Practice Essentials
Coronary artery disease (CAD) is a complex disease that causes reduced or absent blood flow in 1 or more of the arteries that encircle and supply the heart. The disease may be focal or diffuse. Apart from rare congenital anomalies (birth defects), coronary artery disease is usually a degenerative disease, uncommon as a clinical problem before the age of 30 years and common by the age of 60 years. One in four people will have a heart attack. The first recognized symptom may be death. The term coronary is derived from crown, referring to the way these arteries sit on the heart.
CAD is one of the major cardiovascular diseases affecting the global human population. This disease has proved to be the major cause of death in both developed and developing countries. Lifestyle, environmental factors, and genetic factors pose as risk factors for the development of cardiovascular disease. The prevalence of risk factors among healthy individuals elucidates the probable occurrence of CAD in the near future. Risk factors for CAD include diabetes mellitus, hypertension, smoking, hyperlipidemia, obesity, chronic kidney disease, cigarette smoking, diet, family history, homocystinuria, and psychosocial stress. [1, 2]
The American College of Radiology notes that CAD has a long asymptomatic latent period and that early targeted preventive measures can reduce mortality and morbidity. Imaging modalities for evaluating patients at increased risk for CAD include radiography, fluoroscopy, multidetector computed tomography (CT), ultrasound, magnetic resonance imaging (MRI), cardiac perfusion scintigraphy, echocardiography, and positron emission tomography (PET). [3]
Newer technologies such as CT fractional flow reserve, CT angiography with perfusion, and whole-heart coronary magnetic resonance angiography with perfusion, which can provide both anatomic and functional information in the same test, obviate the need for multiple diagnostic tests to obtain a comprehensive assessment of both plaque burden and downstream ischemia. [4]
Coronary revascularization is the most important treatment strategy for CAD. Percutaneous coronary intervention (PCI) has become the most frequently performed procedure. The evolution of stents plays an important role in the results of this procedure. Coronary artery bypass grafting (CABG) is the most effective revascularization approach for stenotic coronary arteries. Conduits selected and surgical techniques applied are important determinants of patient outcomes. Multidisciplinary decision-making should analyze current evidence, consider the clinical condition of the patient, and determine the safety of and necessity for coronary revascularization with either PCI or CABG. [5]
(See the images below depicting the coronary arteries and CAD.)
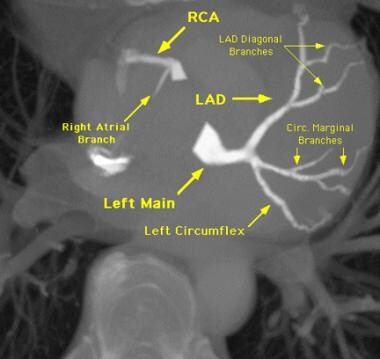
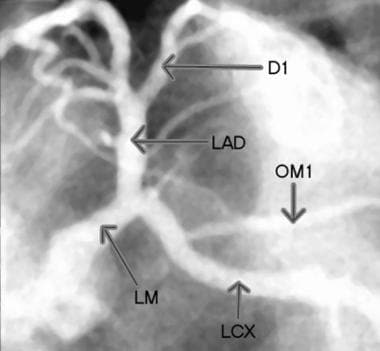 Selective injection image of the left coronary arteries. D1 = first diagonal, LAD = left anterior descending artery, LCX = left circumflex, LM = left main coronary artery, and OM1= first obtuse marginal.
Selective injection image of the left coronary arteries. D1 = first diagonal, LAD = left anterior descending artery, LCX = left circumflex, LM = left main coronary artery, and OM1= first obtuse marginal.
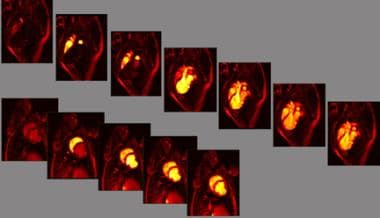 Contrast-labeled blood to the heart is used to identify the territory at risk. The results of this assessment of the delayed arrival compares favorably to the findings of radionuclide stress imaging, and stress induction of ischemia is not required to identify the zone at risk.
Contrast-labeled blood to the heart is used to identify the territory at risk. The results of this assessment of the delayed arrival compares favorably to the findings of radionuclide stress imaging, and stress induction of ischemia is not required to identify the zone at risk.
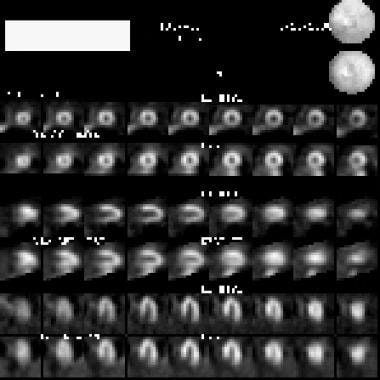 Compared with radionuclide images of blood delivery, MRIs and CT scans improve resolution, depiction of the functional effect and the relationship to the coronary supply, and identification of the area at risk without stress. The advantage of radionuclide imaging is primarily its predictive value; stress echocardiography has similar predictive value. MRI and CT have been less available than other studies; therefore, data on their value are relatively limited.
Compared with radionuclide images of blood delivery, MRIs and CT scans improve resolution, depiction of the functional effect and the relationship to the coronary supply, and identification of the area at risk without stress. The advantage of radionuclide imaging is primarily its predictive value; stress echocardiography has similar predictive value. MRI and CT have been less available than other studies; therefore, data on their value are relatively limited.
Stable and unstable lesions
Lesions that cause blockages in the coronary arteries may be stable or unstable.
For chronic stable CAD, the treatment objective is a combination of symptomatic and prognostic improvement. Cardiovascular magnetic resonance (CMR) has emerged as a highly accurate technique for diagnosis and risk stratification in stable CAD and has been incorporated into national and international guidelines. [6]
Unstable lesions activate blood clotting and/or vascular spasm. Indications that CAD may be unstable include recent onset or familiar symptoms that are increasing in frequency, in duration, or in severity, and decreasing tolerance with exertion or at rest. The term "chest pain" is a code phrase—symptoms of CAD do not have to occur in the chest and do not have to include pain. The phrase "heart warning" symptoms is often preferred. When a warning light is activated, one should resolve the problem quickly, even if it is low in intensity.
Unstable symptoms of CAD may represent a threatened heart attack. After as little as 5 minutes, a wall of the heart may stop functioning but may still be salvageable—this is called stun. After as little as 10-20 minutes, permanent damage can occur. If symptoms are new, if they are familiar but unstable, or if they are not reliably fully resolved in 5 minutes, emergency help is recommended because "time is muscle." Intervention completed within 60 minutes improves outcome. The symptoms of a threatened heart attack may be very mild.
