Practice Essentials
Abdominal wall defects range from the mild umbilical cord hernia to the highly complex limb-body wall syndrome. The most common defects are gastroschisis and omphalocele; rarer ones include the exstrophy complex, pentalogy of Cantrell, and limb-body wall syndrome. Although all have a common feature of viscera herniation through a defect in the anterior body wall, their imaging features and, more important, postnatal management differ widely. Correct diagnosis of each entity is imperative if appropriate and accurate prenatal counseling and postnatal management are to be provided. [1]
Gastroschisis represents a herniation of abdominal contents through a paramedian full-thickness abdominal fusion defect. The abdominal herniation is usually to the right of the umbilical cord. No genetic association exists. A gastroschisis usually contains small bowel and has no surrounding membrane. The herniated bowel is not rotated and is devoid of secondary fixation to the posterior abdominal wall. [2, 3, 4]
Gastroschisis occurs in 1 in 2000 births and is ordinarily detected during prenatal ultrasound scanning. Fetal outcomes vary from uncomplicated surgical correction to stillbirth or neonatal death. Early markers on ultrasound indicating increased mortality include abdominal circumference less than the fifth percentile and an abnormal gastric bubble. [5] Survival rates are partially contingent on intestinal complications and time to establishing feeding. Enhancements in prenatal imaging have given better insight into postnatal outcomes. [6]
Fetal development of gastroschisis is a dynamic process lasting until birth. The typical morphology of gastroschisis changes from the second to the third trimester because the intra-abdominal bowel becomes eventrated by the end of the second trimester. This process of eventration is stopped in cases of intestinal stenosis/atresia caused by narrowing of the abdominal wall defect, resulting in different lengths of intra-abdominal bowel. The time when this occurs may correlate with the amount of viable bowel in cases of intestinal atresia. [7]
(See the images below.)
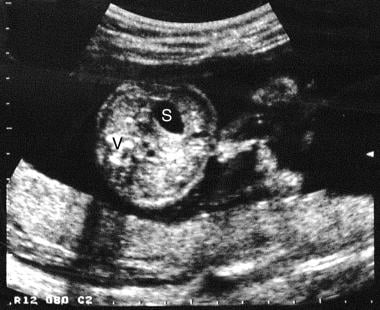 Axial sonogram through the mid to upper abdomen. This image shows free-floating exteriorized bowel in relation to the anterior abdominal wall. S = stomach; V = spine.
Axial sonogram through the mid to upper abdomen. This image shows free-floating exteriorized bowel in relation to the anterior abdominal wall. S = stomach; V = spine.
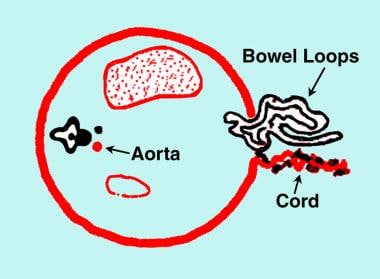 Diagram of the transverse section of the fetal abdomen showing gastroschisis. Note the bowel herniation in the right paramedian/paraumbilical region. The cord is inserted in the normal location to the left of the herniation. No membranous covering exists over the herniated bowel.
Diagram of the transverse section of the fetal abdomen showing gastroschisis. Note the bowel herniation in the right paramedian/paraumbilical region. The cord is inserted in the normal location to the left of the herniation. No membranous covering exists over the herniated bowel.
Because the herniated bowel is bathed by amniotic fluid, alpha-fetoprotein (AFP) levels in both maternal serum and amniotic fluid are elevated, more so than in exomphalos. Thus, gastroschisis is found incidentally or when maternal AFP level is elevated—a finding in 77-100% of cases. Rarely, polyhydramnios may prompt an antenatal sonographic examination. Fetal growth restriction is a frequent association. Oligohydramnios is rare. Chromosomal anomalies are not associated with gastroschisis, and familial occurrence is exceptionally rare.
Gastroschisis usually is detected in the second trimester by means of antenatal sonography. [8, 9, 10, 11, 12, 13, 14, 15, 16] The diagnosis is often made when antenatal sonography is performed before 20 weeks' gestation. With transvaginal sonograms, the diagnosis has been made as early as 12 weeks' gestation.
