Practice Essentials
Pulmonary alveolar proteinosis (PAP) is a rare, diffuse lung disease that is characterized by the alveolar and interstitial accumulation of a periodic acid-Schiff (PAS) stain-positive phospholipoprotein that is derived from surfactant. The lung architecture is otherwise normal, and any associated inflammation or fibrosis is limited in extent. High-resolution CT (HRCT) scanning is superior to conventional CT scanning and to chest radiography for demonstrating the morphologic characteristics of PAP. Crazy paving (see the second image below) is the characteristic finding in PAP on HRCT scanning; it consists of patchy, bilateral geographic areas of ground-glass opacity, which are associated with interlobular septal thickening. [1, 2, 3, 4, 5, 6, 7, 8]
There are 3 types of PAP: primary, secondary, and congenital. The most frequent form is primary PAP, which includes autoimmune PAP; it is defined by the presence of circulating anti-GM-CSF antibodies. Secondary PAP is primarily caused by hematologic disease, infections, or inhaling toxic substances. Congenital PAP affects children almost exclusively. [1]
(A radiograph and a CT scan of PAP are shoiwn below.)
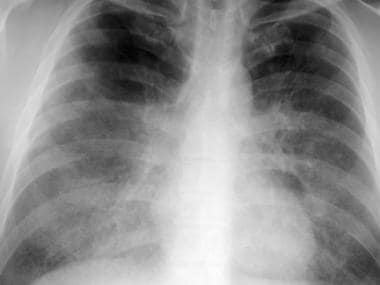 Frontal chest radiograph from a patient with pulmonary alveolar proteinosis. This image demonstrates bilateral perihilar and infrahilar ground-glass opacity without evidence of mediastinal widening, pleural effusion, or adenopathy.
Frontal chest radiograph from a patient with pulmonary alveolar proteinosis. This image demonstrates bilateral perihilar and infrahilar ground-glass opacity without evidence of mediastinal widening, pleural effusion, or adenopathy.
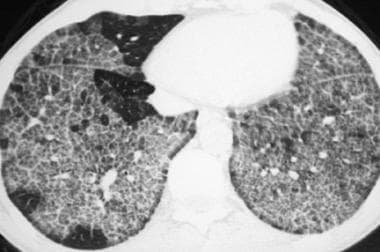 High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with acute silicoproteinosis. This image demonstrates crazy paving, which refers to bilateral ground-glass opacity that is associated with marked interlobular septal thickening and a sharp nonanatomic demarcation between normal and abnormal lung.
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with acute silicoproteinosis. This image demonstrates crazy paving, which refers to bilateral ground-glass opacity that is associated with marked interlobular septal thickening and a sharp nonanatomic demarcation between normal and abnormal lung.
Primary PAP is caused by disruption of granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling, either by GM-CSF autoantibodies (ie, autoimmune PAP)or genetic mutations involving the GM-CSF receptor (ie, hereditary PAP). Secondary PAP occurs in a heterogeneous group of conditions that reduce numbers or functions of alveolar macrophages and thereby surfactant clearance. Congenital PAP is caused by mutations in genes required for normal surfactant production. Over 90% of cases are autoimmune. Hereditary PAP accounts for approximately 3% of cases, secondary PAP for 4%, and congenital PAP for 1.5%. [9, 10]
Peferred examination
Although CT scan (in particular, high-resolution CT [HRCT] scan) findings are often characteristic of PAP, fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy are sufficient for confirming the clinical diagnosis in most patients. Chest radiographs alone, while occasionally suggestive, are rarely diagnostic in patients with PAP. CT scanning, especially HRCT scanning, often displays findings that are characteristic of, although not pathognomonic for, PAP. [11, 12, 13, 14, 15, 16, 5] Indeed, Johkoh et al include at least 15 different entities in the differential diagnosis for the characteristic HRCT scan findings of PAP. [17]
Few data are available regarding the MRI appearance of pulmonary alveolar proteinosis (PAP). Because of magnetic susceptibility effects and the short echo times of lung parenchyma, lung tissue is challenging to image with MRI.
