Practice Essentials
Focal nodular hyperplasia (FNH) is the second most common tumor of the liver, surpassed in prevalence only by hepatic hemangioma. The incidence of FNH is estimated to be 3-5%, and it occurs most often in women in the third through fifth decades of life [1, 2] The liver is the only self-regenerative internal organ in the human body, and this regenerative ability puts the liver at risk for development of atypical masses. FNH is thought to be the result of increased hepatocytes caused by hypoperfusion or hyperperfusion from anomalous arteries in the hepatic lobule. Comorbid conditions can make diagnosis and management difficult. FNH is a hyperplastic process in which all the normal constituents of the liver are present but in an abnormally organized pattern. Results of liver function tests in these patients are usually within the reference range. [3]
(See the image below.)
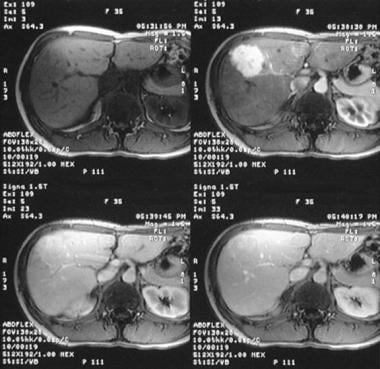 Dynamic MRIs in a 36-year-old woman referred for a gallbladder sonography, during which the patient was found to have a vague ill-defined hypoechoic mass in the right lobe of the liver (not shown). (Top left) Gadolinium-enhanced T1-weighted MRI demonstrates an ill-defined low-signal-intensity mass. (Top right) The mass enhances intensely in the arterial phase after the administration of contrast medium. (Bottom left) Minor enhancement persists in the portal venous phase. (Bottom right) The lesion becomes isointense relative to the liver on delayed images.
Dynamic MRIs in a 36-year-old woman referred for a gallbladder sonography, during which the patient was found to have a vague ill-defined hypoechoic mass in the right lobe of the liver (not shown). (Top left) Gadolinium-enhanced T1-weighted MRI demonstrates an ill-defined low-signal-intensity mass. (Top right) The mass enhances intensely in the arterial phase after the administration of contrast medium. (Bottom left) Minor enhancement persists in the portal venous phase. (Bottom right) The lesion becomes isointense relative to the liver on delayed images.
Although the use of contraceptive agents has not been implicated in the pathogenesis of FNH, their use is associated with an increase in the risk of complications for patients with FNH, and they may be a factor in the development of FNH. In symptomatic females, hemorrhagic foci or infarctions may occur within the FNH; these are aggravated by administration of contraceptive agents. The rare complication of a spontaneous rupture into the peritoneum has also been associated with contraceptive use.
In most patients, the clinical course is silent, and FNH is incidentally discovered during cross-sectional imaging, angiography, radionuclide liver scanning, or surgery. In most cases, FNH occurs as a solitary lesion (80-95%), but multiple lesions may occur. [4] Although FNH usually has no clinical significance, recognition of the radiologic characteristics of FNH is important to avoid unnecessary surgery, biopsy, and follow-up imaging.
Malignant transformation of FNH has not been reported. FNH must be differentiated from a fibrolamellar variant of hepatocellular carcinoma, with which it shares imaging and gross features. FNH has been reported in a liver transplant patient and should be included in the differential diagnoses of hepatic nodules of posttransplant livers.
Imaging modalities
Increasingly, focal nodular hyperplasia is being recognized as an incidental finding because of the widespread use of diagnostic imaging for unrelated conditions. For imaging of the right upper quadrant, ultrasonography (US) is more widely used than other modalities; usually, US findings raise the possibility of FNH. [1] Ultrasonography, particularly when combined with duplex Doppler US, may be the only type of imaging required. However, further confirmation may be required, particularly in patients in whom cancer is suspected at other sites. In this setting, CT, MRI, angiography, and radionuclide imaging may be used to increase diagnostic confidence. [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]
The diagnosis of focal nodular hyperplasia (FNH) is made on the basis of the demonstration of a central scar; however, a typical central scar is not demonstrated in every patient. In as many as 20% of patients, a scar may not be visible. Moreover, a central scar may be found in some patients with fibrolamellar hepatocellular carcinoma, hepatic adenoma, or intrahepatic cholangiocarcinoma. This limitation applies to all cross-sectional imaging techniques, including US, CT, and MRI.