Ischemia
When the blood supply to the heart is inadequate (ie, ischemia), pressure may be felt in the chest that moves to the left arm; one may feel weak, sweaty, short of breath, or nauseated; palpitations (ie, changes in heart rhythm) may occur; or there may be a sensation of pressure or tightness in just the chest, neck, or arms.
Many patients mistake heart warning symptoms for heartburn or gas. If symptoms occur that may represent inadequate blood supply to the heart, one should rest immediately and take nitroglycerin, if available. If symptoms last longer than 5 minutes, if they occur at rest, or if they keep coming back, one should call 911, chew a full-sized aspirin (325 mg) if not allergic, and continue taking nitroglycerin every 5 minutes as long as this does not cause dizziness or light-headedness.
Severity of CAD
One can define the severity of CAD in several ways, including the following:
-
Anatomically, by visualizing blood vessel branches and any blockages to blood flow along the pathways
-
Functionally, by estimating delivery of blood to tissue supplied by each branch vessel
-
Clinically, by identifying symptoms that correspond to inadequate blood delivery, discerning the level of activity that causes them, determining what relieves these symptoms, and ascertaining the patterns of occurrence
Such patterns are described as unstable if they show variable or accelerating frequency, variable or increasing severity or changing character, or a variable or decreasing exercise threshold, or if symptoms continue or recur just after a heart attack.
One must examine the consequences, including location and extent of reversible and permanent impairment, motion and thickening of affected heart segments, and whether the damage is causing or sustaining life-threatening arrhythmias.
One must also evaluate the patient's overall cardiac performance, which typically is expressed as the ejection fraction (EF), or the percentage of contents that the left ventricle pumps forward in a heartbeat, along with exertion tolerance, graded from 1-4 (1=normal, 4=bedridden).
TIMI (Thrombolysis in Myocardial Infarction) risk score looks at 7 factors that tend to worsen outcomes:
Age 65 years or older
At least 3 risk factors for CAD
Prior coronary stenosis of 50% or more
ST-segment deviation on electrocardiogram at presentation greater than 0.5 mm
At least 2 anginal events in the prior 24 hours
Use of aspirin in the prior 7 days
Elevated serum cardiac markers
TIMI risk scores show the following risks of all-cause mortality, new or recurrent MI, or severe recurrent ischemia requiring urgent revascularization within the first 2 weeks: 1=5%, 2=8%, 3=13%, 4=20%, 5=26%, and 6/7=41%. [7]
Imaging in CAD
At present, achieving the best resolution on images of the coronary arteries requires catheterization, injection of an iodinated contrast agent, and use of a radiographic technique. As an alternative, multidetector-row CT (MDCT) or magnetic resonance imaging (MRI) may be used to clarify coronary anatomy and to determine whether a vessel is occluded.
Stress imaging has a complementary role in depicting zones with inducible ischemia (blood supply inadequate for tissue demands). Stress may be produced with exercise, with infusion of a medication that increases the strength of cardiac contractions (eg, dobutamine), or with infusion of a medication that dilates the vessels, thereby reducing delivery of blood to diseased branches (eg, adenosine, dipyridamole).
MRI has proved capable of imaging the coronary arteries and revealing stenoses without catheterization or injection of contrast material. [8] MDCT offers a fast and useful alternative for defining coronary anatomy. [9] MRI takes more time than MDCT and generally provides less detail of coronary anatomy, but it avoids the use of ionizing radiation and iodinated contrast agents.
Advances in MRI and CT have markedly improved the speed and resolution of imaging, making these modalities useful in the clinical evaluation of CAD, while improving their safety and convenience. In addition to defining anatomy, both MRI and CT can be used to identify zones of impaired blood supply by timing the arrival of contrast agent–labeled blood.
MRI is useful in identifying the location and thickness of myocardial scars. Although neither MRI nor CT has replaced coronary angiography (XRA) as the clinical standard for diagnosis of coronary stenosis, their use in determining whether a vessel is open is increasing. Studies report that 64-slice multidetector-row CT angiography (CTA) has shown potential as an alternative to coronary angiography for identification of coronary blockage. [10] In a study of 15,207 intermediate likelihood patients without known CAD, the severity of CAD on coronary CTA was predictive of the need for invasive coronary artery catheterization or revascularization. This suggests that coronary CTA may be an effective gatekeeper for invasive catheterization. [11]
Assessment of tissue viability
The extent of impairment or damage caused by stenosis obstructing a coronary artery depends on how much of the myocardium the vessel supplies, the severity of stenosis and of any superimposed spasm, and the level of demand and the condition of tissue supplied by the vessel.
When demand exceeds supply, the tissue becomes ischemic, which means blood supply is insufficient to maintain normal metabolism. Myocardial ischemia may cause chest pain, fatigue, shortness of breath, or another sign of reduced exertion tolerance.
Ischemia may cause no symptoms but may be detected as impaired blood delivery, impaired contractile function (wall motion or wall-thickening abnormality on dynamic cardiac imaging series), or interference with movement of ions (resulting in depolarization and repolarization abnormalities seen on electrocardiogram [ECG] as shifts in the ST-segment, or as changes in ST and T waves or rhythm abnormalities); or ischemia may be detected when a blood test shows release of enzymes (creatine kinase-MB [CK-MB], troponin-I, troponin-T) from the heart muscle.
Ischemia may deplete high-energy phosphate carriers (eg, creatine, adenosine) needed for muscle contraction. Depletion may occur to the point that impaired motion may persist even when ischemia is relieved. Transiently impaired contractile function of muscle that persists after relief from ischemia is called stun, and long-term dysfunction of viable muscle is called hibernation.
Dead tissue converted to scar likewise loses contractile function. Therefore, a key issue when a region of the heart wall shows loss of function involves determination of whether the myocardium is still viable. Persistent wall motion abnormality at rest shown on imaging (echocardiography, MRI, CT, coronary angiography) can raise the issue of tissue viability and, in particular, can guide decisions on whether repairing a blockage in the blood supply is likely to be beneficial.
A region that is thin and akinetic (no motion) is more likely to scar (dead myocardium) than a region that is not. Viability testing is helpful. For example, one can assess viability by performing phosphorus-31 MRI and by reporting for each region the relative concentrations of creatine phosphate; inorganic phosphate; and adenosine monophosphate, diphosphate, and triphosphate.
