporal bone after schwannomas. About 80% of all glomus tumors are carotid body tumors or glomus jugulare tumors. [2, 3, 4, 5, 6, 7, 8]
Haller introduced glomus tumors of the head and neck into the medical record in 1762, when he described a mass at the carotid bifurcation that had a glomus body–like structure. In 1950, Mulligan renamed this type of neoplasm as a "chemodectoma" to reflect its origins from chemoreceptor cells. In 1974, Glenner and Grimley renamed the tumor "paraganglioma" on the basis of its anatomic and physiologic characteristics. They also created a classification method based on location, innervation, and microscopic appearance of tumors. [9]
Imaging modalities
MRI is frequently the imaging study of choice for primary diagnosis, followed by contrast-enhanced CT scanning. Angiography remains of paramount importance if the diagnosis is obscure or if embolization is contemplated. [10, 11, 12]
CT imaging is excellent for demonstrating cervical masses along the course of the carotid artery, but findings of skull base soft tissue details can be limited. However, CT imaging is superb for revealing characteristic bony destructive skull base changes. CT scanning is also best in the diagnosis of glomus tumors when a satisfactory bolus of contrast material is administered. If peak tumor opacification is missed at CT scanning, the mass can be misconstrued for a nonenhancing schwannoma or a nodal lesion. [10, 11, 12]
MRI can reveal soft tissue masses and their relationships to adjacent structures in multiple imaging planes. This capability is particularly helpful in skull base imaging, by which both extracranial and intracranial components can be evaluated. MRI can fail to depict enhancement if the contrast agent bolus is inadequate. MRI is inherently limited in its ability to show subtle areas of bony destruction, which may be important for proper diagnosis. [12]
Angiography is typically reserved for patients who are undergoing preoperative evaluation and for those in whom the presence of neovascularity can help in focusing the differential diagnosis. Angiography is a minimally invasive test and therefore is not the imaging study of choice. Rarely, diagnostic angiography can show soft tissue neovascularity in other types of abnormalities such as those encountered with hypervascular lymphadenopathy or nodular fasciitis. These tumors can have profound neovascularity that mimics that of glomus tumors. [11]
A combination of contrast-enhanced computed tomography (CT) scanning, magnetic resonance imaging (MRI), and angiography is ideal for proper diagnosis and localization of these tumors. Lesions show a characteristic signature on images, which is based on their locations. [13, 14, 15, 16, 17, 18, 19, 2, 20]
(Glomus tumors are displayed in the images below.)
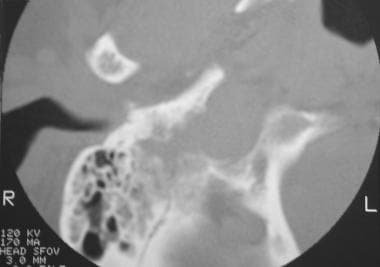 Glomus jugulare tumor. Computed tomography scan shows a permeative destructive skull base mass with involvement of mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Computed tomography scan shows a permeative destructive skull base mass with involvement of mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
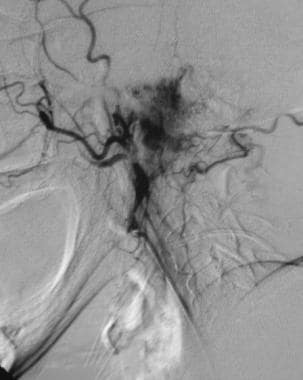 Glomus jugulare tumor. Selective external carotid angiogram reveals a vascular skull base mass. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Selective external carotid angiogram reveals a vascular skull base mass. Reprinted with permission. Copyright Springer-Verlag.
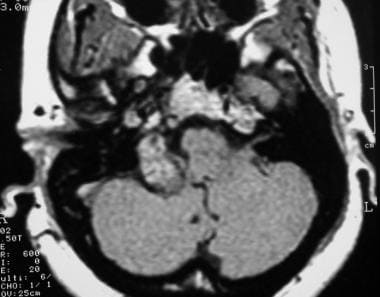 Glomus jugulare tumor. Axial contrast-enhanced T1-weighted magnetic resonance image shows an enhancing right skull base mass. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Axial contrast-enhanced T1-weighted magnetic resonance image shows an enhancing right skull base mass. Reprinted with permission. Copyright Springer-Verlag.
