Practice Essentials
Abdominal aortic aneurysms (AAAs) are segmental dilatations of the aortic wall that cause the vessel to be larger than 1.5 times its normal diameter or that cause the distal aorta to exceed 3 cm. These can continue to expand and rupture spontaneously, exsanguinate, and cause death. AAA rupture is an important cause of unheralded deaths in people older than 55 years.
Ideally, in a hemodynamically stable patient, nonenhanced and enhanced helical or spiral CT of the thorax, abdomen, and pelvis should be expeditiously performed. This examination provides key information about the extent of aneurysmal disease, and it can be used to confirm and localize the site of rupture (see the image below).
The most commonly used noninvasive methods to identify abdominal aortic aneurysm are ultrasound and computed tomography. CT has been shown to have slightly higher sensitivity, and ultrasonography to have higher specificity for diagnosis of AAA. [1, 2, 3]
In the patient with an unstable presentation, an emergency operation is indicated. Time may permit only rapid bedside ultrasonography (US) and Doppler study of abdominal aorta and iliac arteries to confirm the presence of aneurysms.
The maximal aneurysm diameter is adequately assessed by using B-mode ultrasonography, CT scanning, and MRI. [4, 5, 6] Aortography reveals only the lumen of the abdominal aortic aneurysm because laminated clot obscures the outer limit of the aneurysm wall. Therefore, it often causes underestimation of the true aortic diameter.
Key pathobiologic processes of AAA progression and rupture include neovascularization, necrotic inflammation, microcalcification, and proteolytic degradation of the extracellular matrix. Future ancillary imaging techniques may therefore employ the use of MRI with ultrasmall superparamagnetic particles of iron oxide that may identify and track hotspots of macrophage activity. Positron emission tomography (PET) with a variety of targeted tracers may detect areas of inflammation, angiogenesis, hypoxia, and microcalcification. [7, 8, 9]
(See the AAA image below.)
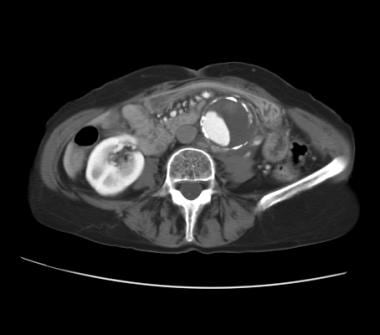 Contrast-enhanced abdominal CT in an elderly patient who presented with severe back pain but was hemodynamically stable. CT reveals an abdominal aortic aneurysm (AAA) with eccentric mural thrombus. A disruption of the calcific rim of the AAA toward the left quadrant appears with adjacent isoattenuating soft tissue anterior to the left psoas muscle. Clinical and radiologic findings are consistent with a diagnosis of contained AAA rupture with left retroperitoneal hematoma.
Contrast-enhanced abdominal CT in an elderly patient who presented with severe back pain but was hemodynamically stable. CT reveals an abdominal aortic aneurysm (AAA) with eccentric mural thrombus. A disruption of the calcific rim of the AAA toward the left quadrant appears with adjacent isoattenuating soft tissue anterior to the left psoas muscle. Clinical and radiologic findings are consistent with a diagnosis of contained AAA rupture with left retroperitoneal hematoma.
Screening
The US Preventive Services Task Force (USPSTF) recommendations include the following statements [3, 10, 11] :
-
One-time screening for AAA with ultrasonography in men aged 65-75 years who have ever smoked.
-
Selectively offer screening for AAA with ultrasonography in men aged 65-75 years who have never smoked rather than routinely screening all men in this group.
-
Recommendation against routine screening for AAA with ultrasonography in women who have never smoked and have no family history of AAA.
The American College of Cardiology/American Heart Association guidelines include a class I, level B recommendation that patients with infrarenal or juxtarenal AAAs measuring 5.5 cm or larger undergo repair to eliminate the risk of rupture, and a class I, level A recommendation that patients with infrarenal or juxtarenal AAAs measuring 4.0 to 5.4 cm in diameter be monitored by ultrasound or CT scans every 6 to 12 months to detect expansion. [12]
In the Cardiovascular Health Study, aneurysm dilatation of 3 cm or greater on a single screening ultrasound exam identified 68% of all AAA repairs over the next 10 years and 6 of the 10 AAA-related deaths in 4% of the total population; dilatation of 2.5 cm or more identified 91% of all AAA repairs and 9 of the 10 deaths in 10% of the total population. [13]
The American College of Radiology in its Appropriateness Criteria on pulsating abdominal masses noted that imaging studies are important in diagnosing the cause of a pulsatile abdominal mass and, if an AAA is found, in determining its size and involvement of abdominal branches. The ACR has noted the following regarding AAA screening [14] :
-
Ultrasound (US) is the initial imaging modality of choice when a pulsatile abdominal mass is present.
