Practice Essentials
Hypersensitivity pneumonitis is an inflammatory syndrome of the lung characterized by repetitive inhalation of antigenic agents in a susceptible host. [1] Hypersensitivity pneumonitis has been traditionally classified into acute, subacute, and chronic phases. However, there are only 2 clinical phases or syndromes: acute and subacute/chronic. Most affected patients present acutely with flu-like illness with cough. Patients can also present subacutely with recurrent pneumonia or chronically with exertional dyspnea, productive cough, and weight loss. Most patients recover completely after exposure to the inciting antigen ceases. [2, 3, 4, 5, 6]
Chest radiography is readily and universally available and has the added advantage of portability. The chest radiograph is abnormal in most patients with hypersensitivity pneumonitis. It is also useful for differential diagnosis in patients presenting with respiratory symptoms. Chest radiography, however, may show no abnormalities in up to 20% of cases of HP. In conjunction with the patient's clinical presentation, radiographic findings are generally sufficient to diagnose hypersensitivity pneumonitis, although high-resolution CT (HRCT) scanning is commonly performed to confirm the diagnosis and to rule out other possibilities. HRCT is often performed in the setting of chronic parenchymal lung disease. [7, 8, 9, 10, 11]
In acute HP, HRCT may be normal or may show diffuse ground-glass or centrilobular ground-glass nodules. Subacute HP often features ground-glass opacification, poorly defined centrilobular nodules, and areas of air trapping at the level of the secondary pulmonary lobule, with mid-lung and upper-lung predominance. [5, 6, 12, 13, 14]
(Images of hypersensitivity pneumonitis are provided below.)
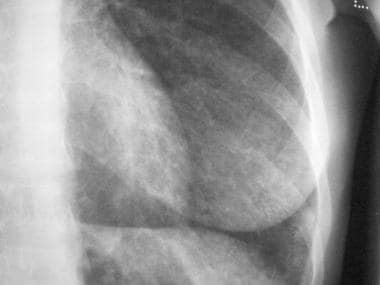
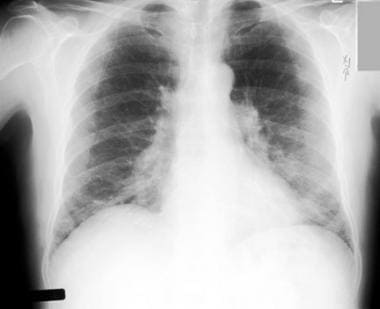 Chest radiograph in a 60-year-old dairy farmer who had an 8-year history of intermittent dyspnea shows bilateral reticulonodular interstitial infiltration secondary to subacute hypersensitivity pneumonitis. Courtesy of Sat Sharma, MD.
Chest radiograph in a 60-year-old dairy farmer who had an 8-year history of intermittent dyspnea shows bilateral reticulonodular interstitial infiltration secondary to subacute hypersensitivity pneumonitis. Courtesy of Sat Sharma, MD.
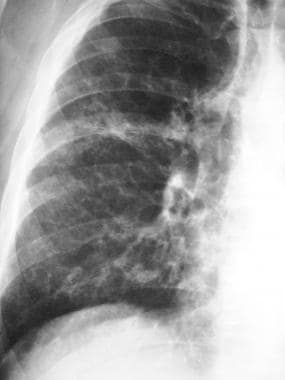
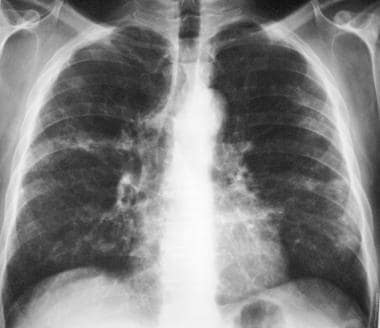 Posteroanterior (PA) chest radiograph in a patient with chronic hypersensitivity pneumonitis (HP)—a pigeon fancier—shows reticular-nodular opacification.