Anatomy
Glomus tumors of the head and neck are associated with 4 primary locations: jugular bulb, middle ear cavity, vagus nerve, and carotid body. They are highly vascular, locally invasive, slow-growing tumors that frequently involve critical neurovascular structures. [2, 6]
Tumors in the jugular bulb region are commonly called "glomus jugulare tumors" [21, 22, 23, 24, 25, 26] ; they arise in the adventitia of the dome of the jugular bulb and represent the most common type of glomus tumor of the head and neck. [7, 21, 22]
Tumors in the area of the middle ear cavity are commonly called "glomus tympanicum tumors" (see the image below) [14, 26, 27, 28, 29] ; they arise from the glomus bodies that run with the tympanic branch of the glossopharyngeal nerve. Although glomus tympanicum tumors are the most common primary neoplasms of the middle ear, they are the rarest of head and neck glomus tumors. [20, 3, 27, 30]
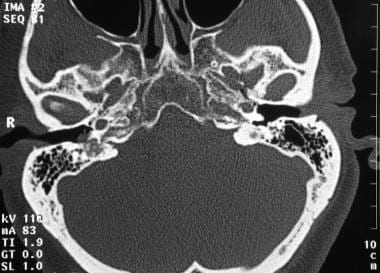 Glomus tympanicum tumor. Axial computed tomography image shows a small vascular mass along the right tympanic membrane. Note adjacent opacified mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
Glomus tympanicum tumor. Axial computed tomography image shows a small vascular mass along the right tympanic membrane. Note adjacent opacified mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
Tumors in the region of the vagus nerve are commonly called "glomus vagale tumors" because of their usual close association with the vagus nerve. [4, 31, 32, 33] Specifically, they arise infratemporally along the course of the cervical vagus nerve.
(See the image below.)
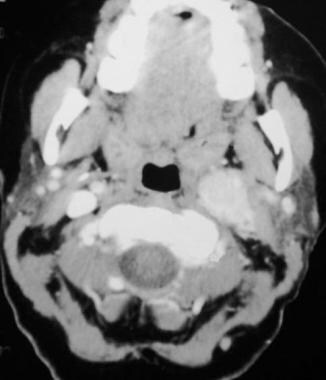 Glomus vagale tumor. Contrast-enhanced computed tomography scan showing a large vascular mass along the course of the left internal carotid artery and jugular vein above the level of the carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
Glomus vagale tumor. Contrast-enhanced computed tomography scan showing a large vascular mass along the course of the left internal carotid artery and jugular vein above the level of the carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
Carotid body glomus tumors, also called "carotid body tumors," occur at the bifurcation of the common carotid artery and arise from tissue of the normal carotid body. [3, 4, 5, 34] Recurrence is more common with glomus jugulare tumors and is less common with carotid body tumors. [35]
(See the image below.)
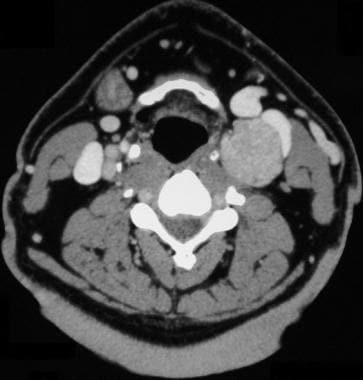 Carotid body tumor. Computed tomography scan shows an enhancing carotid bifurcation mass. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Computed tomography scan shows an enhancing carotid bifurcation mass. Reprinted with permission. Copyright Springer-Verlag.