The detection of lesions by use of radionuclide scans with technetium-99m sulfur colloid depends on the concentration of Kupffer cells in the FNH. If the concentration of Kupffer cells is low, FNH may appear as a photon-deficient mass that is indistinguishable from other liver mass lesions. On angiograms, the characteristic spokelike appearance is demonstrated in only 33% patients; moreover, FNH may be avascular in 10% of patients.
Plain radiographs have little to offer in the diagnosis of focal nodular hyperplasia (FNH). It has low specificity and sensitivity in the diagnosis of focal nodular hyperplasia .Radiographs may demonstrate other causes of abdominal pain in symptomatic patients, including gallstones, nonspecific hepatomegaly, and other soft tissue masses. The presence of calcification in a liver lesion suggests a diagnosis other than FNH, because only 1% of patients with FNH have calcification. [16]
The diagnosis of FNH is achieved by use of several complementary imaging techniques. In patients for whom the diagnosis is not clearly determined with imaging findings, open biopsy or surgical resection may be needed; findings on needle biopsy may substantially overlap with those of well-differentiated hepatocellular carcinoma. [17, 18, 19, 20, 21]
Classification
Focal nodular hyperplasia is subdivided into 2 types: classic (80%) and nonclassic (20%). [22] Nonclassic FNH is further divided into 3 subtypes: telangiectatic FNH, FNH with cytologic atypia, and mixed hyperplastic and adenomatous FNH.
In classic FNH, all 3 characteristic features are present: abnormal nodular architecture, malformed vessels, and cholangiolar proliferation. In nonclassic FNH, 2 of the 3 characteristic features are present; bile duct proliferation is always present. [22]
Telangiectatic FNH shares several morphologic patterns with hepatocellular adenomas. Paradis et al conducted a study in which they attempted to reclassify telangiectatic FNH on the basis of molecular analysis. [23] Their results showed that the molecular pattern seen in cases of telangiectatic FNH closely resembles that of hepatocellular adenomas. They suggested that telangiectatic FNH be referred to as telangiectatic hepatocellular adenoma.
Computed Tomography
On nonenhanced CT scans, focal nodular hyperplasia may appear as an isoattenuating or slightly hypoattenuating mass. Nonenhanced images are important because FNH may be missed without a precontrast study. For the optimal evaluation of FNH, a helical CT scan with a 4-phase study should be performed. This evaluation should include nonenhanced and hepatic arterial, portal venous, and delayed-phase examinations. [18, 24, 25, 26, 27, 2, 8]
(See the images below.)
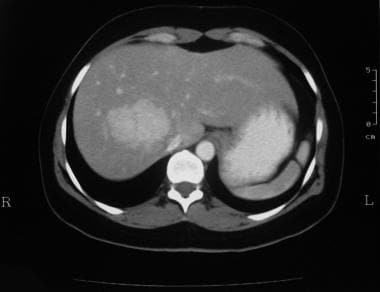 Enhanced axial CT scan through the liver in the arterial phase in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 2 images). The mass demonstrates intense enhancement. (See also the next image.)
Enhanced axial CT scan through the liver in the arterial phase in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 2 images). The mass demonstrates intense enhancement. (See also the next image.)
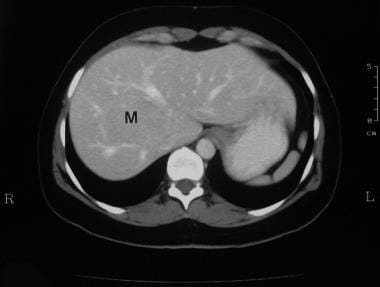 Delayed portal venous phase enhanced axial CT scan in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 3 images). Image shows stretching of the portal vein and the right hepatic vein around the mass (M). (See also the next image.)
Delayed portal venous phase enhanced axial CT scan in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 3 images). Image shows stretching of the portal vein and the right hepatic vein around the mass (M). (See also the next image.)