A study by Moore et al addressed the appearances of diffuse air-space disease on MRI and found that air-space opacity in PAP has a short T1 value. This opacity demonstrated relatively little increased signal intensity with T2-weighted imaging. [18] Presumably, the short T1 signal of PAP reflects the relative lack of water within the lipoproteinaceous material that fills the air spaces in this disease.
A serum GM-CSF autoantibody test is highly sensitive and specific for autoimmune PAP. A negative GM-CSF autoantibody test excludes autoimmune PAP but does not exclude other PAP-causing diseases. However, additional blood-based tests to measure GM-CSF receptors/signaling and other genetic tests are available and permit the rapid, accurate diagnosis of the PAP-causing disease in more than 95% of patients. Because lung biopsy is associated with significant procedure-related morbidity, it should be reserved for patients in whom identification of the cause of PAP remains uncertain after completion of disease-specific, noninvasive serologic, blood-based, and genetic tests. [19]
Limitations of techniques
The differential diagnosis of PAP syndrome is challenged by its low prevalence, nonspecific symptoms (dyspnea, cough, chest tightness, and fatigue), common radiographic appearance (diffuse, patchy, ground-glass opacification and septal thickening), and normal routine laboratory tests. Many patients with PAP undergo transbronchial biopsy, surgical lung biopsy, or both, and histologic examination fails to identify the presence of PAP in 28% of cases because of patchy involvement. PAP is often misdiagnosed as pneumonia initially until the failure of multiple courses of empirical antibiotic therapy prompts diagnostic reconsideration and referral, thereby delaying accurate diagnosis by a median of 1.5 years (range, 0.5-13.5 years). [19]
Radiography
The classic chest radiographic finding of pulmonary alveolar proteinosis (PAP) is bilateral, symmetrical air-space opacity, which appears either as ground-glass or frank consolidation, with a perihilar or basilar predominance (see the images below). Air bronchograms are uncommon.
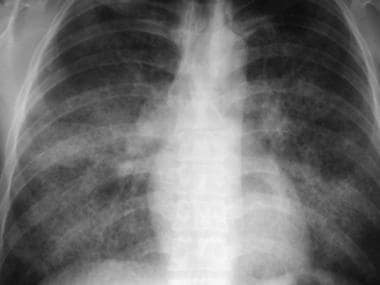 Frontal chest radiograph in a patient with pulmonary alveolar proteinosis. This image reveals bilateral air-space opacity without evidence of effusion or mediastinal widening. A faintly reticular pattern is present, representing thickened, interlobular septa.
Frontal chest radiograph in a patient with pulmonary alveolar proteinosis. This image reveals bilateral air-space opacity without evidence of effusion or mediastinal widening. A faintly reticular pattern is present, representing thickened, interlobular septa.
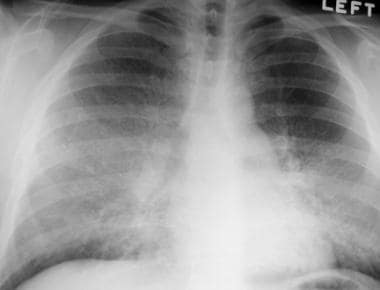 Frontal chest radiograph in a patient with pulmonary alveolar proteinosis that was subsequently treated with bronchoalveolar lavage. This image reveals bilateral, symmetrical air-space opacity without pleural effusion or mediastinal widening.
Frontal chest radiograph in a patient with pulmonary alveolar proteinosis that was subsequently treated with bronchoalveolar lavage. This image reveals bilateral, symmetrical air-space opacity without pleural effusion or mediastinal widening.
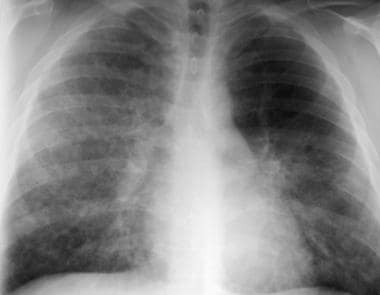 Chest radiograph in a patient after bronchoalveolar lavage. This image demonstrates improvement in the bilateral air-space opacity. These opacities subsequently cleared completely, and the patient remained in remission.