Although glomus tumors usually appear as solitary lesions at a single site, multiple lesions at multiple sites are not uncommon (see the image below). Because they are components of the neuroendocrine system, these tumors are highly vascularized. Clusters of tumor cells (type I cells interspersed with type II cells), called zellballen, are surrounded by a dense network of capillary caliber blood vessels.
Radiation may lead to lower risk of recurrence or progression than surgery for some paraganglioma types. Metastasis is rare but is more likely with recurrent disease. Surveillance neck imaging is recommended every several years for decades after treatment. [35]
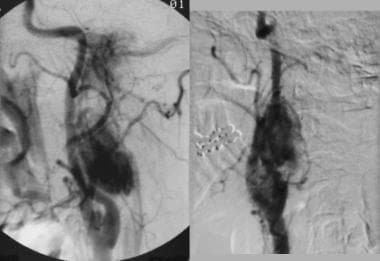 Multiple glomus tumors. Angiogram obtained in a female patient with bilateral carotid body tumors, bilateral glomus vagale tumors, and left glomus jugulare tumors with corresponding angiographically enhancing masses. Reprinted with permission. Copyright Springer-Verlag.
Multiple glomus tumors. Angiogram obtained in a female patient with bilateral carotid body tumors, bilateral glomus vagale tumors, and left glomus jugulare tumors with corresponding angiographically enhancing masses. Reprinted with permission. Copyright Springer-Verlag.
Radiologic intervention
Embolization is commonly available as the lone treatment option or as a precursor to surgical excision. As a result of the highly vascular nature of these neoplasms, embolization is an effective technique that is aimed at starving the lesion of its blood supply and inducing necrosis. This is the primary and, at times, the only treatment option for glomus jugulare tumors because of the difficulty associated with excising many of these tumors. In combination with surgical excision, embolization is often used to reduce blood loss. It has proved highly effective. [8]
With bilateral lesions, especially of the vagale type, embolization is often required as the sole course of treatment for a single lesion, in tandem with surgical excision of the other. This approach is used because of the proximity of the lesion to the vagus nerve, in an attempt to prevent occasionally inevitable perioperative damage to the nerve during excision.
As a result of their inherent neovascularity, catheter-directed embolization is appropriate in the treatment of chemodectomas, particularly if surgical removal is contemplated. The use of microcatheters allows precise delivery of embolic agents into masses and embolization of multiple small feeding trunks. Although glomus tumors may arise from multiple arterial territories, embolization is typically limited to feeders of the external carotid branch artery.
In the author's practice, glomus tumor embolization is performed in a preoperative setting. Nevertheless, some clinicians suggest that embolization alone may be beneficial in the treatment of these tumors.
Special concerns
Whenever external carotid embolization is performed, care must be taken to avoid inadvertent extracranial-intracranial embolization and subsequent risk of stroke. Occult occipital-vertebral connections may be present or open, or they may become apparent on angiography, only after partial embolization is performed.
Because pterygopalatine–internal carotid collaterals, as well as middle meningeal–middle cerebral communications, may exist, these should be recognized before embolization. In addition, the ophthalmic artery should be identified before external carotid embolization is performed, and care should be taken to identify any existing ethmoidal-ophthalmic arterial communications.
Computed Tomography
Contrast-enhanced CT scans demonstrate enhancing soft tissue masses at characteristic locations key to diagnosis. Nonenhanced CT imaging can reveal glomus tumors, but identification of a strongly enhancing mass is typical in the diagnosis of a glomus tumor. [14, 10]
CT scanning shows carotid body tumors at the level of the carotid bifurcation, respectively splaying internal and external carotid arteries medially and laterally. These tumors can vary in size, but their location within the bifurcation is critical for diagnosis. [16]
(See the image below.)
 Carotid body tumor. Computed tomography scan shows an enhancing carotid bifurcation mass. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Computed tomography scan shows an enhancing carotid bifurcation mass. Reprinted with permission. Copyright Springer-Verlag.
Glomus vagale tumors are masses with similarly strong enhancement. [33] Glomus valgale tumors are paragangliomas involving the vagus nerve. These tumors are seen along the course of the jugular vein and the internal carotid artery above the level of the carotid bifurcation but below the skull base. They vary in size and can displace adjacent vascular structures. [33]
(See the image below.)
