Practice Essentials
Germinal matrix hemorrhage (GMH) and intraventricular hemorrhage (IVH) are the most common and most important neurologic injuries in preterm neonates. The brain of a premature infant lacks the ability to autoregulate cerebral blood pressure; thus, fluctuations in cerebral blood pressure and flow can rupture the primitive germinal matrix vessels or lead to infarction of the metabolically active germinal matrix. The damage can extend into the periventricular white matter, resulting in significant neurologic sequelae, including cerebral palsy, intellectual disability, and seizures. Injury to the germinal matrix has substantial mortality and morbidity rates. [1, 2, 3, 4]
The suspected primary causes of intraventricular hemorrhage are the fragile, thin vessels in the germinal matrix and the immature cerebral autoregulation mechanism in preterm neonates. [4, 5, 6, 7, 8, 9, 10, 11, 12, 13] In a study by Coskun et al of 144 preterm infants with a gestational age of 24-34 weeks, low gestational age, low Apgar score, and having a bleeding diathesis were the most important risk factors for IVH. [14]
Prognosis and mortality of IVH are related to the extent of injury as measured by grades I through IV (mortality of 4%, 10%, 18%, and 40% respectively). The degree of bleeding is based on ultrasound findings, as follows [4, 5, 6, 7, 8] :
-
Grade 1 – Germinal matrix alone
-
Grade 2 – IVH without dilation
-
Grade 3- IVH with dilation (>50% clot in ventricle)
-
Intraparenchymal Lesion (Grade 4) – Extension periventricular/intraparenchymal hemorrhage
Approximately 50% of cases of GMH/IVH occur during the first 24 hours after birth, and up to 90% of cases occur in the first 72 hours after birth. Risk factors for GMH/IVH include gestational age, very low birth weight, male sex, and low Apgar scores. [15, 14, 16] GMH occurs in approximately 3 live births per 1,000. It is associated with a 20-30% mortality and accounts for 1.7% of neonatal deaths in the United States. [17, 18, 19]
The risk of GMH/IVH increases with decreasing gestational age, as follows [9, 10, 13] :
-
In infants born at 24 weeks’ gestation, the incidence of the most severe lesions ranges from 10 to 25%.
-
Severe injury is diagnosed in less than 5% of infants born after 28 weeks’ gestation.
-
GMH/IVH rarely occurs in infants older than 32 weeks’ gestation.
In addition to being the leading cause of morbidity and mortality in premature infants, GMH is the leading cause of acquired infantile hydrocephalus. Severity of GMH is graded on a scale of I-IV on the basis of extent and localization of bleeding. [17, 18, 19]
A common lesion that characterizes the neuropathology of GMH/IVH is bleeding into the subependymal germinal matrix, with or without subsequent rupture into the lateral ventricle (see the images below). Sequelae of GMH/IVH include germinal matrix destruction, periventricular hemorrhagic infarction with subsequent encephalomalacia, and posthemorrhagic hydrocephalus. [20, 21, 22, 23]
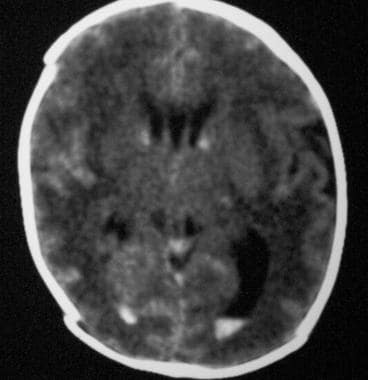 Axial nonenhanced computed tomography scan. The image shows bilateral grade 2 subependymal and intraventricular germinal matrix hemorrhage without hydrocephalus.
Axial nonenhanced computed tomography scan. The image shows bilateral grade 2 subependymal and intraventricular germinal matrix hemorrhage without hydrocephalus.