In early pregnancy, the bowel loops can be seen floating in the amniotic fluid. Thickness and diameter of the bowel are normal. Later in pregnancy, bowel obstruction, peritonitis, bowel perforation, and fetal growth restriction may occur. Intrauterine growth restriction (IUGR) occurs in 38-77% of fetuses, usually secondary to nutrient loss through exposed bowel. Approximately 48% of infants with gastroschisis are small for gestational age. [17]
Bowel diameter greater than 17 mm usually represents significant bowel dilatation, and bowel diameter greater than 11 mm is usually associated with a greater number of postnatal bowel complications. Sonographic findings of bowel abnormalities are associated with difficult abdominal wall repair and an increased incidence of complications.
Approximately 50% of fetuses with gastroschisis are small for gestational date. Fetal abdominal circumference, which is regarded as a standard reference for assessment of fetal size, does not apply to this group of fetuses; therefore, obstetric management may be difficult.
The mortality rate of gastroschisis is approximately 17%. Surgical repair should be offered within the first day after delivery to avoid infection. The outcome is no different in infants delivered in tertiary obstetric centers than in infants delivered in smaller peripheral hospitals, although delivery within easy access of a neonatal surgical unit is advised. Cesarean delivery is performed in many mothers of fetuses with gastroschisis, although this does not convey any advantage over vaginal delivery.
A meta-analysis by South and associates shows that the overall incidence of intrauterine fetal death (IUFD) in gastroschisis is much lower than was previously reported. The largest risk of IUFD occurs before routine and elective early delivery would be acceptable. This analysis concluded that risk of IUFD should not be the primary indication for routine elective preterm delivery in pregnancies that are affected by gastroschisis. [18]
Imaging modalities
Antenatal sonography is the key imaging modality available, with detection rates of 70-72%. Prenatal sonography is the primary imaging modality in pregnancy because it is noninvasive and rapid, and it allows real-time fetal examination. Plain radiographs and bowel contrast studies may be indicated in the postnatal postoperative period for assessment of bowel complications.
In studies exploring the association between antenatal ultrasound signs and outcomes in gastroschisis, significant positive associations were identified between intra-abdominal bowel dilatation (IABD) and bowel atresia, polyhydramnios and bowel atresia, and gastric dilatation and neonatal death. No other ultrasound sign was significantly related to any other outcome. [4]
With the use of antenatal sonography, a surgically treatable malformation is diagnosed before birth in an increasing number of fetuses. This allows fetal intervention, in utero transfer, planned delivery in a specialized unit, and antenatal counseling of parents regarding the likely prognosis and outcome. [19]
Fetal magnetic resonance imaging (MRI) is increasingly used in congenital abdominal wall defects. In gastroschisis, the role of fetal MRI in surgical therapy is poorly understood. Currently, the type of repair is determined primarily by clinical presentation and by institutional preference. [20]
Limitations of techniques
Sonography remains operator dependent, and artifacts are a problem. Despite the straightforward nature of the defect, a diagnosis of gastroschisis can be missed.
Misdiagnosis of exomphalos as gastroschisis has occurred in 5% of patients. This misdiagnosis has serious implications because exomphalos is often associated with chromosomal and other severe anomalies, and karyotyping is not performed in patients with gastroschisis.
In one case series, gastroschisis was misdiagnosed as exomphalos at a rate of 14.7%. This misdiagnosis results in unnecessary amniocentesis, which exposes the fetus to the risks involved in amniocentesis and the mother to psychological trauma.
Assessment of fetal size via abdominal circumference measurements is difficult in the presence of gastroschisis. Postnatal plain radiographs and bowel contrast studies lack specificity and expose the infant to a radiation burden. However, Siemer et al have developed a sonographic weight formula for fetuses with abdominal wall defects. [17] These authors evaluated their formula in a group of 97 fetuses with either gastroschisis or omphalocele and concluded that it provided significantly greater accuracy in estimating fetal weight than a more commonly used formula. More data will be necessary to determine the utility of this formula.