Although MRI of phosphorylated metabolites and positron emission tomography (PET) of metabolic activity (to assess glucose utilization) can be used to assess tissue viability, an alternative method of equal, if not better, clinical value consists of using MRI with contrast to identify retention of contrast by damaged myocardium.
Another way to identify viability is to examine wall motion at rest and with light stress. Dobutamine stress imaging may be performed with MRI or echocardiography. Dobutamine stress tests are used to detect viability by demonstrating dose-related increases in contractility if the tissue is viable. An increase in the dose of dobutamine may subsequently elicit a decline in contractility associated with induced ischemia—that is, a biphasic response, indicating viable but threatened myocardium.
Early in the development of perfusion imaging, [12, 13] retention of gadolinium contrast by injured myocardium was observed. Normally, a bolus of contrast agent washes out of the heart walls within 5-10 minutes. Any contrast agent seen in the heart after the agent has washed out of normal zones demarcates injured myocardium.
This technique has come to be called MRI scar mapping, or delayed enhancement imaging. The fraction of wall thickness that retains gadolinium-based contrast agent 10-20 minutes after a bolus infusion of 20 mL/75 kg indicates viability. The result is an excellent predictor of potential for functional recovery. A scar that is less than one-third the thickness of the wall suggests improvement with revascularization; a scar that is more than two-thirds the thickness of the wall suggests unlikely improvement after revascularization.
MRI scar maps depict contrast retention due to cell disruption. Although acute injury results in slightly enlarged zones of retained contrast agent on MRI after a week, the defined zone appears the same months to years later and corresponds to pathology of dead tissue.
In patients with poor renal function, gadolinium contrast may stay in the body long enough to cause a potentially disabling inflammatory reaction called nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy (NSF/NFD). NSF/NFD has been linked to all gadolinium-based contrast agents. This disease has occurred in patients with moderate to end-stage renal disease after they were given a gadolinium-based contrast agent to enhance MRI or magnetic resonance angiography (MRA) scans.
Appropriate and timely treatment
When symptoms suggestive of a possible threatened heart attack are present (persistent chest pain or pressure radiating to 1 or both arms or to the jaw; unexplained shortness of breath, weakness, sudden sweating; or serious arrhythmia), an electrocardiogram (ECG) should be obtained promptly, with continual monitoring for arrhythmia or ischemia (impaired blood supply).
Ambulances are equipped with both ECG and rhythm and oxygenation monitoring equipment, as are emergency departments. ECG can show ST-segment shifts and/or T-wave inversions as signs of heart ischemia or injury. However, standard monitors have electrically silent areas. A 12-lead ECG does not detect all of the electrical warning signs of heart damage; more extensive thoracic coverage is desirable.
Antiplatelet agents, nitrates, beta blockers, calcium antagonists, and ranolazine are some of the few therapeutic agents used for relief of symptomatic angina associated with CAD. [1]
Education, habitual modification, and social support matter a lot for reducing cardiac morbidity and mortality. Patients with moderate to severe symptoms and complex lesions should be considered for revascularization. Practical management of revascularization should take individual characteristics, preference, and compliance into consideration as well. [14]
Coronary revascularization is the most important treatment strategy for coronary artery disease. Percutaneous coronary intervention (PCI) has become the most frequently performed procedure for CAD. The evolution of stents plays an important role in the results of this procedure. Coronary artery bypass grafting (CABG) is the most effective revascularization approach for stenotic coronary arteries. Conduits selected and surgical techniques applied are important determinants of patient outcomes. Multidisciplinary decision-making should analyze current evidence, consider the clinical condition of the patient, and determine the safety of, and the necessity for, coronary revascularization with either PCI or CABG. [5]
Preferred examination
If a patient has symptoms, suggestive ECG findings, or imaging results that indicate the need for intervention, coronary angiography by means of catheterization is the preferred method of identifying the culprit lesions and, often, of providing an interventional remedy during a single session.
The patient's clinical history (age, symptoms, risk factors) can be used to estimate the likelihood of disease. The basic screening test is stress ECG, which can guide adjustment of prognosis based on the pretest likelihood of disease.
Generally, if the patient has no symptoms and if resting and stress ECGs are normal, risk of mortality in the next year is low. However, the predictive accuracy of ECG even at peak stress as part of stress testing overall is not good, and as many as 50% of all cases of disease are missed. The simple addition of stress testing of B-type natriuretic peptide (BNP) levels in the blood markedly improves predictive accuracy, [15] as does use of nuclear imaging, echocardiography, MRI, or CT.
Stress nuclear imaging is widely used to assess exercise tolerance and to identify zones of inducible ischemia (jeopardized myocardium), providing useful information, even after coronary angiography is performed. PET offers similar rest-stress data and is superior for identifying viable myocardium. Jeopardy and viability are important issues because if the myocardium is not at risk, or if it is not viable, revascularization (bypass or angioplasty) will not eliminate problems in this portion of the heart.
Echocardiography to identify wall motion abnormalities has similar predictive accuracy in patients with intermediate suspicion of CAD, estimated at 80-90%. Echocardiography avoids radiation exposure, which may cause up to 1 new cancer for every 1000 patients studied, but radionuclide imaging (thallium, sestamibi) is preferred if the patient has old wall motion abnormalities or poor echo windows (ie, the lung blocks the view).
Exercise stress echo may be performed before and after treadmill exercise or during exercise on a supine bicycle. The latter requires more cooperation but allows imaging at every stage, so false-negatives from rapid recovery or from involvement of all areas (balanced ischemia) may be avoided.
MRI and CT have markedly improved depiction of zones of impaired blood supply. MRI and CT do not require stress; they offer sensitivity and specificity similar to those of nuclear imaging but with better resolution; and they show 3-dimensional (3-D) coronary anatomy. [16] Therefore, MRI and CT complement the combination of stress test and catheterization and in some settings may replace it (eg, by revealing normal results).
Electron beam CT (EBT) offers similar value. In EBT, an electron beam, rather than the entire x-ray source, is rotated around the patient. EBT and CT have been used to screen for calcifications in the coronary arteries of young patients as a marker for risk of coronary disease.
To monitor angiogenesis, collateral-sensitive and MRI delayed-arrival MRI appear to be far more sensitive than any other techniques.
Collateral-sensitive MRI generates a dark flare of susceptibility effect due to sparse neovascular development at an early stage while suppressing a similar effect from the left ventricle (LV). This finding is a strong predictor (r = 0.93) of improved blood delivery.