 Glomus vagale tumor. Contrast-enhanced computed tomography scan showing a large vascular mass along the course of the left internal carotid artery and jugular vein above the level of the carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
Glomus vagale tumor. Contrast-enhanced computed tomography scan showing a large vascular mass along the course of the left internal carotid artery and jugular vein above the level of the carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumors are enhancing soft tissue masses at the skull base, but skull base artifact can mask their presence. These tumors are seen within the jugular foramen; bony erosion of the jugular foramen and of the petrous apex is often a key finding for diagnosis. Careful review of bone windows is necessary.
(See the image below.)
 Glomus jugulare tumor. Computed tomography scan shows a permeative destructive skull base mass with involvement of mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Computed tomography scan shows a permeative destructive skull base mass with involvement of mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
(Glomus tympanicum tumors are shown in the images below.)
 Glomus tympanicum tumor. Axial computed tomography image shows a small vascular mass along the right tympanic membrane. Note adjacent opacified mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
Glomus tympanicum tumor. Axial computed tomography image shows a small vascular mass along the right tympanic membrane. Note adjacent opacified mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
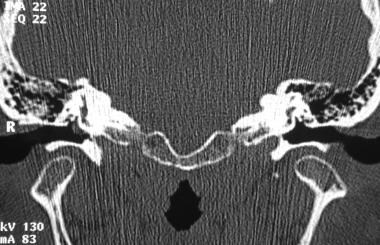 Glomus tympanicum tumor. Coronal computed tomography scan confirms the presence of a known hypotympanic mass consistent with glomus tympanicum. Reprinted with permission. Copyright Springer-Verlag.
Glomus tympanicum tumor. Coronal computed tomography scan confirms the presence of a known hypotympanic mass consistent with glomus tympanicum. Reprinted with permission. Copyright Springer-Verlag.
The degree of confidence is high. The presence of strongly enhancing neck masses in typical perivascular locations leads to a high degree of confidence regarding the diagnosis.
Hypervascular lymphadenopathy may result in false-positive findings, which can be seen in a variety of disorders such as metastatic papillary carcinoma of the thyroid gland. In these instances, location is a key finding.
Lack of sufficient contrast enhancement can be troublesome and may result in false-negative findings. In this case, glomus tumors can mimic schwannomas, neurofibromas, or nonenhancing lymphadenopathies. Small vascular tumors may be missed if they are not easily distinguishable from adjacent vascular structures.
Magnetic Resonance Imaging
Similar to CT imaging, contrast-enhanced MRI reveals enhancing soft tissue masses at characteristic locations; these findings are important for diagnosis. Nonenhanced MRI can demonstrate glomus tumors, but identification of a strongly enhancing mass is typical in the diagnosis of a glomus tumor. [16, 10, 11, 12]
As with most soft tissue tumors, glomus tumors are isointense on T1-weighted MRI and hyperintense on T2-weighted MRI, relative to skeletal muscle.
(See the images below.)
 Glomus jugulare tumor. Axial contrast-enhanced T1-weighted magnetic resonance image shows an enhancing right skull base mass. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Axial contrast-enhanced T1-weighted magnetic resonance image shows an enhancing right skull base mass. Reprinted with permission. Copyright Springer-Verlag.
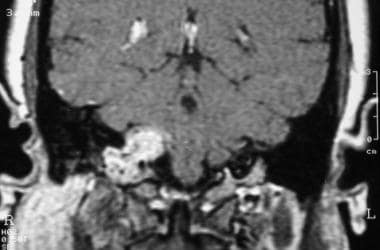 Glomus jugulare tumor. Coronal T1-weighted contrast-enhanced magnetic resonance image shows a mass protruding into the jugular foramen. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Coronal T1-weighted contrast-enhanced magnetic resonance image shows a mass protruding into the jugular foramen. Reprinted with permission. Copyright Springer-Verlag.