Chest radiograph in a patient after bronchoalveolar lavage. This image demonstrates improvement in the bilateral air-space opacity. These opacities subsequently cleared completely, and the patient remained in remission.
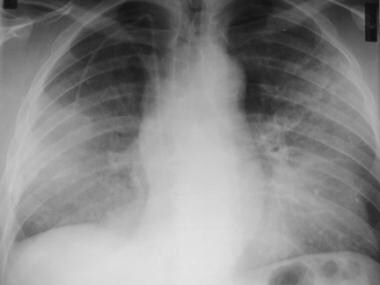 Frontal chest radiograph in a patient with the diffuse form of bronchioloalveolar carcinoma. This image shows the presence of bilateral, hazy, ground-glass attenuation; a normal mediastinal width; and no pleural effusion. This appearance resembles alveolar proteinosis.
Frontal chest radiograph in a patient with the diffuse form of bronchioloalveolar carcinoma. This image shows the presence of bilateral, hazy, ground-glass attenuation; a normal mediastinal width; and no pleural effusion. This appearance resembles alveolar proteinosis.
Radiographic opacities are often vaguely nodular and may be accompanied by fine, linear opacities or reticulation. Serial radiographs may demonstrate persistence of this pattern over time, resulting in a more limited differential diagnosis of chronic air-space opacity.
Unlike in hydrostatic pulmonary edema, the mediastinum is not widened, the heart is not enlarged, and pleural effusions are not common. Adenopathy is not a feature of the disease.
Atypical manifestations include a linear or reticular pattern that is unaccompanied by air-space opacity, lower lobe predominant consolidation, or poorly defined nodules as the primary radiographic manifestation of disease.
The differential diagnosis of PAP on chest radiographs includes all entities that result in diffuse air-space disease, such as hydrostatic and noncardiogenic pulmonary edema, diffuse infections (particularly Pneumocystis pneumonia), pulmonary hemorrhage, interstitial pneumonia, the diffuse form of bronchioloalveolar carcinoma, toxic lung injuries and hypersensitivity pneumonitis, eosinophilic pneumonia, drug or radiation-induced pulmonary disease, and lipoid pneumonia.
Chest radiograph and CT scan findings of treated PAP have also been described. [20, 21, 22]
The initial radiographs after BAL reveal increased opacity in the lobes in which the BAL fluid was instilled; this opacity is transient, clearing in hours to a few days.
After BAL, the aeration of the washed lung gradually improves. By 6 weeks after the BAL procedure, chest radiographs usually demonstrate improved aeration over preprocedure radiographs.
Rarely, in the course of resolution, new opacities may develop in either the washed lung or the contralateral lung. Occasionally, these opacities are related to endobronchial obstruction, usually representing atelectasis. Areas of decreased lung opacity, which represent hyperinflation, may also occur by the same mechanism.
Opacity in the contralateral lung may result from spillage of BAL fluid from the washed lung or atelectasis after the BAL procedure.
Complications of the BAL procedure, including pneumomediastinum and pneumothorax, may be evident on immediate postprocedure radiographs.
Degree of confidence
Radiographic findings of PAP are not specific, and as stated above, a large differential diagnosis must be considered. Similar to the case with other diffuse lung diseases, HRCT scanning provides a more specific morphologic evaluation of the disease pattern and may provide a more limited differential diagnosis.
Computed Tomography
Routine CT scans (7-10 mm collimation) may reveal bilateral areas of consolidation and reticulation in the lungs of patients with pulmonary alveolar proteinosis (PAP) (see the images below). [11, 14, 15, 23, 24, 25] The disease distribution of PAP is usually bilateral and patchy.
Occasionally, an underlying linear abnormality that represents thickened interlobular septa may be appreciated.
HRCT scanning is superior to conventional CT scanning and to chest radiography for demonstrating the morphologic characteristics of PAP. [26, 27] Crazy paving is the characteristic finding in PAP on HRCT scanning; it consists of patchy, bilateral geographic areas of ground-glass opacity, which are associated with interlobular septal thickening. The HRCT scan features of PAP are seen in the images below. [12, 16, 17, 5, 7]
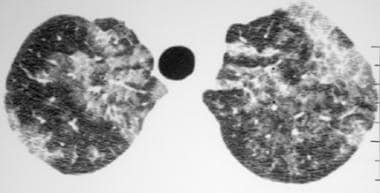 High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. The image reveals bilateral ground-glass opacity that is associated with septal thickening, which is consistent with the crazy-paving pattern.