Posteroanterior (PA) chest radiograph in a patient with chronic hypersensitivity pneumonitis (HP)—a pigeon fancier—shows reticular-nodular opacification.
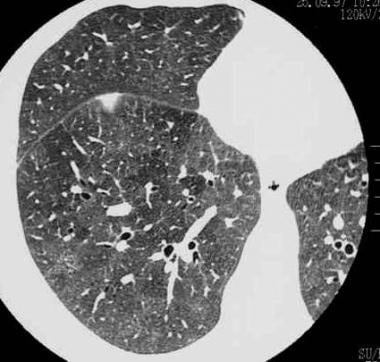
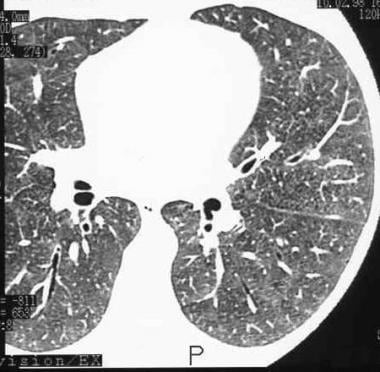 High-resolution CT (HRCT) scan in a man with a clinical diagnosis of hypersensitivity pneumonitis. The diagnosis was not histologically proved, and the patient recovered after steroid therapy. Note the ground-glass appearance and small nodules.
High-resolution CT (HRCT) scan in a man with a clinical diagnosis of hypersensitivity pneumonitis. The diagnosis was not histologically proved, and the patient recovered after steroid therapy. Note the ground-glass appearance and small nodules.
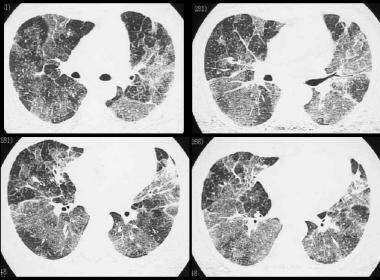 Differential diagnosis of hypersensitivity pneumonitis (HP). High-resolution CT (HRCT) scan in a young African American woman presenting with mild dyspnea. Image shows a ground-glass appearance and small, 1-2 mm nodules due to sarcoidosis.
Differential diagnosis of hypersensitivity pneumonitis (HP). High-resolution CT (HRCT) scan in a young African American woman presenting with mild dyspnea. Image shows a ground-glass appearance and small, 1-2 mm nodules due to sarcoidosis.
The term heykatarr has been defined as "a group of related inflammatory interstitial lung diseases that result from hypersensitivity immune reactions to the repeated inhalation or ingestion of various antigens derived from fungal, bacterial, animal protein, or reactive chemical sources." [15] These antigens are often related to the patient's occupation. The most common antigens are thermophilic actinomycetes and avian proteins, and the most common diseases are farmer's lung and bird fancier's lung. The disease complex is characterized by diffuse inflammation of lung parenchyma and airways in previously sensitized patients.
Crepitant rales can be elicited in some patients. Pulmonary function tests generally reveal a restrictive defect in early disease and a restrictive, obstructive, or mixed defect in late disease. Specific precipitating antibodies are detectable in some cases.
The latent period between exposure to antigen and presentation varies from a few weeks to years. The onset of symptoms after acute exposure is usually between 4 and 12 hours. Some antigens provoke symptoms after repeated exposure; these include bioaerosols of microbial or animal antigens and a few reactive chemicals.