-
Noncontrast computed tomography (CT) may be substituted in patients for whom US is not suitable.
-
When aneurysms have reached the size threshold for intervention or are clinically symptomatic, contrast-enhanced multidetector CT angiography (CTA) is the best diagnostic and preintervention planning study, accurately delineating the location, size, and extent of aneurysm and the involvement of branch vessels.
-
Contrast-enhanced magnetic resonance angiography (MRA) may be substituted if CT cannot be performed.
-
Noncontrast MRA should only be performed at facilities where the needed technical expertise exists.
The following are screening recommendations by Kaiser Permanente for AAA by ultrasonography in the general population [15] :
-
One-time screening for AAA by ultrasonography is recommended in men aged 65 to 75 years.
-
It is an option to limit AAA screening to men aged 65 to 75 years who have never smoked.
-
Routine screening for AAA in women is not recommended.
The following are screening recommendations by Kaiser Permanente for AAA in adults with a family history of AAA [15] :
-
For men aged 50 years and older with a known positive family history of aortic aneurysm in a first-degree relative, AAA screening is recommended.
-
Systematically collecting information on aortic aneurysm family history is not recommended.
Postoperative follow-up
Little objective information is available to guide long-term surveillance after successful emergency repair of AAA rupture. There are data from the prospective Canadian Aneurysm Study in the 1990s, which found that in approximately 15% of patients who underwent elective open repairs, another thoracic or abdominal aneurysm was subsequently detected; a similar percentage of patients had significant iliac aneurysms (following tube AAA graft). [16] These findings led the investigators to recommend routine CT from thorax to pelvis after 5 years.
The ACR Appropriateness Criteria deem abdominal and pelvic CTA the "de facto gold standard" for post-EVAR repair surveillance imaging at 1 month, 12 months, and annually thereafter. MRA is also appropriate, but some stent materials may obscure some images. [17]
Patients who have undergone endovascular aneurysm repair require more frequent and lifelong follow-up. Imaging studies are typically performed 1, 6, and 12 months after EVAR (endovascular aneurysm repair) and annually thereafter. [18] CT angiography is most commonly used. [19] However, studies have found contrast-enhanced ultrasound to be comparable to CT angiography. [20, 21] MRI and magnetic resonance angiography have also been used for surveillance, as has digital subtraction angiography, although this technique is now not as necessary. [19, 22] Intermittent surveillance of the thoracic aorta should be performed, particularly in patients with preexisting aortic ectasia or dilatation elsewhere or for patients (or their siblings) with connective tissue disease (eg, Marfan or Ehlers-Danlos), bicuspid aortic valve, or familial aortopathies.
Surveillance imaging should be considered especially for suggestive symptoms, even for grafts older than 10 years, because surgical polyester graft degradation is not uncommon and has been documented up to 4 decades postoperatively. [23]
Limitation of techniques
CT or MRI can rapidly provides detailed information about the blood vessels and their surrounding structures for treatment planning; the choice between them can be based on which is faster locally. Occasionally, however, these examinations may require too much time for them to be suitable for use in patients whose condition is unstable. Also, when contrast material is used in conjunction with CT to delineate blood-filled structures, it poses a risk of acute renal failure, particularly in hypovolemic elderly patients who may already have baseline nephrosclerosis or diabetic nephropathy.
Sonography is a quick and convenient modality, but it is much less sensitive and specific for the diagnosis of aneurysmal rupture. The absence of sonographic evidence of rupture does not rule out this entity if clinical suspicion is high.
Radiography
A curvilinear calcified rim, often to the left of the midline, is apparent on some plain abdominal radiographs. This may be the first clue to abdominal aortic aneurysms in patients who present with unexplained abdominal pain but are temporarily hemodynamically stable. Lateral radiography can depict large AAAs on the basis of calcification in the wall. This finding can be seen in more than half of patients.
Calcification identified on lateral instant verterbral assessment images has been identified as a risk factor for rupture of an abdominal aortic aneurysm. [24]
The degree of confidence is low. Suspicious findings on the abdominal radiograph should be confirmed by using another imaging modality.
Mural calcification can be radiographically inapparent and lead to a false-negative finding in as many as half of small AAAs. Magnification errors are also possible, leading to overestimation or underestimation of AAA diameter.