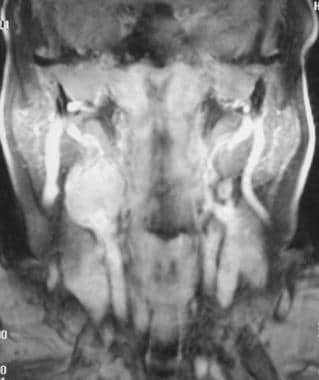 Carotid body tumor. Contrast-enhanced T1-weighted magnetic resonance image shows a strongly enhancing mass overlying the right carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Contrast-enhanced T1-weighted magnetic resonance image shows a strongly enhancing mass overlying the right carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
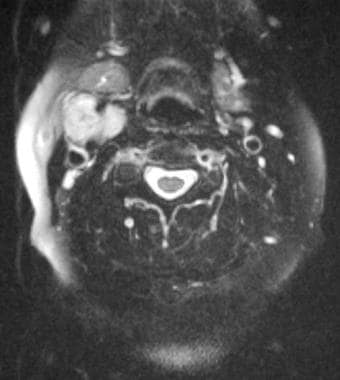 Carotid body tumor. Axial T2-weighted magnetic resonance image reveals splaying of external and internal carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Axial T2-weighted magnetic resonance image reveals splaying of external and internal carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
Contrast-enhanced imaging can show intense tumor enhancement, which again is a key finding for diagnosis. In addition, a salt-and-pepper fine vascular pattern can be seen in tumors; this finding is suggestive of intrinsic tumor neovascularity and is particularly well demonstrated on T2-weighted images.
(See the image below.)
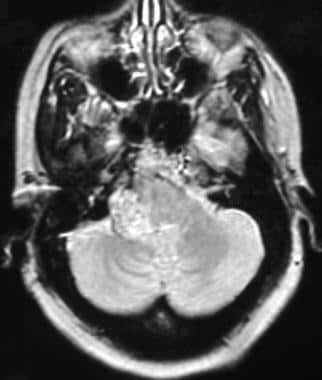 Glomus jugulare tumor. Axial T2-weighted magnetic resonance image shows salt-and-pepper vascularity in the substance of the tumor. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Axial T2-weighted magnetic resonance image shows salt-and-pepper vascularity in the substance of the tumor. Reprinted with permission. Copyright Springer-Verlag.
MRI can show densely enhancing carotid body tumors at the level of the carotid bifurcation, which, respectively, splay internal and external carotid arteries medially and laterally.
(See the images below.)
 Carotid body tumor. Contrast-enhanced T1-weighted magnetic resonance image shows a strongly enhancing mass overlying the right carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Contrast-enhanced T1-weighted magnetic resonance image shows a strongly enhancing mass overlying the right carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
 Carotid body tumor. Axial T2-weighted magnetic resonance image reveals splaying of external and internal carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Axial T2-weighted magnetic resonance image reveals splaying of external and internal carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumors are particularly well demonstrated on MRI, which can show that enhancing soft tissue masses protrude both intracranially and extracranially at the skull base. Direct coronal imaging can show tumoral relationships to adjacent structures such as the brainstem and the skull base, and deep cervical soft tissue structures are extraordinarily well depicted.
The degree of confidence is high. Tumor allocation and intense tumor enhancement are of paramount importance in diagnosis.
Hypervascular lymphadenopathy may result in false-positive findings, which are reported in a variety of disorders such as metastatic papillary carcinoma of the thyroid gland. In particular, MRI findings can be confusing if T2-weighted images show a salt-and-pepper pattern. In such instances, location is a key finding.
As with CT imaging, lack of sufficient contrast enhancement can be troublesome and may result in false-negative findings. In this case, glomus tumors can mimic schwannomas, neurofibromas, or nonenhancing lymphadenopathies if an insufficient amount of contrast material is administered. Small vascular tumors may be missed if they cannot be clearly distinguished from adjacent vascular structures.