Resolution occurs with improvement or complete recovery if exposure is terminated early. Chronic exposure may cause the disease to progress to interstitial fibrosis. [16, 17, 18, 19, 20, 21, 22]
In a study of 117 patients diagnosed with HP, Salisbury et al identified 3 radiologic phenotypes: honeycomb present, non-honeycomb fibrosis (traction bronchiectasis and reticulation) present, and nonfibrotic. Patients with nonfibrotic HP had the longest median survival (>14.3 years). The presence of honeycomb fibrosis resulted in a poor prognosis, with median survival of 2.76 years. Patients with non-honeycomb HP had a median survival of over 7.95 years. [23]
Diagnostic criteria
Various diagnostic criteria have been proposed for hypersensitivity pneumonitis. A modified Delphi survey of 45 international experts in interstitial lung disease achieved consensus on 18 diagnostic items for chronic hypersensitivity pneumonitis. Among those considered to have the highest level of importance were identification of a causative antigen, time relation between exposure and disease, mosaic attenuation on imaging, and poorly formed non-necrotizing granulomas on pathology. [24]
Histologic confirmation
In many cases, lung biopsy is required for histologic confirmation of the diagnosis (see the images below).
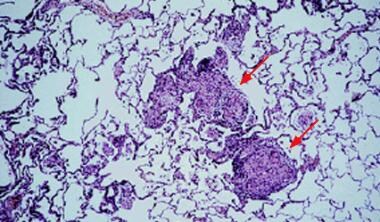 Light microscopy shows mononuclear infiltration and noncaseating granulomas. This finding is usually seen in acute disease, but it can also appear in subacute and chronic disease. Courtesy of Sat Sharma, MD.
Light microscopy shows mononuclear infiltration and noncaseating granulomas. This finding is usually seen in acute disease, but it can also appear in subacute and chronic disease. Courtesy of Sat Sharma, MD.
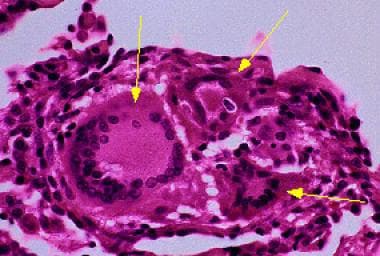 Giant cells are a characteristic feature of hypersensitivity pneumonitis (HP). Courtesy of Sat Sharma, MD.
Giant cells are a characteristic feature of hypersensitivity pneumonitis (HP). Courtesy of Sat Sharma, MD.
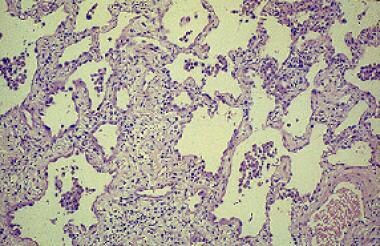 Chronic hypersensitivity pneumonitis results in interstitial inflammation associated with fibrosis. Courtesy of Sat Sharma, MD.
Chronic hypersensitivity pneumonitis results in interstitial inflammation associated with fibrosis. Courtesy of Sat Sharma, MD.
Limitations of techniques
Conventional radiographs are nonspecific; without clinical input, a firm diagnosis of hypersensitivity pneumonitis cannot be made. The chest radiograph may be normal in established acute disease as well as chronic hypersensitivity pneumonitis. Chest radiography should be performed with caution in young or pregnant patients.
HRCT is comparatively expensive and exposes the patient to a radiation dose higher than that of other studies; it is indicated in patients in whom disease or poorly controlled airway disease is clinically suspected when a firm diagnosis has not been determined.
Ill-defined nonbranching centrilobular nodules with an upper lobe predominance or a diffuse distribution with or without ground-glass opacities are characteristic for acute or subacute hypersensitivity pneumonitis. These findings are diagnostic in an appropriate clinical setting, obviating biopsy. However, a normal HRCT scan does not exclude hypersensitivity pneumonitis, as HRCT scans can be normal in up to 50% of patients.
Guidelines
Guidelines on the diagnosis of hypersensitivity pneumonitis, utilizing HRCT, by the American Thoracic Society, Japanese Respiratory Society, and Asociación Latinoamericana del Tórax include the following [25, 26] :
-
In both fibrotic and nonfibrotic HP, 2 series of images acquired in the supine position are obtained: one at deep inspiration and a second after prolonged expiration.