After the administration of contrast material, the lesion becomes hyperattenuating relative to the surrounding liver in the arterial phase; this occurs approximately 20-30 seconds after the bolus of contrast agent is administered. In the portal venous phase, 70-90 seconds after the bolus injection, FNH is less conspicuous and becomes isoattenuating with the rest of the liver. During the delayed phase, approximately 5-10 minutes after the bolus injection, FNH is isoattenuating with normal liver.
In 15-33% of patients, conventional CT scans show the hypoattenuating stellate central scar with a central core and radiating fibrous septa. The central scar may become hyperattenuating on delayed images because of delayed contrast washout from the scar; however, the central scar does not go through a hypoattenuating phase on helical CT scans. The scar is demonstrated as a hyperattenuating region in the portal venous phase. The central artery traversing the central scar may show early enhancement in the arterial phase.
According to Attal et al, there are a number of differences between telangiectatic FNH lesions and typical FNH lesions: atypical FNH features that are often observed in telangiectatic FNH include the lack of a central scar, lesion heterogeneity, hyperintensity on T1-weighted MRI, strong hyperintensity on T2-weighted MRI, and persistent contrast enhancement on delayed contrast-enhanced CT or T1-weighted MRI. The authors described US, CT, and MR features of telangiectatic FNH and correlated their findings with histopathologic findings in 13 cases. [28]
Degree of confidence
When characteristic features are seen in the appropriate clinical setting, one may be fairly confident of the diagnosis. Unfortunately, CT features of other benign and malignant lesions can mimic those of focal nodular hyperplasia.
Because of the nature and pathogenesis of FNH, it is difficult to obtain an accurate diagnosis of FNH on the basis of the clinical presentation and radiographic studies. Shen et al studied 86 patients with pathologically confirmed diagnoses of FNH and found a solitary focus in 80 patients and multiple foci in 6 patients. In 69 patients, the diameter of the tumor was less than 5 cm; in 15 patients, the tumor diameter was 5-10 cm; and in 2 patients, the diameter of the tumor was greater than 10 cm. [29]
Overall, a correct preoperative diagnosis was made in 59.3% of patients (51/86) in the Shen study. Doppler color-flow imaging provided a correct preoperative diagnosis in 32.9%; CT provided a correct diagnosis in 60.3%; and MRI a correct diagnosis in 77.4%. All of the patients underwent tumor resection, and all displayed good curative results. Shen et al concluded that CT and MRI are both important methods for diagnosing FNH but that it is difficult to make a definitive preoperative diagnosis in partial classic and in all nonclassic cases of FNH. As a result of their findings, Shen et al suggested that patients undergo tumor resection if they have clinical symptoms or if the diagnosis is indefinite. [29]
Although triple-phase CT scanning accurately characterizes most FNH lesions, CT findings are not as definitive in some patients with FNH. Rarely, a false-positive diagnosis of FNH occurs in cases of fibrolamellar hepatocellular carcinoma, as well as in cases involving other well-differentiated variants of hepatocellular carcinoma.
Magnetic Resonance Imaging
Focal nodular hyperplasia usually displays a homogeneous signal intensity on MRI (see the image below). [30, 27, 2, 31, 8, 32, 33, 34, 35] In 94-100% of cases of FNH, the FNH lesion is isointense to hypointense on T1-weighted images; in 6% of cases, the signal intensity on T1-weighted images is hyperintense. On T2-weighted images, the lesion is slightly hyperintense to isointense in 94-100% of cases. The central scar of FNH is hypointense on T1-weighted images, but on T2-weighted images, the central scar shows a pattern of variable signal intensity. On T2-weighted images, the scar appears hyperintense in 75% of cases; it appears hypointense in 25% of cases. [16]
 Dynamic MRIs in a 36-year-old woman referred for a gallbladder sonography, during which the patient was found to have a vague ill-defined hypoechoic mass in the right lobe of the liver (not shown). (Top left) Gadolinium-enhanced T1-weighted MRI demonstrates an ill-defined low-signal-intensity mass. (Top right) The mass enhances intensely in the arterial phase after the administration of contrast medium. (Bottom left) Minor enhancement persists in the portal venous phase. (Bottom right) The lesion becomes isointense relative to the liver on delayed images.