Radiography
Conventional radiography is no longer used in assessment of fetal abnormalities because this imaging modality exposes both mother and fetus to an unnecessary radiation burden. However, conventional radiology does have a role in postnatal evaluation, particularly of infants in the postoperative period.
A minority of infants develop complications such as necrotizing enterocolitis, short-bowel syndrome, persistent bowel dysfunction, and cholestatic jaundice. Investigation by means of plain imaging, contrast studies, and sonographic examination is necessary and helpful.
Plain images reveal bowel wall thickening, luminal dilatation, and generalized abdominal distention. Small-bowel enema is considered superior to conventional follow-through in distinguishing mechanical obstruction from functional obstruction in infants with persistent bowel dilatation. [21]
Degree of confidence
Conventional radiographs demonstrate poor resolution between maternal parts and fetal parts, and they have no place in antenatal management. Plain-film images and contrast radiography are excellent noninvasive tools for use in the investigation of persistent postoperative gaseous distention in infants; plain radiography remains the most useful noninvasive procedure in the radiographic diagnosis of small-bowel obstruction despite its limited sensitivity (50-66%). When plain radiographic findings are combined with clinical history and results of physical and laboratory examinations, a diagnosis of small-bowel obstruction can usually be confidently made.
Plain radiographs cannot always be used to differentiate mechanical obstruction from functional obstruction. Differentiating an adynamic ileus from a mechanical small-bowel obstruction may be particularly difficult, especially in the immediate postoperative period. Eventually, most intestinal obstructions (especially those in which strangulation is present) may lead to an adynamic ileus, which is associated with perforation and peritonitis. Under these circumstances, gas may appear in the small bowel proximal to the obstruction or may be retained in the atonic colon, leading to diagnostic confusion. Little or no gas within the small bowel may lead to a false-negative diagnosis.
Magnetic Resonance Imaging
In general, magnetic resonance imaging (MRI) has not been used in the diagnosis of gastroschisis, in part because MRI is time consuming, expensive, and limited in availability. Moreover, to date, image quality has been poor. Some of these problems have been overcome with the use of ultrafast sequences; therefore, MRI can be used as an adjunct to sonography, especially in patients for whom sonographic findings are unclear or are degraded as a result of obesity or oligohydramnios. [8, 22, 23, 24, 25, 26, 27]
Fetal MRI is increasingly used in congenital abdominal wall defects. In gastroschisis, the role of fetal MRI in surgical therapy is poorly understood. Currently, the type of repair to be performed is determined primarily by clinical presentation and institutional preference. [20]
Findings on MRI frequently add information beyond that provided by sonograms. This information commonly changes the selected approach to patient counseling and, at times, patient treatment. [8, 22, 23, 24, 25, 26] MRI provides a global view of the fetus and analysis of the anatomy in multiple planes. This imaging modality is useful for diagnosis of intestinal obstruction and for detection of microcolon, malrotation, and volvulus.
Degree of confidence
Fetal MRI during the first trimester remains controversial secondary to biosafety issues, and its use is limited because of the diminutive fetal size. However, fetal abdominal masses are well depicted on MRI, and the information obtained is not affected by diminished amounts of amniotic fluid. Sufficient experience has not yet been gained with MRI in the diagnosis of anterior abdominal wall defects to determine the existence of false-positive findings.
Ultrasonography
Prenatal sonography is the primary imaging modality in pregnancy because it is noninvasive and rapid, and because it allows real-time fetal examination. [4, 5, 28, 29, 30]
The anterior abdominal wall and the umbilical cord insertion are readily recognized on antenatal scanning because the wall provides a direct interface between itself and the amniotic fluid. The anterior abdominal wall is best demonstrated by axial scans. Assessment of the lower anterior abdominal wall is occasionally made difficult by flexed fetal limbs. The inner aspect of the anterior abdominal wall is difficult to see because its echodensities are identical to the remainder of the abdominal viscera unless fetal ascites is present. [31, 32]
Gastroschisis results from herniation/evisceration of small bowel into the amniotic cavity through a small defect (2-5 cm) in the right paraumbilical region. It has been reported that the defect can be located in the left paraumbilical region, but this site is extremely rare. No covering membrane exists. The large bowel (common), pancreas, stomach, liver (rare), spleen, bladder, uterus, ovaries, and fallopian tubes may also be herniated. The attachment of the umbilical cord is normal.