-
All features of lung infiltration can be depicted on the inspiratory images, except for air trapping, which is an expiratory HRCT finding.
-
Analysis of lung changes at expiration may increase diagnostic confidence in nonfibrotic HP and is necessary for better characterization of heterogeneous lung attenuation in both forms of the disease.
-
Owing to the widespread distribution of lung changes in HP, a third acquisition in the prone position is usually not necessary.
-
The optimal chest HRCT scan for characterizing HP should be a noncontrast examination, except in the context of acute respiratory decline, in which case CT angiography may be justified to detect acute pulmonary embolisms.
-
CT angiography should be preceded by a noncontrast chest HRCT scan to detect new ground-glass changes that raise the probability of acute exacerbation in the absence of pulmonary embolisms.
-
The typical HP pattern relies on the identification of diffusely distributed HRCT findings that include features of lung infiltration (ie, ground-glass opacity [GGO], mosaic attenuation) plus at least one HRCT abnormality suggestive of small airway disease.
-
HRCT features of small airway disease include ill-defined, small (< 5 mm) centrilobular nodules on inspiratory images and air trapping on expiratory images.
-
Mosaic attenuation refers to coexisting areas of varying attenuation within the lung parenchyma on inspiratory HRCT images.
-
In nonfibrotic HP, mosaic attenuation typically reflects coexistent lobules affected by pneumonitis (increased attenuation) interspersed with lobules of normal or slightly decreased attenuation (due to bronchiolar obstruction). These parenchymal patterns are usually bilateral and symmetric with a diffuse distribution, both axially and craniocaudally.
-
Although a combination of parenchymal abnormalities and features of small airway disease is highly suggestive of nonfibrotic HP, isolated air trapping is another pattern that may be seen with HP.
-
Three additional HRCT features have also been described in nonfibrotic HP: uniform and subtle GGO, airspace consolidation, and lung cysts. Each of these features is nonspecific but can be compatible with nonfibrotic HP in the appropriate clinical context.
-
Lung fibrosis in HP most frequently manifests as irregular fine or coarse reticulation with architectural lung distortion, sometimes with septal thickening, that can be seen alone or in association with traction bronchiectasis in areas of GGO.
-
Honeycombing can be present and is often described as minimal, but extensive honeycombing in severe forms of fibrotic HP may also occur.
-
Lung fibrosis is most severe in the mid or mid and lower lung zones or equally distributed in the 3 lung zones with relative basal sparing.
-
On axial images, there is often no central or peripheral predominance of lung fibrosis.
-
Bronchiolar obstruction manifests with several HRCT features in fibrotic HP. Like that observed in nonfibrotic HP, ill-defined centrilobular nodules and mosaic attenuation can be seen.
-
Bronchiolar obstruction is also present in an HRCT pattern combining 3 different lung densities (GGO, lobules of decreased attenuation and vascularity, and normal-appearing lung) that is highly specific to fibrotic HP. “Three-density pattern” describes the presence of these 3 different lung densities, which some radiologists have referred to as the “headcheese sign.” This pattern emphasizes the diagnostic value of lobules with decreased attenuation and vascularity on inspiratory HRCT images, especially when concomitant with air trapping at expiration, both suggesting the presence of severe bronchiolar obstruction. These two individual HRCT features were the highest-ranked radiologic features for fibrotic HP in an International Modified Delphi Survey. [24]
In their classification of HP, acute, subacute, and chronic are replaced by nonfibrotic and fibrotic, and they introduce 3 HRCT patterns: typical, compatible, and indeterminate. [25, 26]
Radiography
The most common abnormality in acute or subacute disease is small, bilateral pulmonary nodules, which are usually 1-5 mm in size (see the images below). The nodules may be well defined or indistinct. In 1 published series, only 2% of the nodules were unilateral. [27]
The nodules have a predilection for the midzones or lower zones, with comparative sparing of the upper zones. The nodules may appear within a few hours after exposure and take weeks or months to resolve. The nodules/opacities may be so small that they give a ground-glass appearance.