Delayed-arrival MRI, which involves delayed enhancement on cardiac MRI, yields additional information on the response of heart rate and blood pressure to exercise and on functional capacity of the heart. [17] Data from quantitative studies on the extent of delayed arrival in humans have validated this method, [12] clearly distinguishing angiogenic treatment from control at 4 weeks after treatment, and showing benefit followed by improvement in wall motion (serial motion assessment by reference tracking [SMART]). [18]
Although invasive coronary angiography has been the gold standard in establishing the diagnosis of CAD, there is a growing shift to more appropriately use the cardiac catheterization laboratory to perform interventional procedures once a diagnosis of CAD has been established by noninvasive imaging modalities, rather than using it primarily as a diagnostic facility to confirm or refute CAD. With ongoing technological advancements, noninvasive imaging plays a pre-eminent role not only in diagnosing CAD but also in informing the choice of appropriate therapies and establishing prognosis, all while containing costs and providing value-based care. [4]
Newer technologies such as CT fractional flow reserve, CT angiography with perfusion, and whole-heart coronary magnetic resonance angiography with perfusion, which can provide both anatomic and functional information in the same test, obviate the need for multiple diagnostic tests to obtain a comprehensive assessment of both plaque burden and downstream ischemia. [4]
Limitations of techniques
Coronary angiography is considered the criterion standard for evaluating coronary artery stenosis. Flow limitations may be estimated from TIMI (Thrombolysis in Myocardial Infarction) score and are confirmed with a flow wire or with intravascular ultrasonography (IVUS). [19] If coronary angiography fails to depict a culprit lesion, and if cardiac ischemia is inducible, the patient may have syndrome X (microvascular disease).
Coronary angiography requires the use of iodine, which may cause serious allergic reactions such as anaphylaxis, or renal failure. Use of large volumes of saline and of the antioxidant acetylcysteine may help prevent renal failure. The catheterization procedure can induce vessel spasm and/or can tear the lining of a vessel, resulting in occlusion and possibly death in a patient who may not have had CAD. The procedure can also result in embolism, which may cause stroke or limb loss; nerve damage, infection, and other complications are possible as well. The death rate is approximately 0.1%.
Nuclear imaging produces low-resolution images that may depict an apparent defect resulting from breast tissue, hiccups, paradoxical septal motion, or other confounding factors. Nuclear imaging may fail to reveal disease due to submaximal stress. Tomographic imaging, attenuation correction, and PET substantively eliminate problems caused by breast attenuation. Newer combinations of nuclear imaging with CT enable the most accurate correction of nuclear event maps for attenuation by overlying tissues.
MRI requires special precautions in patients with a pacemaker or with a recently placed aneurysm clip. Patients with claustrophobia require premedication, mirrors, and/or an open magnet. Many magnets do not accommodate patients who weigh more than 300 lb. Arrhythmias commonly lower image quality.
CT contrast agents usually contain iodine, which may cause an allergic reaction and possibly anaphylaxis. Nonionic contrast material reduces the risk of harm, as does pretreatment with steroids. Gadopentetate dimeglumine, the contrast agent used for MRI, may be used for CT when patients are allergic to iodine-based media.
CT uses x-rays typically equivalent to the dose needed for about 200 chest radiographs. A single routine CT study in a child increases the lifetime risk of cancer by 0.35% per scan. [20] For adults, the lifetime risk of cancer may be as high as 2% with annual CT screening. Because the breast has high radiosensitivity, techniques to reduce tissue exposure, such as displacing the breasts outside the direct x-ray beam and using a lead shield, can reduce the radiation hazard of CTA. [21]
According to the European Association of Nuclear Medicine guidelines for radionuclide myocardial perfusion imaging (MPI) with SPECT, absolute contraindications for iodine contrast include myasthenia gravis, mastocytosis, and post-thyroid carcinoma when follow-up with 131I imaging or 131I therapy is planned within 6 months of CT angiography. [22]
Risk factors that may warrant serum creatinine screening before administration for patients who are scheduled to receive intravascular iodinated contrast medium include the following [22] :
-
Age >60 years
-
History of renal disease, including dialysis, kidney transplant, single kidney, renal cancer, renal surgery
-
History of hypertension requiring medical therapy
-
Diabetes mellitus
The use of contrast media for cardiac imaging is increasing as hybrid cardiac SPECT/CT and PET/CT, as well as coronary CT angiography and cardiac MRI, are used more widely. Hybrid imaging provides a noninvasive assessment of coronary anatomy and myocardial perfusion. [22, 23]
Imaging guidance for interventional procedures
Coronary angiography is widely used to guide interventions such as balloon angioplasty, atherectomy, laser treatment, stent placement, and other procedures. Current practice indicates the use of coronary angiography for patients with potentially treatable lesions to confirm findings and to perform interventions. Both tasks may be accomplished in a single procedure.
Cardiac catheterization is recommended for patients with mild angina (class I or II) plus ejection fraction (EF) less than 45%, including those with noninvasive test results indicating high risk, an uncertain diagnosis after noninvasive testing, or serious ventricular arrhythmias, and those who survive an episode of sudden death. The only indication with submaximal support is mild angina with reduced EF; this is a class IIa recommendation. Classification of indications by the American College of Cardiology reflects the weight of evidence in support of the recommendation. Mild angina with no reduction in EF might be managed with medication as a therapeutic trial.
As an experiment, MRI, CT, or echocardiography may be used to guide interventional procedures. MRI does not involve ionizing radiation; therefore, imaging may be active throughout the procedure. However, special guidewires and other equipment compatible with the magnet and with the rapidly changing magnetic field must be used, and staff must be trained to ensure that no magnetic objects are brought near the magnet.
CT uses ionizing radiation and is slower than coronary angiography; it provides 3-D information that may facilitate localization, especially for newer interventions such as intramyocardial injection of angiogenic growth factors or stem cells. Similarly, 3-D ultrasonography can facilitate accurate injection, with the convenience of portability and without the need for lead shielding from x-rays.
Radiography
Coronary angiography shows where vessels originate, how they branch, whether they have obstructions or dissections or thrombi, the degree of any obstructions, and which territories they supply. (See the x-ray angiograph below.)
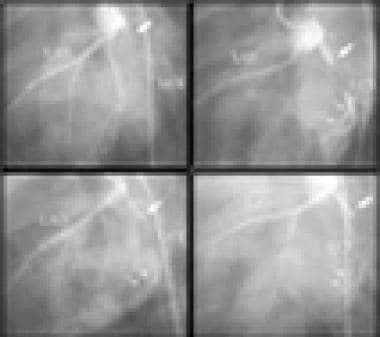 X-ray angiography is the criterion standard for delineating the coronary anatomy, but it is inferior to MRI and CT in identifying myocardium with impaired blood delivery, in assessing the functional consequences, and in identifying the development of microvascular collaterals.