Ultrasonography
Doppler ultrasonography can show cervical masses when images are obtained below the angle of the mandible, above the sternum, or in a superficial location not hidden by bone. Ultrasonography can reveal the extent of masses, as well as their locations. With glomus tumors, diagnosis of carotid body tumors is possible on ultrasonographic cervical imaging, but this modality does not suitably reveal the locations of glomus vagale, jugulare, or tympanicum tumors.
Because of tumor neovascularity, Doppler ultrasonographic sampling of cervical masses such as carotid body tumors can be helpful in diagnosis. In addition, if recognized, increased flow velocities in the external carotid artery or in the jugular vein can provide an indirect clue to diagnosis of a vascular mass that is above the ultrasonographic imaging field.
The degree of confidence is high if these tumors are found on ultrasonography. Hypervascular lymphadenopathy can mimic a glomus tumor and may produce a false-positive result. Many glomus tumors are seen in the neck above the level of the mandibular angle, rendering diagnosis impossible with the use of ultrasonography alone; a false-negative finding can result.
Angiography
Glomus tumors of the head and neck are typically highly vascular, as shown on angiography. This finding differentiates them from other types of neck neoplasia.
(See the images below.)
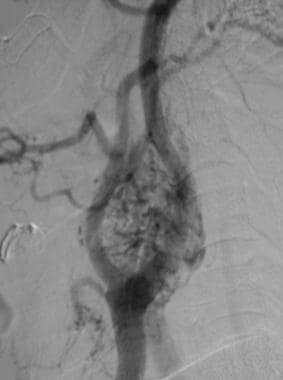 Carotid body tumor. Lateral angiogram shows a densely vascular mass at the level of the carotid bifurcation that causes splaying of internal and external carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Lateral angiogram shows a densely vascular mass at the level of the carotid bifurcation that causes splaying of internal and external carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
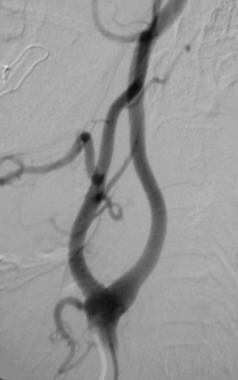 Carotid body tumor. Post embolization with polyvinyl alcohol. Many feeding arteries arising from the occipital artery and superior thyroid arteries were embolized to achieve this result. Reprinted with permission. Copyright Springer-Verlag.
Carotid body tumor. Post embolization with polyvinyl alcohol. Many feeding arteries arising from the occipital artery and superior thyroid arteries were embolized to achieve this result. Reprinted with permission. Copyright Springer-Verlag.
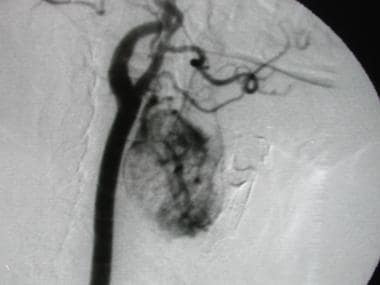 Angiogram in a patient who had a biopsy-proven renal cell carcinoma that metastasized to a laryngeal lymph node and formed a hypervascular mass that mimicked a glomus tumor. This mass was not in a typical location for a glomus tumor, despite its vascular appearance. Reprinted with permission. Copyright Springer-Verlag.
Angiogram in a patient who had a biopsy-proven renal cell carcinoma that metastasized to a laryngeal lymph node and formed a hypervascular mass that mimicked a glomus tumor. This mass was not in a typical location for a glomus tumor, despite its vascular appearance. Reprinted with permission. Copyright Springer-Verlag.
Typical carotid body tumors are situated in the carotid bifurcation and derive their arterial supply from regional external carotid branch arteries. These include ascending pharyngeal and occipital arteries.
Glomus vagale and jugulare tumors are encountered higher in the neck and at the skull base; therefore, these masses concomitantly involve higher external carotid branch vessels.
(See the images below.)