Focal consolidation is found in 10-25% of patients. [28] Interstitial-type change is occasionally seen with accentuated bronchovascular markings and Kerley B lines. Generally, there are no pleural changes, but occasional thickening of minor fissures is noted.
 Chest radiograph in a 60-year-old dairy farmer who had an 8-year history of intermittent dyspnea shows bilateral reticulonodular interstitial infiltration secondary to subacute hypersensitivity pneumonitis. Courtesy of Sat Sharma, MD.
Chest radiograph in a 60-year-old dairy farmer who had an 8-year history of intermittent dyspnea shows bilateral reticulonodular interstitial infiltration secondary to subacute hypersensitivity pneumonitis. Courtesy of Sat Sharma, MD.
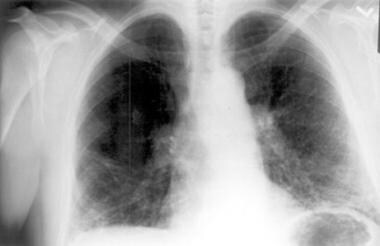 Chest radiograph in a patient with chronic hypersensitivity pneumonitis from pigeon breeder's disease. Bilateral reticulonodular opacities are present. Courtesy of Sat Sharma, MD.
Chest radiograph in a patient with chronic hypersensitivity pneumonitis from pigeon breeder's disease. Bilateral reticulonodular opacities are present. Courtesy of Sat Sharma, MD.
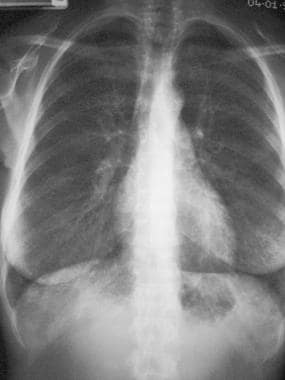 Posteroanterior (PA) chest radiograph shows bilateral reticulonodular shadowing in a patient with hypersensitivity reaction to moldy hay.
Posteroanterior (PA) chest radiograph shows bilateral reticulonodular shadowing in a patient with hypersensitivity reaction to moldy hay.
 Posteroanterior (PA) chest radiograph in a patient with chronic hypersensitivity pneumonitis (HP)—a pigeon fancier—shows reticular-nodular opacification.
Posteroanterior (PA) chest radiograph in a patient with chronic hypersensitivity pneumonitis (HP)—a pigeon fancier—shows reticular-nodular opacification.
Hilar lymphadenopathy is rare but has been reported in mushroom worker's lung, [29] farmer's lung, [30, 31, 32] and bird fancier's lung. [27, 33] In farmer's lung, plain chest radiographs are normal in up to 70% of cases. When the images are abnormal, acute disease is characterized by a pattern of diffuse airspace disease or a ground-glass pattern mimicking that of pulmonary edema. These changes are reversible and improve over several days. Over time, the disease progresses to a granulomatous reaction that may eventually lead to a small nodular pattern, followed by interstitial fibrosis and end-stage honeycomb lung. Pleural effusion is rare, and the disorder is not associated with hilar adenopathy.
Chronic changes reflect healing by fibrosis and may occur after 1 or more attacks. Alternatively, they may develop insidiously in relation to chronic low-grade antigen exposure (eg, with bird fancier's lung related to budgerigars). The characteristic radiologic change in the chronic stage is scarring process with loss of lung volume, which has a marked (85%) upper lobe preponderance. The principal opacities are reticular, sometimes with a definite honeycomb pattern. Larger ring shadows 1-4 mm in diameter are due to bullae, blebs, cysts, or bronchiectasis. Line shadows secondary to scarring are common, particularly in the upper zones. Parallel line shadows are caused by bronchiectasis or simple bronchial wall thickening; in the latter case, they are often transient.