X-ray angiography is the criterion standard for delineating the coronary anatomy, but it is inferior to MRI and CT in identifying myocardium with impaired blood delivery, in assessing the functional consequences, and in identifying the development of microvascular collaterals.
Key questions answered during an examination of the anatomy include the following:
-
Does a coronary artery pass between the aorta and the pulmonary artery, where it may get pinched?
-
Does a segment tunnel under a myocardial bridge?
-
Which pathway supplies the posterior surface? Is it the right, the left circumflex, or both? That is, is it right dominant, left dominant, or circumflex dominant?
-
Does the left anterior descending artery (LAD) wrap around the apex to supply the distal diaphragmatic surface?
-
What vessel supplies the atrioventricular (AV) node? Is its blood supply impaired?
-
If an infarct is present, which is the infarct-related artery?
-
If abnormal wall motion is seen, which branch obstruction accounts for it?
-
Are any bypass graft vessels present? If so, where do they originate (left internal mammary, saphenous vein graft from anterior aortic root)? Are they long or short, where do they connect, and how do they connect (end to side, side to side)?
The caliber of vessels may be estimated by comparing them with the known diameter of the catheter if it appears on the image. The reviewer should take into account the fact that magnifications differ at different distances from the source to the intensifier with x-ray projection angiography.
After the anatomy is described, location, percentage of narrowing, and character of all focal obstructions (stenoses) are discussed.
For each lesion:
-
Is it concentric (symmetric) or eccentric (1-sided)?
-
Is it long or short?
-
Does it abut a branch vessel (which may be lost after intervention)?
-
Is it calcified?
-
Is any thrombus visible?
-
Is evidence of intimal tear shown?
-
Is evidence of vessel spasm apparent?
-
Is diffuse narrowing revealed?
The flow of contrast agent–labeled blood offers useful information. TIMI criteria may be applied to determine whether the distribution of contrast material is TIMI 0 (incomplete—fails to fill branches and the distal portion of the vessel), TIMI 1 (slow but complete), or TIMI 2 (brisk and complete). When imaging is performed at a rate of 30 frames per second, the number of frames it takes for a vessel to completely fill may be assessed. The normal number is approximately 21 frames. Filling takes longer in patients with disease than in healthy people—not only in the diseased vessel but also in normal vessels.
Consider how findings may affect possible interventions, and report them accordingly. Clinically significant narrowing in the left main coronary artery is a medical emergency because of the amount of myocardium at risk. Other patterns of disease can pose similar risk—for example, proximal disease in both the LAD and a dominant right or left circumflex vessel.
-
What is the caliber of distal vessels that may support a bypass graft?
-
Are the distal vessels calcified?
-
Is any stenosis near a branch point (such that balloon angioplasty of the lesion may obstruct a branch artery)?
-
How long is the left main coronary artery?
-
How much myocardium is at risk?
Examine images for ancillary findings.
-
Which calcifications move with the heart?
-
Is the mitral valve annulus calcified?
-
Is the aortic root or the aortic valve calcified?
-
Are valve rings, bypass vessel rings or clips, stents, sternal wires, or other evidence of prior surgeries apparent?
-
If pacer wires are noted, where do they end?
-
Does evidence suggest chamber enlargement, aneurysm, cardiac displacement, abnormal pulmonary venous return, unusual persistence of fetal structures, or other variants?
If left ventriculography is performed, examine left ventricle (LV) function for ejection fraction (EF), for regional wall motion abnormalities, and for valve integrity. Hypokinesis indicates reduced motion, akinesis indicates no motion, and dyskinesis indicates reversed motion, such as ballooning outward during systole. Note any leakage of contrast material back into the left atrium and any restriction of the valve leaflets.
At the time of coronary angiography, the same set of tools can be used to examine other vessels (eg, renal and carotid arteries). [24]
Although it is the method used by most interventional cardiologists to assess the severity of CAD and to guide treatment, coronary angiography has many known limitations, particularly the fact that it is a lumenogram depicting foreshortened, shadowgraph, planar projections of the contrast-filled lumen rather than imaging the diseased vessel itself. Intravascular imaging—intravascular ultrasound and optical coherence tomography—is clinically useful for answering questions such as whether stenosis is clinically relevant, for identifying the culprit lesion, and for indicating whether the patient is at high risk of future adverse events. [25]
Degree of confidence
Coronary angiography is the standard for identifying coronary anatomy and stenoses. In select cases, alternative imaging may appear superior, but one must be careful to distinguish between high-quality or good-looking pictures and reliable results. Coronary angiography may provide a false-negative result if a branch vessel is occluded at its origin, if the disease is asymmetrical, or if the lesion is cracked, such that the contrast agent can extend close to the full diameter of the vessel even though the vessel cross-sectional area is severely reduced (eg, a star-shaped lesion).
It is possible to miss a lesion that is hidden behind another vessel, but this problem is generally resolved by using angled views and by moving the camera (panning) during image collection. If the significance of an obstruction is unclear on coronary angiography, intravascular ultrasound (IVUS) or a flow wire may be used to clarify its spatial extent in relation to the vessel lumen or its impact on flow down a particular branch vessel. A vasodilator may be delivered to assess flow reserve. X-ray angiography is not a good detector of small-vessel disease, epitomized by cardiac syndrome X.
The predictive accuracy of stress ECG can be as low as 50% but rises above 75% if it is combined with proBNP blood testing. [15] Accuracy of stress imaging for detection of CAD ranges from 70 to 90% if the target stress level is achieved while off antianginal medications.
Treadmill or bicycle stress testing is generally preferred, followed by dobutamine stress testing, then adenosine combined with low-level exercise. Adenosine or dipyridamole alone is less reliable. Chest pain during a dipyridamole stress test in the absence of CAD is not uncommon. [26] Target heart rate (peak HR) for exercise or dobutamine stress testing is 85% of age-predicted maximum (85% of peak systolic BP × peak HR). Animal studies have shown that the rate-pressure product is a better predictor of the stress levels that should induce detectible ischemia. A 50% blockage should be detected with more than 50% confidence above a rate-pressure product of 20 kilotorr/min, and with more than 85% confidence above 25 kilotorr/min.
Limitations
Balloon angioplasty can disrupt an obstruction so that the vessel appears to recover its full diameter, when, in fact, the cross-sectional area is improved only minimally and insufficiently. 3-D imaging can be used to examine contrast agent attenuation and percentage of narrowing. On occasion, this condition may be identified by looking at the lesion on different views or by performing IVUS or optical CT.
