Practice Essentials
Epidural hematoma (EDH) is defined as hemorrhage into the potential space between the dura, which is inseparable from cranial periosteum, and the adjacent bone. EDH can occur intracranially or intraspinally and can result in clinically significant morbidity and/or mortality if it is not diagnosed and treated promptly. [1, 2] EDH is considered a neurosurgical emergency in which somnolence, coma, and even herniation can result if it is left untreated. [3] However, with surgical treatment, the mortality of epidural hematomas has been reduced to almost 0% in noncomatose patients. Despite the risks of a serious and untreated EDH, it has become increasingly apparent that many small epidural hematomas resolve with nonsurgical management without neurologic sequelae.
Epidural hematoma often has a traumatic origin and in most of cases is caused by a medial meningeal artery lesion. Although epidural hematomas are relatively uncommon (less than 1% of all patients with head injuries and fewer than 10% of those who are comatose), they should always be considered in evaluation of a traumatic brain injury (TBI). [4] Acute EDH in children accounts for 2-3% of all TBI cases in this population. Because of the atypical occurrence, pediatric EDH poses a significant challenge for diagnosis and is not directly comparable to adult EDH. Mortality after pediatric EDH varies across studies and has been shown to be in the range of 0-12%. [5]
Because of the need for accurate and rapid diagnosis and treatment, prompt transfer of the patient to a facility capable of CT scanning and neurosurgical intervention is necessary.
Preferred Examination
CT scanning is the examination of choice in the evaluation of suspected intracranial epidural hematoma. However, because of volume averaging with adjacent bone, small epidural hematomas can be difficult to detect with CT scanning. [6] MRI should be performed when spinal EDH is considered possible. [7, 8] However, although MRI is sensitive for EDH, this modality is seldom the preferred examination because of its limited availability outside of urban institutions and because of problems with MRI-incompatible equipment that is often needed in treating a patient with trauma or a patient in an unstable condition. [9, 10]
Reimaging of EDH that is managed nonoperatively is common, but it rarely changes management. One study found that limiting reimaging to patients with concerning neurologic findings or mass effect on initial evaluation could reduce imaging by more than 50% and reduce exposure to additional ionizing radiation. [11]
(See the images below.)
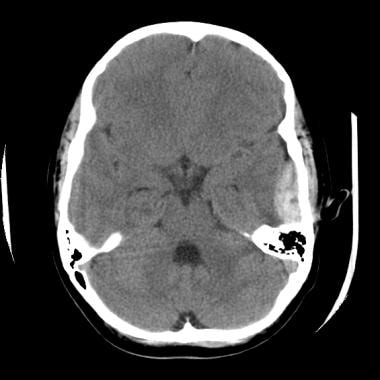 An epidural hematoma demonstrates the classic lenticular configuration that overlies the lateral aspect of the left temporal lobe. Areas of diminished attenuation in the hematoma suggest ongoing hemorrhage.
An epidural hematoma demonstrates the classic lenticular configuration that overlies the lateral aspect of the left temporal lobe. Areas of diminished attenuation in the hematoma suggest ongoing hemorrhage.
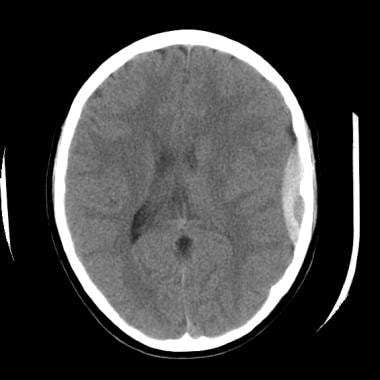 The epidural hematoma shown above extends superiorly to overlie the lateral aspect of the left frontal lobe with associated sulcal effacement, as well as a rightward midline shift of 5-6 mm.
The epidural hematoma shown above extends superiorly to overlie the lateral aspect of the left frontal lobe with associated sulcal effacement, as well as a rightward midline shift of 5-6 mm.