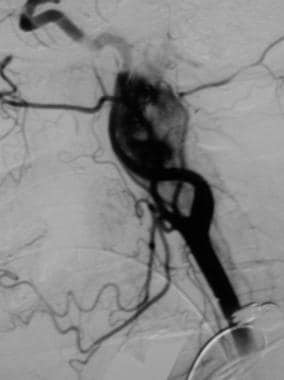 Glomus vagale tumor. Angiogram reveals a densely vascular mass. Reprinted with permission. Copyright Springer-Verlag.
Glomus vagale tumor. Angiogram reveals a densely vascular mass. Reprinted with permission. Copyright Springer-Verlag.
 Glomus jugulare tumor. Selective external carotid angiogram reveals a vascular skull base mass. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Selective external carotid angiogram reveals a vascular skull base mass. Reprinted with permission. Copyright Springer-Verlag.
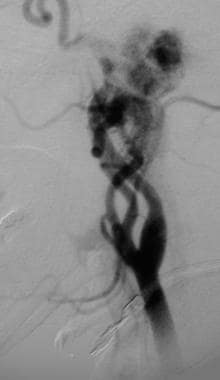 Glomus jugulare tumor. Lateral angiogram reveals a large vascular mass arising from branches of the external carotid artery. Note anterior bowing of the internal carotid artery and extension of the mass through the skull base. Reprinted with permission. Copyright Springer-Verlag.
Glomus jugulare tumor. Lateral angiogram reveals a large vascular mass arising from branches of the external carotid artery. Note anterior bowing of the internal carotid artery and extension of the mass through the skull base. Reprinted with permission. Copyright Springer-Verlag.
 Multiple glomus tumors. Angiogram obtained in a female patient with bilateral carotid body tumors, bilateral glomus vagale tumors, and left glomus jugulare tumors with corresponding angiographically enhancing masses. Reprinted with permission. Copyright Springer-Verlag.
Multiple glomus tumors. Angiogram obtained in a female patient with bilateral carotid body tumors, bilateral glomus vagale tumors, and left glomus jugulare tumors with corresponding angiographically enhancing masses. Reprinted with permission. Copyright Springer-Verlag.
Ascending pharyngeal, tympanic, and occipital arteries dominate the arterial blood supply. Neovascularity may be extremely intense, and arteriovenous fistulae may be present. Rarely, internal carotid and vertebral arteries may contribute feeders to neoplasms. Typically, these tumors are evaluated with attention paid to all potential feeding arteries. Care is taken to evaluate occult contralateral or ipsilateral masses, which occasionally can be overlooked during cross-sectional imaging.
The degree of confidence is high. The hallmark of a glomus tumor is its intrinsic neovascularity. At times, lymph node neovascularity can be difficult to differentiate from that of glomus tumors. In these situations, location is a key feature.
Latest Developments
Head and neck paragangliomas are slowly growing benign tumors that originate from specialized neural crest cells. Choice of treatment depends on size, location, and biologic activity of the tumor, as well as the physical condition of the patient. Glomus tumors can be resected with low mortality and morbidity rates because of developing imaging and microsurgical methods. [36]
Most head and neck paragangliomas (PGLs) are biochemically silent; they often present with recurrence and metastases in association with hereditary syndromes. Whole-body functional imaging is increasingly used to detect tumor extent and to guide planning for treatment of PGLs. [68Ga]-DOTATATE, which targets somatostatin receptor 2 (SSTR2) overexpression, has emerged as a sensitive functional imaging modality in PGLs. [37]
CyberKnife is a robotic stereotactic radiotherapy system that is a good alternative to surgery for treatment of head and neck paragangliomas. Investigators have concluded that CyberKnife seems to be a safe and efficient system with minimal side effects over the long term, and that CyberKnife may have a role as first-line therapy, especially for patients with symptomatic nonfunctional head and neck paragangliomas, to achieve better symptom control. [38, 39]
Cass and colleagues provided an overview of head and neck paragangliomas (HNPGLs) and details of the latest discoveries regarding their molecular genetics, along with updated recommendations for workup, treatment, and surveillance. They noted that recommendations regarding HNPGLs have undergone a fundamental reorientation in the last decade as a result of improved understanding of the genetic and pathophysiologic basis of these disorders. SDHx susceptibility gene mutations, encoding subunits of the enzyme succinate dehydrogenase (SDH), give rise to hereditary pheochromocytoma/paraganglioma syndromes. SDHA, SDHB, SDHC, SDHD, and SDHAF2 mutations all result in unique phenotypes with distinct penetrance and risk for variable tumor development, as well as metastasis. Genetic and biochemical testing is recommended for every patient with HNPGL. Multifocal disease should be managed in multidisciplinary fashion. Patients with SDHx mutations require frequent biochemical screening and whole-body imaging, as well as lifelong follow-up with an expert in hereditary pheochromocytoma and paraganglioma syndromes. [40]