Computed Tomography
General findings
Unlike angiography, CT and MRI provide information about the wall of the aorta, and they delineate the presence of thrombus. [25] They provide detail about surrounding abdominal structures and their relationship to the abdominal aortic aneurysms. Perianeurysmal fibrosis, venous anomalies (eg, retroaortic left renal vein, circumaortic venous collar), and horseshoe kidney are reliably demonstrated. However, these modalities (particularly MRI) are currently time and labor intensive, and they are not suitable for use in patients in unstable condition.
A large baseline aneurysm size and a rapid increase in size over time are associated with a higher risk for rupture on multidetector CT (MDCT). Additional CT findings that reflect aortic aneurysm instability include luminal expansion with lysis of thrombus, intramural hemorrhage (ie, the crescent sign), periaortic hemorrhage, a penetrating atherosclerotic ulcer, and contained rupture (ie, the draped aorta sign). These may be missed if quality control and adequate visualization are not done diligently, because additional time is occasionally required for complete (delayed) opacification. Moreover, consistent and careful review not only of the early arterial phase but also up to the venous phase will enhance detection of subtle signs. The presence of new and focal outward displacement of calcified intimal plaque should trigger a suspicion of disrupted AAA and contained rupture. [26]
The degree of confidence is high. Soft tissue attenuation outside of the abdominal aorta can be related to changes secondary to an inflammatory process. These findings may possibly be related to pathology from other abdominal viscera. The sensitivity and specificity for the detection of AAA rupture decreases if a nonenhanced study is performed.
Helical or spiral CT angiography (CTA) allows reasonable visualization of branches of the aorta in the context of surrounding structures. Postprocessing of images and 3-dimensional reconstruction are possible.
In a retrospective study of 67 consecutive pre-repair AAA CTAs, AAA contrast inhomogeneity was found to be a common observation in first-pass CTA and was associated with rapid aneurysm growth, independent of aneurysm diameter. [27]
Magnetic resonance angiography (MRA) has the advantage of eliminating the need for potentially nephrotoxic contrast agents and ionizing radiation, but its acquisition speed, cost, and image quality still need to be improved. Ferromagnetic implants or severe claustrophobia also can preclude use of MRI and/or MRA.
Findings that indicate possible AAA rupture include soft tissue hyperintensity outside the aortic wall, an indistinct aortic wall, thinning or fracture of a calcified aortic wall segment, penetration of a hematoma into the leaves of the mesentery, or contrast extravasation into the psoas muscle or retroperitoneum.
The nonenhanced study shows a mass or collection that extends into the perirenal spaces or, less commonly, into the pararenal spaces. The AAA is often obscured or anteriorly displaced. Other possible findings include a focally indistinct aortic margin (usually the site of rupture), enlargement or obscuration of the psoas muscle, and anterior displacement of the kidney.
Patients with relatively small AAAs may experience a sealed rupture. Characteristic findings in such cases are a draped aorta and adjacent vertebral erosion. [28]
Apter et al determined that a sealed rupture of an AAA can occur in relatively small aneurysms and that a draped aorta and adjacent vertebral erosion are characteristic CT signs of such a rupture. [29] They reviewed the CT scans of 6 patients with a sealed rupture of an AAA and those reported in the literature for aneurysm size, presence of a draped aorta, and adjacent vertebral erosion.
CT-specific findings
Freshly extravasated blood typically has a high CT attenuation value, whereas an isoattenuating or hypoattenuating hematoma signifies that a leak is days or weeks old.
When intraluminal blood dissects into a thrombus and comes in contact with a weakened aortic wall, the risk of rupture is increased. The crescent sign is of high density within the AAA mural thrombus or wall, which is thought to represent an intramural hematoma. [30] The attenuation should be greater than the aortic lumen on unenhanced scans and should be greater than the psoas muscle on enhanced scans. This was found to be a sign of acute or impending rupture in a retrospective series, with a sensitivity, specificity, and positive predictive value of 77%, 93%, and 53%, respectively. [31] In addition, thrombus fissuration can be seen from the same process. This sign can be observed on enhanced CT studies as linear contrast infiltration from the aortic lumen into intramural thrombus. [32]
Periaortic fibrosis outside of the subintimal calcification is suggestive of inflammatory aneurysm.
Postoperative findings
Follow-up imaging after EVAR is used to monitor the status of the repair. Endoleak (persistent blood flow within the aneurysmal sac) is the most common complication of EVAR, occurring in approximately 25% of patients. Endoleaks are classified into 5 types, according to the source of blood flow into the sac [19] :
-
Type I: from incomplete or ineffective sealing at the graft attachment site; this is usually an early complication.
-
Type II: from retrograde blood flow from aortic collateral vessels.
-
Type III: from disruption of the graft.
-
Type IV: from porosity of the graft.