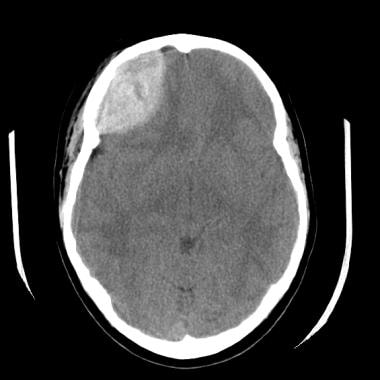 An epidural hematoma overlies the right frontal lobe with right-to-left subfalcine herniation of approximately 7 mm. Areas of low attenuation in the hematoma are again seen. These indicate continued hemorrhage at the time of the examination. Overlying soft-tissue swelling is present in the right frontal aspect of the scalp.
An epidural hematoma overlies the right frontal lobe with right-to-left subfalcine herniation of approximately 7 mm. Areas of low attenuation in the hematoma are again seen. These indicate continued hemorrhage at the time of the examination. Overlying soft-tissue swelling is present in the right frontal aspect of the scalp.
Clot thickness, hematoma volume, and midline shift on preoperative CT scans have been shown to correspond with outcome. In nonoperative patients with EDH, the first follow-up CT scan should be obtained within 6-8 hours after TBI.
A temporal location of EDH has been shown to be associated with failure of nonoperative management and should therefore lower the threshold for surgery (see the image below).
ACR Appropriateness Criteria
The American College of Radiology (ACR) has published appropriateness criteria for imaging head trauma. Key recommendations include the following [12] :
-
In patients who require neuroimaging, noncontrast CT is the most appropriate initial imaging study.
-
In short-term follow-up imaging of acute TBI without neurologic deterioration, noncontrast CT is the most appropriate imaging study, but only in patients with risk factors (eg, subfrontal/temporal intraparenchymal contusions, anticoagulation, age >65 yr, or intracranial hemorrhage with volume >10 mL).
-
In short-term follow-up imaging of acute TBI with neurologic deterioration, delayed recovery, or persistent unexplained deficits, noncontrast CT is the most appropriate imaging study, but MRI has a complementary role when the patient has neurologic findings or symptoms not sufficiently explained by CT.
-
Advanced neuroimaging techniques (SPECT, PET, perfusion CT and MRI, DTI, functional MRI, and MRS) are areas of active research but are not considered routine clinical practice at this time.
Radiography
Skull radiographs may demonstrate the fracture responsible for an epidural hematoma, although vascular channels and suture lines may mimic skull fractures and make the diagnosis difficult. Furthermore, small, minimally displaced skull fractures can be difficult to diagnose.
In the case of spinal EDH, myelography may demonstrate a nonspecific extramedullary lesion that results in compression on the thecal sac or spinal cord to varying degrees.
Because of the nonspecific nature of the radiographic findings, CT scanning should be performed when it is available, and MRI should be performed when spinal EDH is considered.
Computed Tomography
In adults, epidural hematomas may be caused by laceration of arteries or veins running along the inner table of the skull, and injury to these vessels is often accompanied by an associated skull fracture. Bleeding secondary to arterial injury is thought to result in larger and more rapidly growing epidural hematomas than epidural hematomas that occur secondary to venous injury. [6]
Epidural hematomas in children are somewhat different from those in adults. The middle meningeal artery is not as incorporated into bone as in adults, but epidural bleeds from the edges of a fracture easily lead to hematomas. They occur in a wider variety of locations, in part because these are often due to venous rather than arterial hemorrhages. Fractures in the occipital and suboccipital regions are particularly concerning because of the risk of a posterior fossa hematoma, which rapidly causes brainstem compression as well as hydrocephalus by fourth ventricular and aqueduct obstruction. [13]
EDH can be seen in young children without fractures because of the resiliency of the skull. In young children, open sutures and compliant bones result in increased calvarial flexibility, which may permit outward bending of the calvaria without fracture. This bending may lead to separation of the periosteum from the inner table of the skull and disruption of perforating arteries or veins, causing an EDH.
CT scans may demonstrate the responsible fracture in addition to the hematoma (see the images below). [9, 10]
Epidural hematomas can typically be distinguished from subdural hematomas by the biconvex shape of epidural hematomas, versus the crescent shape of the subdural hematoma. In addition, unlike subdural hematomas, epidural hematomas usually do not cross the sutures. [6] If the epidural hematoma is small, it can be difficult to differentiate from subdural hematoma, and EDH may coexist with this condition. [14] With typical biconvex, elliptical, extra-axial fluid collections, the CT scan appearance of EDH depends on the source of bleeding, the time elapsed since injury, the severity of hemorrhage, and the degree of clot organization and breakdown. [15, 16]
Acute, or type 1, EDH may contain both a hyperattenuating clot and a swirling lucency. These findings are believed to represent a mixture of active bleeding and the serum remaining after previous clot formation. Subacute, or type 2, EDH becomes homogeneously hyperattenuating as active bleeding ceases and organized clot forms. Chronic, or type 3, EDH is at least partly hypoattenuating as the clot undergoes breakdown and resorption. Enhancing membrane formation may result from neovascularity and the formation of granulation tissue in the displaced dura during the clot-resorption process.