Dynamic MRIs in a 36-year-old woman referred for a gallbladder sonography, during which the patient was found to have a vague ill-defined hypoechoic mass in the right lobe of the liver (not shown). (Top left) Gadolinium-enhanced T1-weighted MRI demonstrates an ill-defined low-signal-intensity mass. (Top right) The mass enhances intensely in the arterial phase after the administration of contrast medium. (Bottom left) Minor enhancement persists in the portal venous phase. (Bottom right) The lesion becomes isointense relative to the liver on delayed images.
After the administration of a gadolinium-based contrast agent, the enhancement pattern parallels that of contrast-enhanced CT. Dense enhancement is seen in the arterial phase; the lesion becomes isointense during the portal venous phase and isointense on delayed images. Late and prolonged enhancement of the central stellate scar occasionally occurs.
MRI findings are not pathognomonic for FNH, but the use of MRI reticuloendothelial agents, such as superparamagnetic iron oxide (SPIO) and ultrasmall superparamagnetic iron oxide (USPIO), increase the specificity. On SPIO-enhanced T2-weighted images, FNH shows decreased signal intensity because of iron uptake by Kupffer cells. This finding is not specific to FNH, because hepatocellular adenoma and hepatocellular carcinoma also may contain Kupffer cells.
Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI has been shown to be superior to unenhanced MRI alone or spiral CT in the characterization of FNH. [36]
According to Attal et al, telangiectatic FNH differs from typical FNH on imaging: the atypical features that were often observed with telangiectatic FNH were the lack of a central scar, heterogeneity of lesions, hyperintensity on T1-weighted MRI, strong hyperintensity on T2-weighted MRI, and persistent contrast enhancement on delayed contrast-enhanced CT or T1-weighted MRI. [28] The authors described the features of US, CT, and MRI in cases of telangiectatic FNH and compared the findings with histopathologic findings in 13 cases of FNH.
Purysko et al studied the performance of gadoxetate disodium-enhanced MRI to evaluate its performance and potential advantages in the characterization of FNH and hepatocellular adenoma of hepatocyte phase imaging in identifying features that distinguish FNH from hepatocellular adenoma. [37] The authors concluded that gadoxetate disodium-enhanced MRI has accuracy in distinguishing FNH and hepatocellular adenoma, and the hepatocyte phase improved their distinction. The authors found that FNH enhances significantly more than hepatocellular adenoma and suggested that an enhancement ratio, in the hepatocyte phase, can be potentially used to improve diagnostic accuracy.
Portilha et al also concluded in their study that MRI hepatocyte contrast agents are a valuable tool for differentiating FNH from hepatic adenoma, based on the different patterns of uptake and retention of gadoxetic acid. [38]
Nowicki et al found that gadobenate disodium–enhanced MRI is a more effective method than multiphase CT in the differential diagnosis of FNH. In both CT and MRI, the radiologic sign with the highest accuracy was the presence of a central scar (0.93 and 0.96, respectively). [2]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
MRI generally allows differentiation from other hypervascular liver lesions. Moreover, with the development of hepatospecific MRI contrast agents, MRI provides both functional and morphologic information, which is useful in the detection and characterization of FNH lesions. [39]
A false-positive diagnosis of FNH may occur with fibrolamellar hepatocellular carcinoma and other well-differentiated forms of hepatocellular carcinoma. On SPIO-enhanced T2-weighted images, hepatocellular adenoma and hepatocellular carcinoma may show decreased signal intensity, because Kupffer cells may be present.
Ultrasonography
In cases of focal nodular hyperplasia, ultrasonographic findings are variable. The lesion may appear as a homogeneous mass that is isoechoic, hypoechoic, or hyperechoic. FNH has a mass effect that may displace intrahepatic blood vessels. In only 18% of cases is a central scar present [20, 40, 41]
(See the images below.)
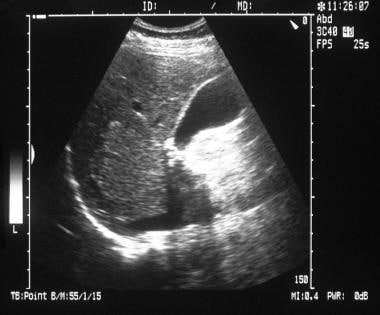 Longitudinal sonogram through the liver and gallbladder in a 38-year-old woman referred for gallbladder scanning. Image shows an ill-defined hyperechoic mass in the right lobe of the liver. (See also the next image.)