Preferred examination
Ultrasonography is the preferred screening and diagnostic tool for GMH. [24, 25] The portability of this modality allows imaging in the nursery with minimal disturbance of the infant. Ultrasonography also depicts GMHs that are larger than 5 mm, with a sensitivity of nearly 100% and specificity of 91%. Smaller GMHs, however, are more difficult to identify. Power and pulsed-wave Doppler ultrasonography can be used to identify preterm neonates who are at risk for GMH and IVH during their first week of life. Using this modality, clinicians can detect autoregulatory fluctuations in the preterm neonate's cerebral blood flow with examination of the lenticulostriate arteries; measurements of the peak velocity, resistive index, and coronal vascular cross-sectional area; and product of the peak velocity and vascular cross-sectional area. [26, 27, 28, 29]
According to the American Academy of Neurology, the preferred modality for diagnosis of IVH is transcranial Doppler ultrasound in neonates younger than 30 weeks’ gestational age. The AAN suggests that the initial ultrasound be performed at 7-14 days of life and repeated at 36-40 weeks of age. [4, 5, 6, 7, 8]
CT scanning and MRI are also used and have better interobserver agreement. [30] Because these modalities more readily depict small GMHs, CT scanning and MRI have a higher sensitivity than that of ultrasonography. However, these 2 imaging modalities require that the infant be moved from the nursery; there is also the possibility that sedation would be required. [26, 31, 32]
Limitations of techniques
All imaging modalities have relatively low negative predictive values (NPVs). Blankenberg et al found negative predictive values of 53% at 2-month follow-up and 59% at 2-year follow-up, irrespective of the modality. [33] However, the absence of neuroimaging abnormalities in the infant does not exclude the possibility of later neurodevelopmental problems.
Normal imaging findings must be viewed with caution. Ultrasonography, CT scanning, and MRI all have low negative predictive values of approximately 60%.
With fetal ultrasonography and fetal MRI, GMH/IVH can be identified in utero, remote in time from delivery. [34]
Intraventricular hemorrhage grading system
The IVH grading system created by Burstein et al relies on the detection of blood in the subependymal germinal matrix and the ventricles, as follows [35] :
-
Grade 1: Hemorrhage that is confined to the germinal matrix
-
Grade 2: Extension of the hemorrhage into the lateral ventricles without hydrocephalus
-
Grade 3: Ventricular hemorrhage with the presence of associated hydrocephalus
-
Grade 4: Parenchymal hemorrhage
Computed Tomography
Intraventricular hemorrhage (IVH) evolves in a predictable pattern. Acutely, it appears to hyperattenuate. After 7-10 days, the hemorrhage becomes isoattenuating relative to the brain parenchyma. Later, with clot retraction, a subependymal hematoma may develop into a fluid-filled cyst. The affected brain parenchyma may undergo atrophy and gliosis (see the image below).
Blankenberg et al found that CT scanning had nearly twice the sensitivity of ultrasonography in the detection of germinal matrix hemorrhage (GMH) and IVH; interobserver agreement with this modality was also improved relative to ultrasonography. [33]
A normal CT scan finding for GMH and IVH does not exclude abnormal neurodevelopment; the negative predictive value is 50-60% at age 2 years.
 Axial nonenhanced computed tomography scan. The image shows bilateral grade 2 subependymal and intraventricular germinal matrix hemorrhage without hydrocephalus.
Axial nonenhanced computed tomography scan. The image shows bilateral grade 2 subependymal and intraventricular germinal matrix hemorrhage without hydrocephalus.
Magnetic Resonance Imaging
In the first 3 days after intraventricular hemorrhage (IVH), subependymal hematomas are isointense to slightly hypointense on T1-weighted MRIs (T1WIs) and markedly hypointense on T2-weighted MRIs (T2WIs). In the early subacute stage during days 4-7, the signal intensity increases on T1WIs. In the late subacute stage during days 7-14, the signal intensity increases on T2WIs. Over the next several months, the hemorrhage becomes hypointense on images obtained with both sequences, and ferromagnetic effects secondary to hemosiderin and ferritin predominate. [31]
As with CT scanning, Blankenberg et al found that MRI had nearly twice the sensitivity of ultrasonography in the detection of germinal matrix hemorrhage (GMH) and IVH, and interobserver agreement with this modality was also improved relative to ultrasonography. [33] A normal image finding for GMH/IVH does not exclude abnormal neurodevelopment. The negative predictive value is 50-60% at age 2 years.
(See the images below.)
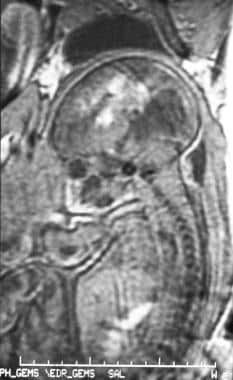 Sagittal spoiled gradient-recalled echo (SPGR) magnetic resonance image (MRI) of a fetus at 34 weeks' gestation in a 29-year-old woman. The MRI was performed to investigate fetal tachycardia; the image demonstrates intraventricular hemorrhage and parenchymal hematoma.