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. The image reveals bilateral ground-glass opacity that is associated with septal thickening, which is consistent with the crazy-paving pattern.
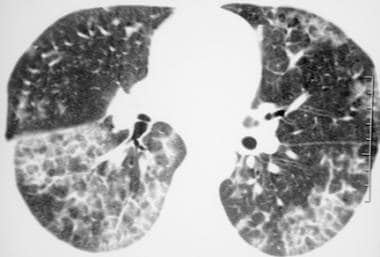
 High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis who received bronchoalveolar lavage. This image reveals regression of the ground-glass opacity, representing partial resolution of the disease.
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis who received bronchoalveolar lavage. This image reveals regression of the ground-glass opacity, representing partial resolution of the disease.
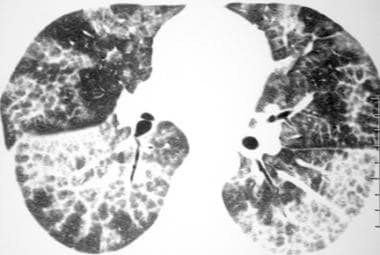
 High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. This image demonstrates the presence of bilateral ground-glass opacity and interlobular septal thickening.
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. This image demonstrates the presence of bilateral ground-glass opacity and interlobular septal thickening.
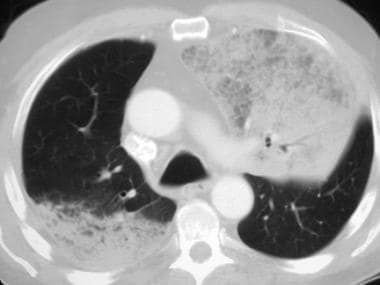
 High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis (PAP) (same patient as in the previous image). This image reveals slight interval worsening of the bilateral ground-glass opacity and interlobular septal thickening that is consistent with progression of PAP.
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis (PAP) (same patient as in the previous image). This image reveals slight interval worsening of the bilateral ground-glass opacity and interlobular septal thickening that is consistent with progression of PAP.
PAP is often distributed uniformly from the lung apex to the base. Interlobular septal thickening may be encountered more frequently in the lower lung zones. Although the ground-glass opacity in PAP is usually patchy, centrilobular nodules have been described in pediatric patients with PAP, as well as in adult patients with acute silicoproteinosis. [13]
Although findings of the interlobular septa are commonly abnormal for pathologic specimens from patients with PAP, septa findings may occasionally be pathologically normal, despite a thickened appearance on HRCT scans. This apparent septal thickening on HRCT scans presumably reflects the aggregation of PAS-positive lipoproteinaceous material immediately adjacent to the interlobular septa.
Classically, the abnormal pulmonary parenchyma is demarcated sharply from normal lung areas without a discernible anatomic boundary. (The images below show no such sharp demarcation and, therefore, suggest a diagnosis other than PAP.)
 Axial computed tomography scan (window width = 1500 Hounsfield units [HU], level = -600 HU) in a patient with the diffuse form of bronchioloalveolar carcinoma. Note the presence of ground-glass opacity that is associated with septal thickening, resembling the crazy-paving appearance of alveolar proteinosis. The space-occupying nature of the process (note that the left major fissure is bowed posteriorly) and the lack of the usually sharp, but nonanatomic, demarcation between the normal and abnormal lung (best seen in the posterior right lung) are findings that are not characteristic of alveolar proteinosis and, thus, should suggest an alternative diagnosis.
Axial computed tomography scan (window width = 1500 Hounsfield units [HU], level = -600 HU) in a patient with the diffuse form of bronchioloalveolar carcinoma. Note the presence of ground-glass opacity that is associated with septal thickening, resembling the crazy-paving appearance of alveolar proteinosis. The space-occupying nature of the process (note that the left major fissure is bowed posteriorly) and the lack of the usually sharp, but nonanatomic, demarcation between the normal and abnormal lung (best seen in the posterior right lung) are findings that are not characteristic of alveolar proteinosis and, thus, should suggest an alternative diagnosis.