Longitudinal sonogram through the liver and gallbladder in a 38-year-old woman referred for gallbladder scanning. Image shows an ill-defined hyperechoic mass in the right lobe of the liver. (See also the next image.)
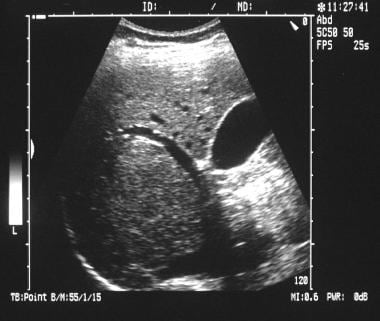 Longitudinal sonogram (more medial section than in the previous image) in a 38-year-old woman referred for gallbladder scanning. Sonogram shows mass effect from the tumor, as demonstrated by the arching of the portal vein anteriorly. (See also the next image.)
Longitudinal sonogram (more medial section than in the previous image) in a 38-year-old woman referred for gallbladder scanning. Sonogram shows mass effect from the tumor, as demonstrated by the arching of the portal vein anteriorly. (See also the next image.)
Doppler sonograms demonstrate an enlarged afferent blood vessel with central arterial hypervascularity and centrifugal filling to the periphery in a spokelike manner. Large draining veins may be seen at the periphery of the mass. High-velocity Doppler signals with arterial pulsatility may be recorded from arteriovenous shunts. Echo-enhanced Doppler US has a high sensitivity for detection of the feeding artery and for depiction of the radial vascular architecture in FNH lesions, especially for lesions that are located in the liver's left lobe. Power Doppler US has increased sensitivity for FNH and may help distinguish FNH from hepatocellular carcinoma.
Dynamic contrast-enhanced US is increasingly being used to diagnose FNH. [5, 7, 42] According to Ungermann et al, contrast-enhanced US may be the final diagnostic method for lesions that are larger than 3 cm and have a typical spoke-wheel structure; however, they concluded that if the spoke-wheel pattern is not present and if there is no central scar, the diagnosis of FNH cannot be made specifically on the basis of contrast-enhanced US alone. [43] Kang et al found that In 62 patients (mean age, 37 yr; range, 20-69 yr; 41 women) with FNH, the majority of patients displayed the spoke -wheel sign on super-resolution US and on contrast-enhanced US (63% [39 of 62] and 71% [44 of 62], respectively). [6]
He et al reported on 28 lesions diagnosed as FNH and found that contrast-enhanced ultrasound enables an accurate diagnosis in FNH smaller than 3 cm using sulfur hexafluoride and perflubutane. [5]
In a study of 13 cases of FNH, Attal et al found that telangiectatic FNH differed from typical FNH. The atypical FNH findings that were often observed with telangiectatic FNH were the lack of a central scar, heterogeneous lesion formation, hyperintensity on T1-weighted MRI, strong hyperintensity on T2-weighted MRI, and contrast enhancement on delayed contrast-enhanced CT or T1-weighted MRI. [28]
The lesion of focal nodular hyperplasia may be difficult to detect, because it is often hypoechoic to normal liver tissue. The specificity of US is low, but the specificity may be increased with Doppler US and echo enhancement. As is the case with other cross-sectional imaging, however, a specific diagnosis is sometimes not possible because the FNH lesion is similar to lesions of other benign and malignant diseases.
The lesion may be missed entirely if the signal is isoechoic. US findings of FNH overlap those of hepatic adenomas and hepatocellular carcinomas, although the sensitivity and specificity improve with the use of Doppler US, particularly power Doppler and echo-enhanced Doppler US.
Nuclear Imaging
The best imaging modalities for characterizing FNH are those modalities that can delineate the lesion's central scar or that can show Kupffer cell activity. The best modalities for identifying the central scar are CT and MRI; Kupffer cell activity is best demonstrated by radionuclide scans. However, MRI superparamagnetic contrast agents may challenge radionuclide scanning.