Sonographic features that suggest gastroschisis
Abdominal wall defects are a complex group of anomalies, and many are incorrectly diagnosed. Evaluation of the defect relative to the umbilical cord insertion site is fundamentally important in differentiating between the various malformations. The 2 most common abdominal wall defects are gastroschisis, in which the defect is on the right side of the normally inserting cord and free-floating bowel loops are present, and omphalocele, in which the cord inserts on a membrane-covered midline defect. With use of an algorithmic approach beginning with discovery of the location of the defect, a more precise diagnosis can be determined that may directly affect prenatal and postnatal management decisions. [33]
Findings include exteriorized bowel in relation to the anterior abdominal wall (see the image below), multiple loops of bowel, and a thickened bowel floating freely in the amniotic fluid. The bowel can be identified by its characteristic sonographic pattern.
 Axial sonogram through the mid to upper abdomen. This image shows free-floating exteriorized bowel in relation to the anterior abdominal wall. S = stomach; V = spine.
Axial sonogram through the mid to upper abdomen. This image shows free-floating exteriorized bowel in relation to the anterior abdominal wall. S = stomach; V = spine.
No covering is present around the bowel loops of a gastroschisis, resulting in a mass with irregular edges (see the image below).
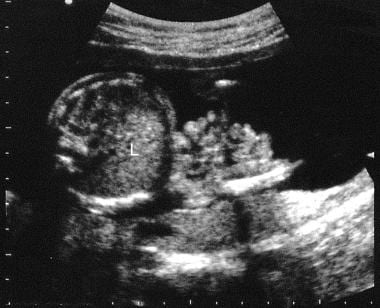 Axial sonogram through the mid abdomen of a fetus. This image shows exteriorized bowel in relation to the anterior abdominal wall. Multiple loops of bowel are depicted. Because the bowel loops are not covered, they have irregular edges. L = liver.
Axial sonogram through the mid abdomen of a fetus. This image shows exteriorized bowel in relation to the anterior abdominal wall. Multiple loops of bowel are depicted. Because the bowel loops are not covered, they have irregular edges. L = liver.
Usually, the small and large bowels are herniated, but occasionally, the stomach, liver, gallbladder, spleen, uterus, adnexa, and urinary bladder may be herniated.
Signs of intestinal obstruction may be noted; examples of these include multiple distended loops of bowel (both intraperitoneal and extraperitoneal), bowel loops greater than 17 mm in diameter, and increased peristalsis. Bowel diameter greater than 17 mm usually represents significant bowel dilatation, and diameter greater than 11 mm is usually associated with a greater number of postnatal bowel complications.
A right paramedian paraumbilical abdominal wall defect is revealed, usually measuring 2-5 cm. Insertion of the umbilical cord is normal. Typically, no ascites is noted. Bowel perforation can cause calcification and an intramesenteric extra-abdominal pseudocyst. Unlike with omphalocele, associated anomalies are uncommon, but if present, most are detectable on antenatal sonography.
Polyhydramnios may ensue in high intestinal obstruction. Polyhydramnios in third-trimester prenatal ultrasonography on babies with gastroschisis can predict complex gastroschisis at birth, whereas the absence of markers in prenatal ultrasonography can suggest uncomplicated disease. Complex gastroschisis is associated with increased time to feeds and length of stay. [6]
A 13-year retrospective analysis of ultrasound images in 59 pregnancies affected by fetal gastroschisis found that the only statistically significant predictor of complex cases of gastroschisis was extra-abdominal bowel dilatation. However, approximately 75% of infants with gastroschisis with associated extra-abdominal bowel dilatation had simple gastroschisis. [28]
In an 11-year retrospective study of of prenatal ultrasound markers for complex gastroschisis in the first 10 days of life, third-trimester intra-abdominal bowel dilatation (IABD) adjusted for gestational age appeared to be the prenatal ultrasound marker most strongly associated with adverse outcomes. [30]
Other considerations
Color Doppler and Doppler velocimetry of the mesenteric circulation have been used in the diagnosis of gastroschisis, but these findings add little to clinical outcomes and are not predictive of a poor neonatal outcome. [34]
Use of 3-dimensional (3-D) sonography in patients with abdominal wall defects can prove helpful in family counseling and in postnatal therapy planning. [10]
Sonography can be used in the postoperative neonatal period, when images may demonstrate features of intestinal obstruction, volvulus, perforation, ascites, and other fluid collections.