Sagittal spoiled gradient-recalled echo (SPGR) magnetic resonance image (MRI) of a fetus at 34 weeks' gestation in a 29-year-old woman. The MRI was performed to investigate fetal tachycardia; the image demonstrates intraventricular hemorrhage and parenchymal hematoma.
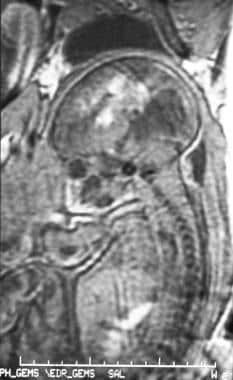
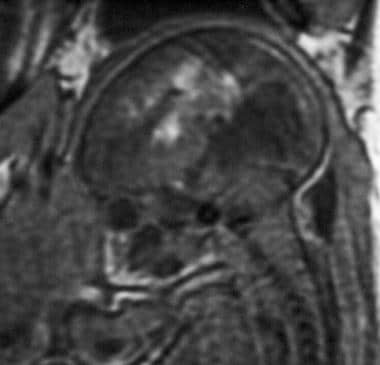 Sagittal spoiled gradient-recalled echo (SPGR) magnetic resonance image (MRI) of a fetus at 34 weeks' gestation in a 29-year-old woman (same patient as in the previous image). An ultrasonogram was initially performed to investigate fetal tachycardia and revealed hydrocephalus. Subsequent SPGR MRI demonstrated a grade 4 germinal matrix hemorrhage in utero.
Sagittal spoiled gradient-recalled echo (SPGR) magnetic resonance image (MRI) of a fetus at 34 weeks' gestation in a 29-year-old woman (same patient as in the previous image). An ultrasonogram was initially performed to investigate fetal tachycardia and revealed hydrocephalus. Subsequent SPGR MRI demonstrated a grade 4 germinal matrix hemorrhage in utero.
 Sagittal spoiled gradient-recalled echo magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
Sagittal spoiled gradient-recalled echo magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
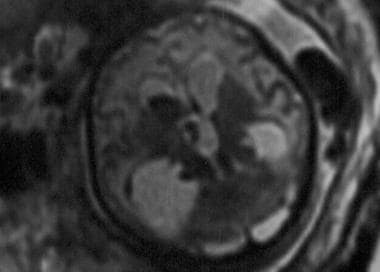 Axial single-shot, fast spin-echo, T2-weighted magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
Axial single-shot, fast spin-echo, T2-weighted magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
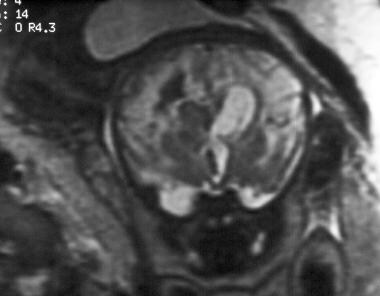 Coronal single-shot, fast spin-echo, T2-weighted magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
Coronal single-shot, fast spin-echo, T2-weighted magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
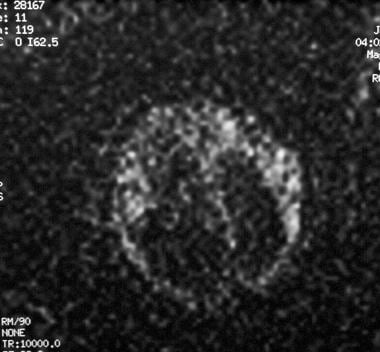 Axial diffusion-weighted magnetic resonance image (MRI) of a fetus. The MRI demonstrates no evidence of an acute infarct. Abnormal parenchymal signal intensity seen on MRIs obtained with other sequences is most compatible with hemorrhage.
Axial diffusion-weighted magnetic resonance image (MRI) of a fetus. The MRI demonstrates no evidence of an acute infarct. Abnormal parenchymal signal intensity seen on MRIs obtained with other sequences is most compatible with hemorrhage.
Ultrasonography
Neurosonography is the primary modality for both screening and follow-up of germinal matrix hemorrhage (GMH) and intraventricular hemorrhage (IVH) in neonates. Ultrasonography is portable, allowing imaging in the comfortable environment of the neonatal intensive care unit (NICU). This modality has negative predictive values similar to those of CT scanning and MRI. Current screening protocols recommend performing ultrasonographic studies on days 7-14 of life and between the fourth and sixth weeks of life. Many centers offer more frequent screening. [27, 28, 36]
On ultrasonograms, acute subependymal hemorrhage appears as a homogeneous echogenic mass, often in the caudothalamic groove (see the image below).