Other changes sometimes seen in chronic stage include persisting small nodules, massive opacities, and evidence of pulmonary heart disease. Pneumothorax is recorded in the fibrotic stage, but this is uncommon.
Degree of confidence
Radiographic findings are often nonspecific but could be suggestive in appropriate clinical setting. Therefore, it is important to ask questions about etiologic, environmental, or occupational exposure when images show changes suggestive of extrinsic allergic alveolitis.The chest radiograph is often normal in patients with hypersensitivity pneumonitis. For this reason, HRCT is recommended in the proper clinical setting if the radiograph is normal.
False positives/negatives
There is some evidence that the radiographic changes are the same whatever the extrinsic provocative agent, but the evidence is incomplete. In the acute phase of the disease, the radiographic changes may be subtle, or the radiograph may appear normal. The radiograph may also appear normal in the chronic stage of the disease despite abnormal diffusing capacity [30, 34] and biopsy-proved allergic granulomatous disease. [31, 32]
Computed Tomography
In hypersensitivity pneumonitis, high-resolution CT (HRCT) findings are related to the stage of disease. With heavy acute antigen exposure, diffuse airspace shadowing may mimic that of pulmonary edema; if present, this finding generally resolves over a few days. The subacute phase occurs from several days to months after exposure. In this phase, diffuse ground-glass opacities and small (1-5 mm) centrilobular and poorly defined nodules often affect all zones, though a middle and upper lobe predominance is often seen. The ill-defined nodules can be found at all 3 stages of the disorder.
In acute HP, HRCT may be normal or may show diffuse ground-glass or centrilobular ground-glass nodules. Subacute HP often features ground-glass opacification, poorly defined centrilobular nodules, and areas of air trapping at the level of the secondary pulmonary lobule, with mid-lung and upper-lung predominance. [5, 6, 12, 13, 14]
(HRCT images depicting hypersensitivity pneumonitis are presented below.
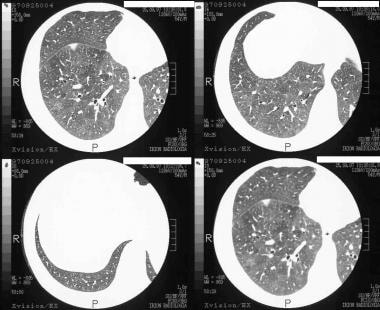 High-resolution CT (HRCT) scans of 50-year-old man. The patient was a physician presenting with mild dyspnea and no other risk factors. The diagnosis of external allergic alveolitis was suggested on HRCT. Note the extensive ground-glass appearance and mosaic attenuation with a patch of consolidation inferior to the lesser fissure. He recovered completely after the air conditioning system of his car was cleaned.
High-resolution CT (HRCT) scans of 50-year-old man. The patient was a physician presenting with mild dyspnea and no other risk factors. The diagnosis of external allergic alveolitis was suggested on HRCT. Note the extensive ground-glass appearance and mosaic attenuation with a patch of consolidation inferior to the lesser fissure. He recovered completely after the air conditioning system of his car was cleaned.
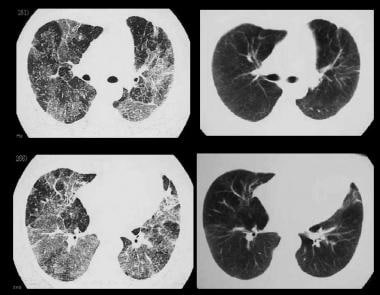 High-resolution CT (HRCT) scans show polyurethane foam–induced pneumonitis before and after cessation of exposure.
High-resolution CT (HRCT) scans show polyurethane foam–induced pneumonitis before and after cessation of exposure.