Prenatal imaging features of ventral body wall defects may be complex and challenging, often requiring from the radiologist a high level of suspicion and familiarity with the imaging patterns. Because appropriate management is dependent on accurate diagnosis and assessment of defects, radiologists should be able to recognize and distinguish between the various defects and their associated anomalies. [35]
Degree of confidence
The sensitivity of abnormality detection with sonography is 75% for gastroschisis and 77.3% for omphalocele. Ultrasound sensitivity in the diagnosis of gastroschisis has improved considerably.
Other anterior abdominal wall defects may mimic gastroschisis, but with the use of modern ultrasound equipment, confusion should not arise. When the liver is intra-abdominal in an omphalocele, a false diagnosis of gastroschisis may be entertained; however, the many anomalies associated with omphalocele should indicate the correct diagnosis. [17, 36] Bladder exstrophy, body stalk anomaly, and periumbilical blood clots are other mimics of gastroschisis.
-
Differentiation from physiologic bowel herniation: Physiologic bowel herniation occurs at 10-13 weeks. The best method for differentiating this from an omphalocele is to obtain a repeat sonogram after 15 weeks' menstrual age. A large defect with the liver exteriorized indicates an omphalocele at any gestational age.
-
Differentiation from umbilical hernia: Umbilical hernia results from a defect in the linea alba; the protruding bowel is covered by subcutaneous tissue and skin. Umbilical hernia is common in the first months in 20% of black neonates and in 3% of white neonates, and it is common in premature infants—specifically, in more than 5% of premature infants weighing less than 1500 g. Sonographic findings include a prominent bulge of the anterior abdominal wall; this bulge may contain omentum and/or bowel, which may protrude into the umbilical cord. Amniotic fluid AFP levels may be elevated when the bowel is herniated into the umbilical cord.
-
Differentiation from bladder exstrophy: Sonographically, bladder exstrophy may present as an external, well-defined, solid or complex mass immediately superior to the fetal genitalia. Prolonged and repeated scans fail to reveal the fetal bladder. The renal collecting system and ureters need not be dilated, and unilateral or horseshoe kidneys may be found. Uterine and adnexal anomalies are relatively frequent. The pubis is abnormally wide, and the umbilical cord insertion may be abnormal.
-
Differentiation from cloacal exstrophy: Cloacal exstrophy consists of a low omphalocele; bladder or cloacal exstrophy; and, frequently, other caudal anomalies. These other conditions may include meningomyelocele, anal atresia, and lower limb anomalies. Most affected fetuses have a single umbilical artery. Sonographic findings include a low anterior abdominal mass below the umbilical cord that is associated with an absent urinary bladder.
-
Axial sonogram through the mid to upper abdomen. This image shows free-floating exteriorized bowel in relation to the anterior abdominal wall. S = stomach; V = spine.
-
Axial sonogram through the mid abdomen of a fetus. This image shows exteriorized bowel in relation to the anterior abdominal wall. Multiple loops of bowel are depicted. Because the bowel loops are not covered, they have irregular edges. L = liver.
-
Diagram of the transverse section of the fetal abdomen showing gastroschisis. Note the bowel herniation in the right paramedian/paraumbilical region. The cord is inserted in the normal location to the left of the herniation. No membranous covering exists over the herniated bowel.
-
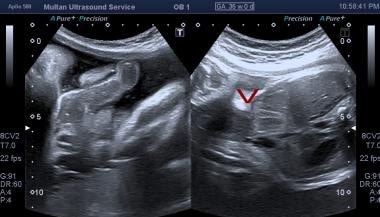
Ultrasound of fetal abdomen showing anterior wall defect.