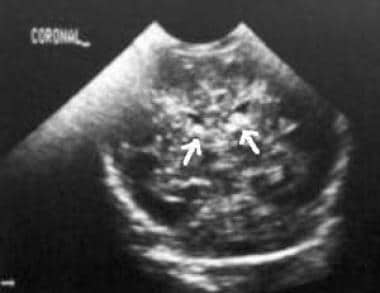 Coronal ultrasonogram of a 1-day-old neonate. The image demonstrates a bilateral grade 2 subependymal hemorrhage. Note the echogenic clot in the left temporal horn (arrow, right side).
Coronal ultrasonogram of a 1-day-old neonate. The image demonstrates a bilateral grade 2 subependymal hemorrhage. Note the echogenic clot in the left temporal horn (arrow, right side).
The hematoma becomes less echogenic over time, beginning with the central portion. Subsequent to eventual clot retraction, a subependymal cyst may develop, or a linear echo may result (see the following image).
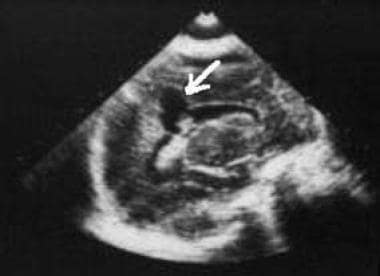 Sagittal ultrasonogram. The image demonstrates a grade 3 germinal matrix hemorrhage and shows a subependymal focus of hyperechogenicity (arrow) that represents hemorrhage, as well as intraventricular hemorrhage and hydrocephalus.
Sagittal ultrasonogram. The image demonstrates a grade 3 germinal matrix hemorrhage and shows a subependymal focus of hyperechogenicity (arrow) that represents hemorrhage, as well as intraventricular hemorrhage and hydrocephalus.
Acutely, IVH also appears echogenic. Cerebrospinal fluid (CSF)–blood fluid levels may be observed. When large, the clot forms a cast of the ventricle (see the image below) and may break up in the ventricle, resulting in low-level echoes that float in the CSF.
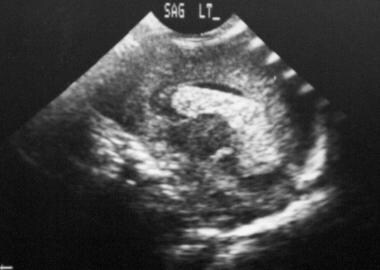 Sagittal ultrasonogram. The image shows intraventricular hemorrhage; the clot forms a cast of the ventricle.
Sagittal ultrasonogram. The image shows intraventricular hemorrhage; the clot forms a cast of the ventricle.
The clot may also move when the patient's head position changes. With clot evolution, the hematoma becomes echolucent, starting centrally (see the following images). Scanning through the posterior fontanelle may optimize visualization of occipital horn clots.
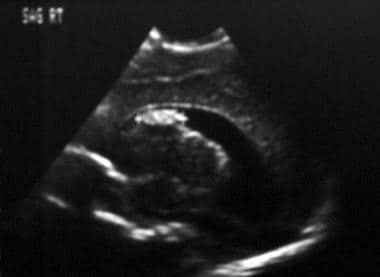 Sagittal neurosonogram. The image demonstrates a grade 3 germinal matrix hemorrhage with an intraventricular clot and hydrocephalus.
Sagittal neurosonogram. The image demonstrates a grade 3 germinal matrix hemorrhage with an intraventricular clot and hydrocephalus.
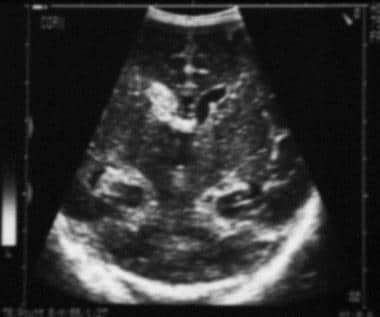 Coronal neurosonogram. The image demonstrates (right) grade 4 germinal matrix and intraventricular hemorrhage and (left) grade 1 germinal matrix hemorrhage.