Otolaryngologists are likely to encounter patients with HNPGL. Keeping abreast of the latest recommendations, especially regarding genetic testing, workup for additional tumors, multidisciplinary approaches to care, and the need for lifelong surveillance, will help otolaryngologists provide appropriate care for these patients. [40]
-
Glomus jugulare tumor. Computed tomography scan shows a permeative destructive skull base mass with involvement of mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus jugulare tumor. Selective external carotid angiogram reveals a vascular skull base mass. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus jugulare tumor. Axial contrast-enhanced T1-weighted magnetic resonance image shows an enhancing right skull base mass. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus jugulare tumor. Axial T2-weighted magnetic resonance image shows salt-and-pepper vascularity in the substance of the tumor. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus jugulare tumor. Coronal T1-weighted contrast-enhanced magnetic resonance image shows a mass protruding into the jugular foramen. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus jugulare tumor. Lateral angiogram reveals a large vascular mass arising from branches of the external carotid artery. Note anterior bowing of the internal carotid artery and extension of the mass through the skull base. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus vagale tumor. Contrast-enhanced computed tomography scan showing a large vascular mass along the course of the left internal carotid artery and jugular vein above the level of the carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus vagale tumor. Angiogram reveals a densely vascular mass. Reprinted with permission. Copyright Springer-Verlag.
-
Carotid body tumor. Computed tomography scan shows an enhancing carotid bifurcation mass. Reprinted with permission. Copyright Springer-Verlag.
-
Carotid body tumor. Contrast-enhanced T1-weighted magnetic resonance image shows a strongly enhancing mass overlying the right carotid bifurcation. Reprinted with permission. Copyright Springer-Verlag.
-
Carotid body tumor. Axial T2-weighted magnetic resonance image reveals splaying of external and internal carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
-
Carotid body tumor. Lateral angiogram shows a densely vascular mass at the level of the carotid bifurcation that causes splaying of internal and external carotid arteries. Reprinted with permission. Copyright Springer-Verlag.
-
Carotid body tumor. Post embolization with polyvinyl alcohol. Many feeding arteries arising from the occipital artery and superior thyroid arteries were embolized to achieve this result. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus tympanicum tumor. Axial computed tomography image shows a small vascular mass along the right tympanic membrane. Note adjacent opacified mastoid air cells. Reprinted with permission. Copyright Springer-Verlag.
-
Glomus tympanicum tumor. Coronal computed tomography scan confirms the presence of a known hypotympanic mass consistent with glomus tympanicum. Reprinted with permission. Copyright Springer-Verlag.
-
Multiple glomus tumors. Angiogram obtained in a female patient with bilateral carotid body tumors, bilateral glomus vagale tumors, and left glomus jugulare tumors with corresponding angiographically enhancing masses. Reprinted with permission. Copyright Springer-Verlag.
-
Angiogram in a patient who had a biopsy-proven renal cell carcinoma that metastasized to a laryngeal lymph node and formed a hypervascular mass that mimicked a glomus tumor. This mass was not in a typical location for a glomus tumor, despite its vascular appearance. Reprinted with permission. Copyright Springer-Verlag.