Although CT scanning is the study of choice in evaluating intracranial EDH, this modality is limited in the evaluation of spinal EDH because of the difficulty in examining long segments of the spine with axial CT images and because of the low attenuation of subacute or chronic EDH.
Magnetic Resonance Imaging
Although it is highly sensitive in the evaluation of spinal EDH, MRI is infrequently the initial modality of choice for assessing intracranial EDH because of the acuteness and severity of EDH. Motion artifact in obtunded patients and the lack of readily available MRI units outside of urban areas also limit its usefulness.
MRI demonstrates a biconvex mass separated from the overlying dura by a thin rim of extruded serum lying between the clot and the dura. This stripe is hyperintense on both T1- and T2-weighted images.
Acute EDH is isointense to minimally hypointense on T1-weighted images and markedly hypointense on T2-weighted images; this appearance corresponds to the deoxyhemoglobin phase. Subacute EDH is hyperintense on T1-weighted images, because deoxyhemoglobin is converted to methemoglobin. On T1-weighted images, the dura may be seen as a thin, hypointense stripe that the hematoma displaces inwardly.
MRI may also demonstrate a fracture with fluid between the fracture margins. This modality may be helpful in demonstrating occlusion of the dural sinus in cases of a fracture-induced intimal flap associated with venous sinus EDH.
In spinal EDH, MRI demonstrates a biconcave, elongated mass in the epidural space with variable degrees of cord compression separated from the spinal cord by low-intensity dura. The signal intensity of spinal EDH varies with the age of the hemorrhage and parallels that of the brain. [9, 17, 18]
Differential considerations for spinal EDH include extradural lipomatosis and spinal angiolipomas, which are usually epidural and located in the midthoracic region. Both of these entities can be differentiated from EDH: lipomatosis demonstrates signal intensity similar to that of fat on MRIs, and angiolipomas often result in bone erosion and pathologic fracture in addition to cord compression.
-
An epidural hematoma demonstrates the classic lenticular configuration that overlies the lateral aspect of the left temporal lobe. Areas of diminished attenuation in the hematoma suggest ongoing hemorrhage.
-
The epidural hematoma shown above extends superiorly to overlie the lateral aspect of the left frontal lobe with associated sulcal effacement, as well as a rightward midline shift of 5-6 mm.
-
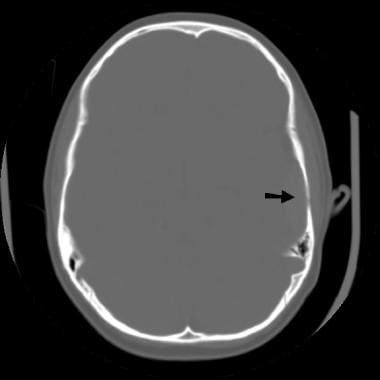
A nondisplaced linear fracture is present in the left temporoparietal region.
-
An epidural hematoma overlies the right frontal lobe with right-to-left subfalcine herniation of approximately 7 mm. Areas of low attenuation in the hematoma are again seen. These indicate continued hemorrhage at the time of the examination. Overlying soft-tissue swelling is present in the right frontal aspect of the scalp.
-
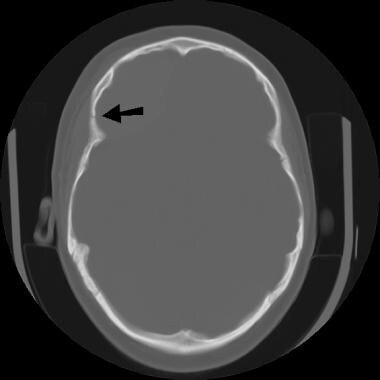
Image depicts a fracture of the right frontal bone anterior to the coronal suture.