-
Type V: expansion of the aneurysm without an obvious endoleak, termed endotension.
Techniques
In a study of multidetector-row CTA in patients with aortic aneurysms, Kubo et al found that optimal contrast enhancement was produced by using 75 mL of contrast medium followed by 20 mL of saline flush. [33]
Iezzi et al found that in patients undergoing multidetector-row CTA for surveillance after EVAR, the delayed phase should be acquired 300 seconds after injection of contrast medium. [18, 20]
In contrast, Hong et al reported that endoleaks detected only in the delayed phase of CT resolved spontaneously; they therefore suggested eliminating the delayed phase of acquisition, so as to minimize radiation exposure. [34]
Magnetic Resonance Imaging
MRI is a valuable alternative to CT in patients with renal insufficiency in whom contrast material–induced nephropathy is a concern. [35] MRI is also helpful in further delineating the aorta in the context of a large retroperitoneal collection that obscures the borders between adjacent structures, as well as laminated clot or atherosclerotic debris on the aneurysmal wall. [9]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
The degree of confidence is high. Soft tissue intensity changes outside of the abdominal aorta can be related to changes secondary to an inflammatory process. These findings might actually be related to pathology from other abdominal viscera.
Ultrasonography
Unlike most other modalities (eg, aortography, CT, MRI), abdominal US can be performed expeditiously and at the bedside. [36] For partially encapsulated hematomas, a hypoechoic or anechoic para-aortic space-occupying lesion may be detected.
Color-flow Doppler can aid in detecting the site of leak or extravasation, although adjustment to low-velocity scales may be necessary to register leaks with low flow rates. Contrast-enhanced US has been advocated for surveillance after EVAR; the recommended dose of contrast medium for such studies is 2.4 mL. [20, 21]
The degree of confidence is high for the detection of abdominal aortic aneurysms and low for the detection of AAA rupture. Although duplex US is competitive with CT or MRI for the detection of an AAA (>95% sensitivity), the visualization of surrounding structures is poor, and the sensitivity and specificity for rupture are low. For detection of endoleak after aneurysm repair, contrast-enhanced US imaging was shown to be significantly more accurate than unenhanced US studies (89.3% vs 63.1%). [20]
Conditions that limit the detection and measurement of AAAs or its branches include excessive bowel gas, obesity, and recent abdominal surgery. If a ruptured AAA is clinically suspected, other diagnostic modalities should be pursued even if the sonographic results are negative. [7]
Three-dimensional ultrasonography has demonstrated acceptable reproducibility and good agreement with 3D-CT. Using 3D reconstructions and perhaps center-line multiplanar reconstruction techniques may lower interobserver and interval variability of AAA size comparisons. [37, 38]
Angiography
Digital subtraction angiography (DSA) provides high-spatial-resolution images of the lumen of the vascular tree and permits the quantification of significant stenoses in renal, mesenteric, and iliofemoral arteries. [39] CT also depicts aberrant vessels well. [27]
Angiographic findings of abdominal aortic aneurysms rupture include the following:
-
Contained rupture: Circumscribed extraluminal contrast enhancement is present. Additional views (oblique or lateral) may be required to detect this finding.
-
Leaking aneurysm: Frank extravasation of contrast material with poor washout is observed. This is rarely demonstrated because the patients are typically in unstable condition and are transported directly to the operating room.
-
Displacement of the visceral arteries or kidneys and ureters: Sometimes, this finding can be evident in larger collections of blood.
-
Contrast enhancement: Contrast material may flow into these structures from the AAA in the rare circumstance of rupture into the GI tract or IVC.
The degree of confidence is low. Angiography is rarely used in the setting of suspected rupture and is relatively contraindicated except when endovascular stent grafting is planned because this is an experimental procedure. However, unusual manifestations of AAA rupture, such as into the IVC or GI tract (aortoenteric fistula) is demonstrated well with angiography.
The absence of extraluminal contrast cannot be used to totally rule out a small leak or stabilized rupture. If the degree of suspicion is high, a tomographic imaging modality should be performed.
Laminated or mural thrombus may give the false arteriographic impression that no AAA is present. A calcified aortic shell and the absence of lumbar arteries are clues to the presence of an aneurysm.
-
Contrast-enhanced abdominal CT in an elderly patient who presented with severe back pain but was hemodynamically stable. CT reveals an abdominal aortic aneurysm (AAA) with eccentric mural thrombus. A disruption of the calcific rim of the AAA toward the left quadrant appears with adjacent isoattenuating soft tissue anterior to the left psoas muscle. Clinical and radiologic findings are consistent with a diagnosis of contained AAA rupture with left retroperitoneal hematoma.