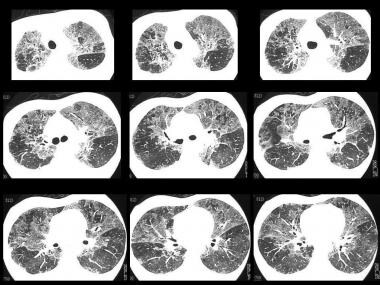 Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) scan shows a patchy ground-glass appearance and airtrapping.
Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) scan shows a patchy ground-glass appearance and airtrapping.
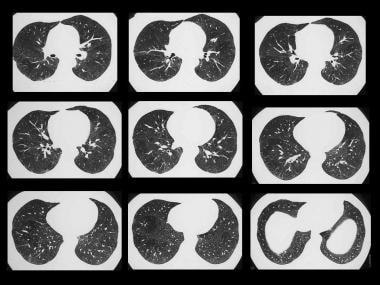 Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) shows a patchy ground-glass appearance on inspiratory scans.
Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) shows a patchy ground-glass appearance on inspiratory scans.
Associated areas of ground-glass opacification are common (86%) and often produce a mosaic pattern of attenuation. Less commonly, there is widespread ground-glass attenuation, which is indistinguishable from that of desquamative interstitial pneumonitis. The areas of ground-glass attenuation are usually diffuse, but they may spare the periphery. A mixed appearance with both regions of airtrapping (best seen on expiratory images) and ground-glass attenuation can be seen in patients with hypersensitivity pneumonitis.
About 20% of patients have perivascular interstitial thickening, which is often irregular.
Chronic inflammatory infiltrates along small airways produces bronchiolar narrowing that results in airtrapping.
Chronic hypersensitivity pneumonitis develops as a result of continuous or intermittent antigenic exposure over several months or years and commonly demonstrates intralobular interstitial thickening (evidence of fibrosis) predominantly involving the mid and upper lung zones. Usually present is relative sparing of the lung apices and the costophrenic sulci, which aids in distinguishing this disorder from idiopathic pulmonary fibrosis (IPF). [35]
Honeycombing is found in about 75% of patients with end-stage lung disease resulting from hypersensitivity pneumonitis.
Degree of confidence
The finding of poorly defined centrilobular nodules on HRCT scans in an appropriate clinical setting should prompt consideration of this disease. The sensitivity of HRCT for the detection of hypersensitivity pneumonitis reported in a population-based study is greater than that of chest radiography.
There are several causes of nodular and ground-glass attenuation on HRCT. Causes of a nodular pattern include sarcoidosis, silicosis, coal worker's pneumoconiosis, and pulmonary histiocytosis X. Causes of a ground-glass pattern include IPF, desquamative interstitial pneumonia, and alveolar proteinosis.
Micronodules may also be identified in respiratory bronchiolitis, but many patients with micronodules have a smoking history, which is generally not associated with hypersensitivity pneumonitis.
Nodules associated with sarcoidosis are usually larger, better defined, and denser than those seen in hypersensitivity pneumonitis. Ill-defined centrilobular micronodules can help distinguish hypersensitivity pneumonitis from IPF, whereas honeycombing and a lower lung zone or peripheral involvement suggest IPF.
Although patients with bronchiolitis obliterans organizing pneumonia (BOOP) may have a clinical presentation similar to that of hypersensitivity pneumonitis, the areas of parenchymal opacification are typically denser and more patchy than those associated with hypersensitivity pneumonitis.
A normal HRCT scan does not exclude hypersensitivity pneumonitis, as HRCT scans can be normal in up to 50% of patients.
-
Chest radiograph in a 60-year-old dairy farmer who had an 8-year history of intermittent dyspnea shows bilateral reticulonodular interstitial infiltration secondary to subacute hypersensitivity pneumonitis. Courtesy of Sat Sharma, MD.
-
Light microscopy shows mononuclear infiltration and noncaseating granulomas. This finding is usually seen in acute disease, but it can also appear in subacute and chronic disease. Courtesy of Sat Sharma, MD.