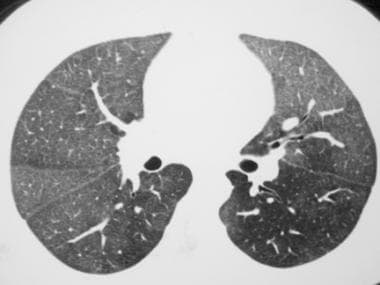 Axial computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with diffuse pulmonary hemorrhage. This image reveals diffuse, bilateral, ground-glass opacity that is associated with mild, interlobular septal thickening. The lack of the sharp demarcation between the normal and abnormal pulmonary parenchyma, which is characteristic of crazy paving, suggests a diagnosis other than alveolar proteinosis. In addition, the septal thickening in alveolar proteinosis is usually more prominent.
Axial computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with diffuse pulmonary hemorrhage. This image reveals diffuse, bilateral, ground-glass opacity that is associated with mild, interlobular septal thickening. The lack of the sharp demarcation between the normal and abnormal pulmonary parenchyma, which is characteristic of crazy paving, suggests a diagnosis other than alveolar proteinosis. In addition, the septal thickening in alveolar proteinosis is usually more prominent.
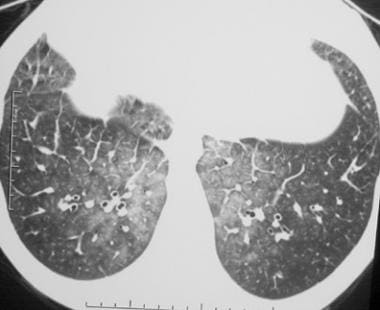 Axial computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) obtained through the lower lobes in a patient with pulmonary edema. This image reveals the bilateral, ground-glass opacity that is associated with smooth, interlobular, septal thickening. Although by definition these findings resemble crazy paving, the appearance is nevertheless quite distinct from alveolar proteinosis. Note also the absence of the sharp demarcation between the normal and abnormal lung that is usually a characteristic of crazy paving in alveolar proteinosis.
Axial computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) obtained through the lower lobes in a patient with pulmonary edema. This image reveals the bilateral, ground-glass opacity that is associated with smooth, interlobular, septal thickening. Although by definition these findings resemble crazy paving, the appearance is nevertheless quite distinct from alveolar proteinosis. Note also the absence of the sharp demarcation between the normal and abnormal lung that is usually a characteristic of crazy paving in alveolar proteinosis.
Similar to the case with other diffuse lung diseases, HRCT scanning provides a more specific morphologic evaluation of the disease pattern and may provide a more limited differential diagnosis. HRCT scanning is also useful for targeting the optimal site for tissue sampling.
Generally, PAP in children is often more acute and severe than PAP in adults. Chest radiographic and HRCT scan findings in childhood forms of PAP are few but are likely similar to their adult counterparts.
McCook et al suggested that a miliarylike pattern is encountered more frequently in the pediatric entity than in the adult form and that basilar predominant linear and reticular abnormalities may be observed more often on CT scans in children. [28]
HRCT scan descriptions of childhood PAP by Zontsich et al suggest that the same crazy-paving pattern seen in adults may be seen in children. [21]
Pleural effusions and adenopathy are uncommon in PAP, and the clinician should suspect a superimposed infection or malignancy if these are present. Occasionally, pleural effusions may be encountered in uncomplicated cases of PAP shortly after BAL.
Degree of confidence
Although the appearance of crazy paving is highly suggestive of PAP, this finding is not pathognomonic for PAP. Many other entities may present with a pattern similar to crazy paving on HRCT scans, including the following:
-
Hydrostatic pulmonary edema
-
Diffuse alveolar damage from any number of causes
-
Pulmonary hemorrhage
-
Diffuse pulmonary infections (including Mycobacterium tuberculosis, M pneumoniae, other bacterial pneumonias)
-
Diffuse form of bronchioloalveolar carcinoma
-
Acute respiratory distress syndrome (ARDS)
-
Drug-induced pneumonitis
-
Radiation pneumonitis
-
Bronchiolitis obliterans organizing pneumonia
-
Chronic eosinophilic pneumonia
-
Obstructive pneumonitis
-
Acute interstitial pneumonia
-
Lipoid pneumonia
Atypical manifestations of PAP also occur. Do not expect all instances of PAP to present as crazy paving on HRCT scans.