The introduction of a catheter or a wire can cause intimal dissection (a tear in the lining of a vessel), which may be mistaken for vascular spasm, thrombosis, or a long stenosis on cursory examination. A tissue flap in the endothelial lining may alternate between an open position and an obstructive one, mimicking a spasm; however, it is not responsive to nitrates. The distinction may be a matter of life or death. If clinically significant, stent placement, bypass, placement of a perfusion catheter, or other emergency treatment is typically required to treat a dissection. Sudden obstruction due to dissection can be deadly and does not respond to medication.
Myocardial bridges, or small bands of muscle overlying a vessel, may be mistaken for stenoses; however, these are not amenable to angioplasty. Obstruction from a myocardial bridge is smooth and eccentric. Observation throughout the cardiac cycle shows that the obstruction occurs during systole.
Computed Tomography
Computed tomography (CT) imaging of the coronary arteries can be achieved with fast CT and with electron beam CT (EBCT) systems triggered or gated by electrocardiography (ECG) to accumulate data when the heart is in diastole. The newest technology uses 64-section multidetector-row CT. [23, 27, 28, 29, 30]
With section thickness of 1 or 0.5 mm or less, the coronary anatomy is laid out in a 3-D volume. Image processing can greatly facilitate visualization of the course of vessels and branches and the presence and degree of stenoses. The coronary artery tree may be viewed as a solid rendering of the surface of the heart, but portions may be obstructed from view.
Proper viewing of each coronary artery branch should include clean views in which the LV blood pool, the aortic root, and all extracardiac structures are removed; vascular projections are limited to zones that include the vessel of interest and a margin for partial-volume effects.
One should not rely on threshold-based renderings, which can cause false stenosis and false obstruction, causing an intravascular thrombus to be missed. Use of a pair of volumes before and after administration of contrast material for elastic matching [31] greatly facilitates evaluation by automatically isolating the coronary tree without thresholding. [16]
CT also enables superb evaluation of blood delivery. In principle, CT combined with catheterization permits accurate definition of the extent of collateral-dependent myocardium. [16] (See the CT image below.)
 Elastic-match imaging automatically identifies differences between image volumes. The acquisition of 1 set of contrast-enhanced chest CT images via the coronaries and a nonenhanced set provides a 3-dimensional view of the coronary-artery tree. The nonenhanced volume data were rendered as holographic projections to provide the anatomic context, and the elastic-match coronary tree was overlaid. In addition to automation, this method avoids thresholding so that small branches and filling defects, if present, are represented properly.
Elastic-match imaging automatically identifies differences between image volumes. The acquisition of 1 set of contrast-enhanced chest CT images via the coronaries and a nonenhanced set provides a 3-dimensional view of the coronary-artery tree. The nonenhanced volume data were rendered as holographic projections to provide the anatomic context, and the elastic-match coronary tree was overlaid. In addition to automation, this method avoids thresholding so that small branches and filling defects, if present, are represented properly.
Pizzuto et al found that transthoracic Doppler echocardiography can improve the diagnostic accuracy of multidetector computed tomography (MDCT) for detecting left anterior descending coronary artery (LAD) stenosis. In 144 consecutive patients, coronary anatomy was assessed with MDCT, and echocardiography was used to calculate coronary flow reserve (CFR) by measuring the ratio of hyperemic to baseline peak flow velocity; results of both methods were verified by invasive coronary angiography. [32]
In a univariate model, prediction of significant LAD stenosis was slightly, but significantly, better with CFR (sensitivity 90%, specificity 96%, positive predictive value 84%, negative predictive value 97%, diagnostic accuracy 94%) than with MDCT (sensitivity 80%, specificity 93%, positive predictive value 71%, negative predictive value 95%, diagnostic accuracy 90%). [32]
When findings from transthoracic Doppler echocardiography and MDCT were consistent, diagnostic accuracy was increased (96%). Among 13 patients whose condition was missed by MDCT, transthoracic Doppler echocardiography proved 100% accurate for predicting significant LAD stenosis. [32]
In a study of myocardial CT perfusion imaging versus single-photon emission CT (SPECT) perfusion imaging for diagnosis of CAD, overall performance was better for myocardial CT perfusion imaging. Sensitivity and specificity of CT perfusion imaging for diagnosis of CAD were 88% and 55%, respectively, versus 62% and 67% for SPECT. Sensitivities for left main, 3-vessel, 2-vessel, and 1-vessel disease were 92%, 92%, 89%, and 83%, respectively, for CT perfusion imaging, and 75%, 79%, 68%, and 41%, respectively, for SPECT. [33]
Degree of confidence
The ability of MRI and CT to depict anatomy and the absence of notable obstructions is improving rapidly, but performance is not uniform. The value of MRI and CT must be assessed in a truly double-blind fashion until standardized, reliable methods are widely established.
Whether MRI and CT results match in terms of the percentage of stenosis is relatively unimportant. Most important is whether MRI and CT reliably depict normal tissue and culprit lesions, and whether they establish severity and territories supplied by the culprit vessel. Both MRI and CT offer the significant advantage of direct assessment of zones of impaired blood delivery.
Limitations
MRI shows calcifications as black or signal voids, whereas CT shows calcifications as white and similar to contrast-filled blood. These appearances can influence the estimation of stenoses.
Heavy calcification causes a beam-hardening artifact on CT that can interfere with visualization. Stents cause a local disturbance stronger on MRI than on CT. Also, with 3-D MRI or CT, one must be certain to understand how images account for local curvature in and out of the imaging planes. In finding the best plane to show a vessel, radiologists can mistake a local curve that is out of plane for an apparent stenosis. Proper image processing helps to resolve this problem.
Magnetic Resonance Imaging
Coronary magnetic resonance imaging (MRI) has improved from early methods [8] and equipment sufficient to identify normal proximal coronary arteries and courses, but it is not a clinical replacement for coronary angiography, apart from ruling out aberrant coronary origins, demonstrating graft or native vessel patency, or following up on specific lesions.