-
Giant cells are a characteristic feature of hypersensitivity pneumonitis (HP). Courtesy of Sat Sharma, MD.
-
Chronic hypersensitivity pneumonitis results in interstitial inflammation associated with fibrosis. Courtesy of Sat Sharma, MD.
-
Clinical diagnostic criteria for hypersensitivity pneumonitis (HP).
-
Chest radiograph in a patient with chronic hypersensitivity pneumonitis from pigeon breeder's disease. Bilateral reticulonodular opacities are present. Courtesy of Sat Sharma, MD.
-
High-resolution CT (HRCT) scan of the lungs shows ground-glass and mosaic attenuation opacification in the acute phase of hypersensitivity pneumonitis. Courtesy of Sat Sharma, MD.
-
Image obtained during the chronic phase of hypersensitivity pneumonitis shows honeycombing in the right upper lobe (RUL) and traction bronchiectasis. Courtesy of Sat Sharma, MD.
-
High-resolution CT (HRCT) scan in a patient with chronic hypersensitivity pneumonitis demonstrates centrilobular nodules. These nodules are unlike those of sarcoidosis, where nodules are subpleural and present along the bronchovascular bundles. Courtesy of Sat Sharma, MD.
-
High-resolution CT (HRCT) scan shows a ground-glass appearance and reticulonodular opacities in subacute phase of hypersensitivity pneumonitis (HP) secondary to moldy hay.
-
Posteroanterior (PA) chest radiograph shows bilateral reticulonodular shadowing in a patient with hypersensitivity reaction to moldy hay.
-
Magnified view of the left lung base shows reticulonodular shadowing.
-
High-resolution CT (HRCT) scan shows bilateral fine pulmonary nodules in a patient—a malt worker—with hypersensitivity pneumonitis (HP); this condition was due to exposure to moldy barley.
-
Posteroanterior (PA) chest radiograph in a patient with chronic hypersensitivity pneumonitis (HP)—a pigeon fancier—shows reticular-nodular opacification.
-
Magnified view of the right lung base shows reticular shadowing.
-
High-resolution CT (HRCT) scans of 50-year-old man. The patient was a physician presenting with mild dyspnea and no other risk factors. The diagnosis of external allergic alveolitis was suggested on HRCT. Note the extensive ground-glass appearance and mosaic attenuation with a patch of consolidation inferior to the lesser fissure. He recovered completely after the air conditioning system of his car was cleaned.
-
Magnified high-resolution CT (HRCT) scan.
-
High-resolution CT (HRCT) scans show polyurethane foam–induced pneumonitis before and after cessation of exposure.
-
Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) scan shows a patchy ground-glass appearance and airtrapping.
-
Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) shows a patchy ground-glass appearance on inspiratory scans.
-
Young female patient with mollusk-shell hypersensitivity. High-resolution CT (HRCT) shows a patchy ground-glass and airtrapping on expiratory scans.
-
Differential diagnosis of hypersensitivity pneumonitis (HP). High-resolution CT (HRCT) scan shows clearing of the abnormalities from the lung fields after treatment.
-
High-resolution CT (HRCT) scan in a man with a clinical diagnosis of hypersensitivity pneumonitis. The diagnosis was not histologically proved, and the patient recovered after steroid therapy. Note the ground-glass appearance and small nodules.
-
Differential diagnosis of hypersensitivity pneumonitis (HP). High-resolution CT (HRCT) scan in a young African American woman presenting with mild dyspnea. Image shows a ground-glass appearance and small, 1-2 mm nodules due to sarcoidosis.
-
Differential diagnosis of hypersensitivity pneumonitis (HP) Pneumocystis carinii pneumonia (PCP) in an immunocompetent, HIV-negative patient. High-resolution CT (HRCT) scan shows ground-glass appearance due to PCP. Bronchoscopic lavage confirmed PCP infection.