Coronal neurosonogram. The image demonstrates (right) grade 4 germinal matrix and intraventricular hemorrhage and (left) grade 1 germinal matrix hemorrhage.
Intraparenchymal hemorrhage is usually located in the frontal and parietal lobes and appears acutely as an echogenic homogeneous mass. As the hemorrhage evolves, an echogenic rim with a sonolucent center forms. After 2-3 months, a porencephalic cyst (if the lesion communicates with a ventricle) or encephalomalacia may develop (see the image below).
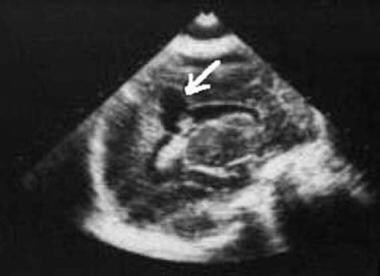 Sagittal ultrasonogram. The images shows cystic encephalomalacia as a sequela of previous germinal matrix hemorrhage. In a region of an earlier parenchymal hemorrhage, a cerebrospinal fluid-filled cyst has formed (arrow).
Sagittal ultrasonogram. The images shows cystic encephalomalacia as a sequela of previous germinal matrix hemorrhage. In a region of an earlier parenchymal hemorrhage, a cerebrospinal fluid-filled cyst has formed (arrow).
Power and pulsed-wave Doppler ultrasonography may be useful in identifying preterm neonates who are at risk of GMH/IVH during their first week of life. The ultrasonograms may depict autoregulatory fluctuations in cerebral blood flow. Neurosonography depicts GMHs that are larger than 5 mm, with a sensitivity of 100% and a specificity of 91%. IVH may blend imperceptibly with the choroid plexus, which has a similar echo texture; thus, asymmetric thickness of the choroid plexus should be viewed with suspicion. The lack of abnormality with ultrasonography does not exclude the possibility of later neurodevelopmental problems.
-
Axial nonenhanced computed tomography scan. The image shows bilateral grade 2 subependymal and intraventricular germinal matrix hemorrhage without hydrocephalus.
-
Coronal ultrasonogram of a 1-day-old neonate. The image demonstrates a bilateral grade 2 subependymal hemorrhage. Note the echogenic clot in the left temporal horn (arrow, right side).
-
Sagittal spoiled gradient-recalled echo (SPGR) magnetic resonance image (MRI) of a fetus at 34 weeks' gestation in a 29-year-old woman. The MRI was performed to investigate fetal tachycardia; the image demonstrates intraventricular hemorrhage and parenchymal hematoma.
-
Sagittal spoiled gradient-recalled echo (SPGR) magnetic resonance image (MRI) of a fetus at 34 weeks' gestation in a 29-year-old woman (same patient as in the previous image). An ultrasonogram was initially performed to investigate fetal tachycardia and revealed hydrocephalus. Subsequent SPGR MRI demonstrated a grade 4 germinal matrix hemorrhage in utero.
-
Sagittal spoiled gradient-recalled echo magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
-
Axial single-shot, fast spin-echo, T2-weighted magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
-
Coronal single-shot, fast spin-echo, T2-weighted magnetic resonance image of a fetus. The image demonstrates a grade 4 germinal matrix hemorrhage in utero.
-
Axial diffusion-weighted magnetic resonance image (MRI) of a fetus. The MRI demonstrates no evidence of an acute infarct. Abnormal parenchymal signal intensity seen on MRIs obtained with other sequences is most compatible with hemorrhage.
-
Sagittal ultrasonogram. The image demonstrates a grade 3 germinal matrix hemorrhage and shows a subependymal focus of hyperechogenicity (arrow) that represents hemorrhage, as well as intraventricular hemorrhage and hydrocephalus.
-
Sagittal ultrasonogram. The image shows intraventricular hemorrhage; the clot forms a cast of the ventricle.
-
Sagittal neurosonogram. The image demonstrates a grade 3 germinal matrix hemorrhage with an intraventricular clot and hydrocephalus.
-
Coronal neurosonogram. The image demonstrates (right) grade 4 germinal matrix and intraventricular hemorrhage and (left) grade 1 germinal matrix hemorrhage.
-
Sagittal ultrasonogram. The images shows cystic encephalomalacia as a sequela of previous germinal matrix hemorrhage. In a region of an earlier parenchymal hemorrhage, a cerebrospinal fluid-filled cyst has formed (arrow).