Coronary MRI may be performed by using 3-D volume, but the trade-off in time and resolution favors imaging in selective planes that address each branch of interest. At 3-D volume, MRI may show the coronary tree in a way similar to the methods described for CT. Background tissue may be suppressed with fat saturation, tissue saturation, magnetization transfer, and/or T2 preparation (90°-180°-180°- ... -180°-90°). [34]
The vessel plane approach is as follows: Any desired target plane can be obtained by specifying 3 points for inclusion in the plane, by drawing lines of intersection with 2 previous images at different angles, or (commonly) by drawing a single line of intersection with a previous image that is perpendicular to the desired view. For example, to obtain a short-axis view of the coronary sinus, one must first obtain a long-axis view of the left ventricle (LV) parallel to the septum and perpendicular to the atrioventricular (AV) groove, then prescribe a plane in the AV groove perpendicular to that view, passing through the 2 observed points of intersection on the first view with the coronary sinus, seen as bright dots anterior and posterior to the mitral valve.
Other points regarding MRI for evaluation of CAD include the following:
-
A transverse stack of images covering the aortic root depicts the origin of the right coronary artery (RCA) and the left main coronary artery. Typical section thickness should be 3 mm or less. A bright- or dark-blood technique can be applied with the use of single frames or with a dynamic movie series.
-
An additional distal transverse image shows a cross-section of the right coronary artery (RCA), the left anterior descending artery (LAD), and the left circumflex artery (LCX).
-
From 2 points along the proximal vessel and from 1 point in the distal vessel, a plane that captures the desired segment is selected. The plane may be adjusted to be thick enough to encompass out-of-plane bends. As an alternative, it may be subdivided into a stack of thin imaging planes for a localized 3-D stack of images.
-
The course of the RCA in the atrioventricular (AV) groove can be ascertained quickly from a 4-chamber long-axis view of the heart by obtaining 1 preliminary image perpendicular to the AV groove and parallel to the septum through the mid right ventricle (RV). This provides 2 points of intersection with the RCA: 1 anterior and 1 posterior in the AV groove. Prescribing a plane through these 2 points from the long-axis image gives the desired view.
-
The posterior descending artery requires a different imaging plane, as do the LAD, the LCX, and major branches. The course of the LCX in the AV groove is assessed similarly to the RCA, by acquiring a scout image parallel to the septum to identify 2 points for inclusion in 1 final short-axis image. However, in this case, the scout image should be laterally displaced to the outer third because the distal LCX is often hard to identify.
-
Clinicians routinely identify the proximal course of the coronary arteries to look for aberrant origins in young patients who have had syncope. Complete absence of abnormalities suggests a good prognosis.
-
MRI with contrast is an excellent method of identifying myocardial scar (infarction) as small as 1% of the myocardium, which is a very strong prognostic factor, [35] while also assessing perfusion and precise function of left and right ventricles. It can be combined with stress testing and coronary imaging for a "1-stop shop."
-
MRI is the preferred test for right ventricular injury or infarction.
-
Apparent stenosis must be distinguished from an out-of-plane bend.
-
A signal void from flow disturbance may exaggerate apparent stenosis.
-
MRI is well established as a means to assess the patency of a bypass graft.
One study reported that infarct tissue heterogeneity identified by cardiac MRI is associated with mortality beyond that of left ventricular EF in patients who have both CAD and left ventricular dysfunction. Researchers found that this is particularly true for patients with mild or moderate left ventricular dysfunction. They have suggested that additional studies incorporating cardiac MRI in clinical decision-making for defibrillator therapy are warranted. [36]
Cardiovascular magnetic resonance (CMR) assesses cardiac function, ischemia, viability, and tissue characterization, all within a single scan. CMR has emerged as a highly accurate technique for diagnosis and risk stratification in stable CAD and has been incorporated into national and international guidelines. Furthermore, clinical pathways utilizing CMR have been shown to be the most cost-effective in several health care systems. [6]
Degree of confidence
According to one study, infarct heterogeneity identified by cardiac MRI is associated with mortality beyond left ventricular ejection fraction (LVEF) in patients with CAD and left ventricular dysfunction. Researchers have found that this is particularly true for patients with mild or moderate left ventricular dysfunction. They have suggested that additional studies incorporating cardiac MRI in clinical decision-making for defibrillator therapy are warranted.
MRI offers high sensitivity to changes in wall function (eg, wall thickening, radial motion). [18] MRI may be useful for identifying and quantifying impaired blood delivery and wall function in response to interventions. [12, 37, 38, 39, 40, 41, 42, 43] Using such applications is perhaps more vital than visualizing the percentage of stenosis.
Confidence in the data depends on speed and quality of the imaging method, cooperation of the patient (shallow regular breathing or several matching breath holds), accuracy of ECG triggering or gating, and anatomic knowledge and judgment of the person directly supervising data collection.
The usual ECG signal in MRI is markedly distorted by competing signals resulting from movement in a magnetic field and by moving magnetic fields, particularly from blood flow in the great vessels, called the magnetohydrodynamic effect. This distortion makes it difficult to perform electrographic safety monitoring for ischemic changes.
Cardiac MRI by the vessel-chasing approach requires highly informed decision-making as data are being acquired. If the operator acquiring the data understands what the coronary angiogram demonstrates, views may be manipulated for the best match. This consideration is not necessarily positive because the operator may exaggerate the agreement.
Cardiac MRI is a powerful diagnostic method, supporting robust measurements of crucial markers of cardiac structure and function, myocardial perfusion, and scar, and providing detailed insight into myocardial tissue. Accurate and informative diagnostic readouts can help with guiding therapy, monitoring disease progress, and tailoring response to treatment. [44]
The ability of MRI and CT to identify anatomy and the absence of clinically significant obstructions is improving rapidly, but it is not uniform. The value of MRI and CT must be assessed in a truly double-blind fashion until standardized and reliable methods are widely established.
Whether MRI and CT results match in terms of the percentage of stenosis is relatively unimportant. Most important is whether MRI and CT reliably depict normal tissue and culprit lesions, and whether they help in establishing disease severity and in depicting territories supplied by the culprit vessel. Both MRI and CT offer the notable advantage of enabling direct assessment of zones with impaired blood delivery.
Limitations
In an apparent stenosis, one must be certain that the finding is not a partial-volume artifact or a velocity-shear effect. Because local differences in velocity can cause a signal void, estimates of stenosis may be exaggerated.
Magnetic susceptibility artifacts may produce signal voids. Stents, clips, and wires cause local disturbances.
The presence of a pacemaker wire is considered a relative contraindication to MRI because rapidly changing magnetic fields may induce a voltage that can trigger an arrhythmia, induce a burn, or shorten battery life.
When the patient enters and leaves the magnet, the magnetic reed switch on most pacemakers will switch it to fixed mode, and the temperature in metal devices may rise. For example, a pacemaker generator may warm by 1-2°C. However, with informed consent, careful pulse monitoring, and readiness to promptly abort a pulse sequence if an arrhythmia is induced, patients with pacers have undergone MRI with no apparent consequence and no change in pacer thresholds. Of the dozen reports of mishaps related to pacemakers and MRI, none were caused by MRI.