-
Frontal chest radiograph from a patient with pulmonary alveolar proteinosis. This image demonstrates bilateral perihilar and infrahilar ground-glass opacity without evidence of mediastinal widening, pleural effusion, or adenopathy.
-
Frontal chest radiograph in a patient with pulmonary alveolar proteinosis. This image reveals bilateral air-space opacity without evidence of effusion or mediastinal widening. A faintly reticular pattern is present, representing thickened, interlobular septa.
-
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with acute silicoproteinosis. This image demonstrates crazy paving, which refers to bilateral ground-glass opacity that is associated with marked interlobular septal thickening and a sharp nonanatomic demarcation between normal and abnormal lung.
-
Frontal chest radiograph in a patient with pulmonary alveolar proteinosis that was subsequently treated with bronchoalveolar lavage. This image reveals bilateral, symmetrical air-space opacity without pleural effusion or mediastinal widening.
-
Chest radiograph in a patient after bronchoalveolar lavage. This image demonstrates improvement in the bilateral air-space opacity. These opacities subsequently cleared completely, and the patient remained in remission.
-
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. The image reveals bilateral ground-glass opacity that is associated with septal thickening, which is consistent with the crazy-paving pattern.
-
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis who received bronchoalveolar lavage. This image reveals regression of the ground-glass opacity, representing partial resolution of the disease.
-
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. This image demonstrates the presence of bilateral ground-glass opacity and interlobular septal thickening.
-
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis (PAP) (same patient as in the previous image). This image reveals slight interval worsening of the bilateral ground-glass opacity and interlobular septal thickening that is consistent with progression of PAP.
-
High-resolution computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with pulmonary alveolar proteinosis. This image shows that the sharp demarcation between the normal and abnormal lung parenchyma that is characteristic of the crazy-paving pattern is less conspicuous than usual, although it is evident in the anterior segment of the right upper lobe. Ground-glass opacity that is associated with interlobular septal thickening is the dominant finding on this image.
-
Frontal chest radiograph in a patient with the diffuse form of bronchioloalveolar carcinoma. This image shows the presence of bilateral, hazy, ground-glass attenuation; a normal mediastinal width; and no pleural effusion. This appearance resembles alveolar proteinosis.
-
Axial computed tomography scan (window width = 1500 Hounsfield units [HU], level = -600 HU) in a patient with the diffuse form of bronchioloalveolar carcinoma. Note the presence of ground-glass opacity that is associated with septal thickening, resembling the crazy-paving appearance of alveolar proteinosis. The space-occupying nature of the process (note that the left major fissure is bowed posteriorly) and the lack of the usually sharp, but nonanatomic, demarcation between the normal and abnormal lung (best seen in the posterior right lung) are findings that are not characteristic of alveolar proteinosis and, thus, should suggest an alternative diagnosis.
-
Axial computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) in a patient with diffuse pulmonary hemorrhage. This image reveals diffuse, bilateral, ground-glass opacity that is associated with mild, interlobular septal thickening. The lack of the sharp demarcation between the normal and abnormal pulmonary parenchyma, which is characteristic of crazy paving, suggests a diagnosis other than alveolar proteinosis. In addition, the septal thickening in alveolar proteinosis is usually more prominent.
-
Axial computed tomography scan (window width = 1000 Hounsfield units [HU], level = -700 HU) obtained through the lower lobes in a patient with pulmonary edema. This image reveals the bilateral, ground-glass opacity that is associated with smooth, interlobular, septal thickening. Although by definition these findings resemble crazy paving, the appearance is nevertheless quite distinct from alveolar proteinosis. Note also the absence of the sharp demarcation between the normal and abnormal lung that is usually a characteristic of crazy paving in alveolar proteinosis.