Detection of Kupffer cells in FNH has historically been achieved using technetium-99m (99mTc) sulfur colloid scanning (see the image below). In 60-70% of FNH patients, these scans show normal or increased uptake of 99mTc sulfur colloid. In 30-40% of patients, Kupffer cells are not sufficiently concentrated in the FNH lesion; the lesion may even be photon deficient. [16]
The uptake of 99mTc–hepatoiminodiacetic acid (HIDA) is normal or increased in 40-70% of patients, but the lesion may be photon deficient in as many as 60% of patients. With 99mTc-tagged RBCs, uptake is increased during the early phase; subsequently, the uptake is decreased. [16]
Although Tc-99m sulfur colloid uptake in patients with FNH depends on the concentration of Kupffer cells in the FNH lesion, other hepatocellular neoplasms, such as a hepatocellular adenoma and hepatocellular carcinoma, may also have Kupffer cells and demonstrate 99mTc sulfur colloid uptake. Hepatic adenoma, hemangioma, hepatoblastoma, liver herniation, and hepatocellular carcinoma may be similar in appearance on 99mTc sulfur colloid scans.
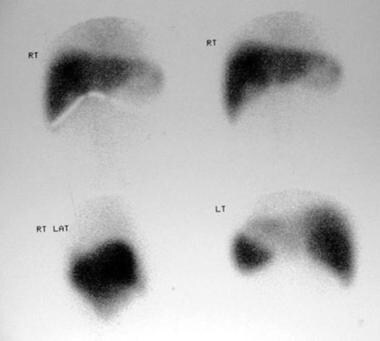 Technetium-99m sulfur colloid scans in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 4 images). Images show complete filling of the mass depicted on sonograms and CT scans.
Technetium-99m sulfur colloid scans in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 4 images). Images show complete filling of the mass depicted on sonograms and CT scans.
Angiography
Angiographic findings demonstrate a discretely marginated mass. When the mass is small, the arteries supplying the mass break up into small branches, which appear to permeate the FNH and form a reticular pattern. These branches are not dilated, but the overall impression is that of increased vascularity. Vascularity may be decreased within the central stellate scar.
In the parenchymal phase, a fine, homogeneous granularity is demonstrated; occasionally, a lucent ring is seen around the mass.
In large tumors, the dilated main feeding artery perforates the center of the tumor. Peripheral arteries arise from the central artery; these are arranged in a spoke-wheel pattern.
Although the typical angiographic findings are present in only 33% of patients, the diagnosis of FNH may still be suggested. [16] FNH may appear similar to an adenoma. An incorrect diagnosis, such as a hepatocellular adenoma, may be made in up to 67% of patients. [16]
-
Dynamic MRIs in a 36-year-old woman referred for a gallbladder sonography, during which the patient was found to have a vague ill-defined hypoechoic mass in the right lobe of the liver (not shown). (Top left) Gadolinium-enhanced T1-weighted MRI demonstrates an ill-defined low-signal-intensity mass. (Top right) The mass enhances intensely in the arterial phase after the administration of contrast medium. (Bottom left) Minor enhancement persists in the portal venous phase. (Bottom right) The lesion becomes isointense relative to the liver on delayed images.
-
Longitudinal sonogram through the liver and gallbladder in a 38-year-old woman referred for gallbladder scanning. Image shows an ill-defined hyperechoic mass in the right lobe of the liver. (See also the next image.)
-
Longitudinal sonogram (more medial section than in the previous image) in a 38-year-old woman referred for gallbladder scanning. Sonogram shows mass effect from the tumor, as demonstrated by the arching of the portal vein anteriorly. (See also the next image.)
-
Enhanced axial CT scan through the liver in the arterial phase in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 2 images). The mass demonstrates intense enhancement. (See also the next image.)
-
Delayed portal venous phase enhanced axial CT scan in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 3 images). Image shows stretching of the portal vein and the right hepatic vein around the mass (M). (See also the next image.)
-
Technetium-99m sulfur colloid scans in a 38-year-old woman referred for gallbladder scanning (same patient as in the previous 4 images). Images show complete filling of the mass depicted on sonograms and CT scans.