On MRI, calcification is depicted as a black area or a signal void, whereas CT shows calcifications as white—similar to blood filled with contrast agent. These appearances can influence the estimation of stenoses. Also, with 3-D MRI or CT, one must be certain to understand how the images account for local curvature in and out of the imaging planes. In finding the best MRI plane for showing a vessel, radiologists can mistake a local curve that is out of plane for an apparent stenosis. Proper image processing can help to resolve this problem.
With MRI, flow disturbances that cause velocity shear (range of phases in each picture element or pixel resulting from different rates of motion of blood) cause a local decrease in signal intensity, which may create or exaggerate an apparent stenosis.
Ultrasonography
Echocardiography can be used to identify the left main coronary artery. In some patients, much of the right coronary artery (RCA) and the left anterior descending artery (LAD) can be viewed; however, in most patients, the imaging window is inadequate for useful coronary imaging from outside the chest.
In the catheterization laboratory, intravascular ultrasonography (IVUS) may be performed to examine coronary arteries from the inside and to characterize plaque. However, the diameter of the device limits the ability to pass through tight stenoses. Also, injection of a sonographic contrast agent (eg, agitated Renografin) into the coronary arteries, combined with transthoracic or esophageal ultrasonography, can be useful in identifying perfusion territories.
Pizzuto et al found that transthoracic Doppler echocardiography can improve the diagnostic accuracy of multidetector computed tomography (MDCT) in detecting LAD coronary artery stenosis. In 144 consecutive patients, coronary anatomy was assessed with MDCT, and echocardiography was used to calculate coronary flow reserve (CFR) by measuring the ratio of hyperemic to baseline peak flow velocity; results of both methods were verified with invasive coronary angiography. [32]
In a univariate model, prediction of significant LAD stenosis was slightly, but significantly, better with CFR (sensitivity 90%, specificity 96%, positive predictive value 84%, negative predictive value 97%, diagnostic accuracy 94%) than with MDCT (sensitivity 80%, specificity 93%, positive predictive value 71%, negative predictive value 95%, diagnostic accuracy 90%). When findings from transthoracic Doppler echocardiography and MDCT were consistent, diagnostic accuracy was increased (96%). Among 13 patients with disease missed by MDCT, transthoracic Doppler echocardiography proved 100% accurate in predicting significant LAD stenosis. [32]
Nuclear Imaging
Nuclear imaging does not depict the coronary arteries, but it does demonstrate various metabolites useful for identifying perfusion defects and tissue viability. Thallium-201 and technetium-99m sestamibi are widely used and may be combined to shorten the study of myocardial uptake of radioactive tracer at rest and during stress. [45]
Although a rest-and-stress thallium study takes longer than 4 hours, a combined study performed with thallium and sestamibi may be completed in less than 2 hours.
When positron emission tomography (PET) is used, a rest-and-stress study with rubidium-82 may be completed in 30 minutes because the agent has a half-life less than 5 minutes. A defect during stress that is not evident at rest indicates a zone of induced ischemia. A defect at rest and also during stress indicates persistent metabolic dysfunction resulting from infarction (scar) or from hibernation (prolonged dysfunction). PET with ammonia, fluorinated glucose, or other agents may be used to determine if tissue with a defect at rest is viable. [45]
Degree of confidence
Nuclear medicine tests for CAD improve predictive accuracy over stress tests alone to approximately 90%. The utility of these tests depends on the previous probability of disease, and on whether they are being used to identify CAD or to clarify the pathophysiology of known disease.
Breast attenuation may cause an apparent defect on radionuclide images. Attenuation correction and multiplanar imaging mitigate the problem.
Unusual motion, such as that from a bundle branch block or from coughing during imaging, may cause false-positive results. A persistent defect is commonly interpreted as a fixed defect or a scar, but it may represent prolonged yet still-reversible ischemic impairment of tracer uptake.
The low resolution of nuclear medicine studies compared with other modalities may result in false-negative results. Also, global disease may be missed because defects generally are identified by comparing them to regions with high uptake of the tracer.
-
Selective injection image of the left coronary arteries. D1 = first diagonal, LAD = left anterior descending artery, LCX = left circumflex, LM = left main coronary artery, and OM1= first obtuse marginal.
-
MRIs of the coronaries can be used to build 4-dimensional images (3-dimensional beating heart). These images show a single frame, including a cutaway view to show the cardiac interior, the outer surface (no thresholding), and the extracted coronary artery tree including the aortic root.
-
Elastic-match imaging automatically identifies differences between image volumes. The acquisition of 1 set of contrast-enhanced chest CT images via the coronaries and a nonenhanced set provides a 3-dimensional view of the coronary-artery tree. The nonenhanced volume data were rendered as holographic projections to provide the anatomic context, and the elastic-match coronary tree was overlaid. In addition to automation, this method avoids thresholding so that small branches and filling defects, if present, are represented properly.
-
Elastic-match imaging can be used to identify collateral-dependent myocardium. Left and middle images are baseline and peak-arrival collateral-sensitive MRIs demarcating microvascular development. Right image, based on CT imaging of the heart, was obtained with and without back pressure to nullify collateral-dependent perfusion; white volume on represents collateral-dependent myocardium. The extent of collateral-dependent myocardium corresponds well on MRI and CT (r = 0.95).
-
Contrast-labeled blood to the heart is used to identify the territory at risk. The results of this assessment of the delayed arrival compares favorably to the findings of radionuclide stress imaging, and stress induction of ischemia is not required to identify the zone at risk.
-
Space-time maps show the history of blood arrival to all layers of myocardium on a 2-dimensional map. The indentation indicates the severity of the defect in blood delivery, and the length indicates the size as a percentage of the myocardium, without the need for stress induction of ischemia. In addition to the safety advantage, this method is also more reproducible than stress testing, which is useful in assessing the effect of therapy.
-
Compared with radionuclide images of blood delivery, MRIs and CT scans improve resolution, depiction of the functional effect and the relationship to the coronary supply, and identification of the area at risk without stress. The advantage of radionuclide imaging is primarily its predictive value; stress echocardiography has similar predictive value. MRI and CT have been less available than other studies; therefore, data on their value are relatively limited.
-
X-ray angiography is the criterion standard for delineating the coronary anatomy, but it is inferior to MRI and CT in identifying myocardium with impaired blood delivery, in assessing the functional consequences, and in identifying the development of microvascular collaterals.









