Practice Essentials
Constrictive pericarditis (CP) is a reduction in elasticity, or stiffening, of the pericardium—a sack-like covering that surrounds the heart—resulting in impaired filling of the heart with blood. Diagnosing CP can be challenging, and a variety of imaging techniques may be necessary. Symptoms of CP appear insidiously, with patients displaying peripheral edema, anasarca, and elevated right-sided heart pressures. Patients with CP typically present with symptoms related to right-sided heart failure such as cardiac ascites. Spontaneous bacterial peritonitis (SBP) usually arises in association with ascites secondary to hepatic cirrhosis. [1] Patients frequently present with symptoms that are not thought to be due to cardiac disease, such as exercise intolerance, dyspnea, liver failure, and renal failure. [2, 3, 4, 5, 6, 7, 8]
(See the images of constrictive pericarditis below.)
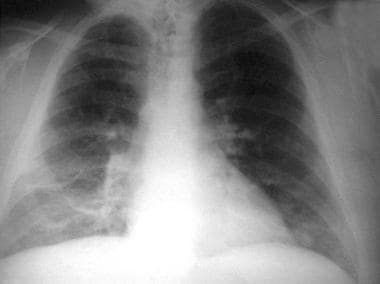 Constrictive pericarditis. A man presented with an 8-year history of slow worsening of exercise intolerance, weight gain, and dyspnea. His chest radiograph shows a normal-sized heart. Calcifications are not visible in the pericardium on the frontal view. The azygos vein is moderately distended.
Constrictive pericarditis. A man presented with an 8-year history of slow worsening of exercise intolerance, weight gain, and dyspnea. His chest radiograph shows a normal-sized heart. Calcifications are not visible in the pericardium on the frontal view. The azygos vein is moderately distended.
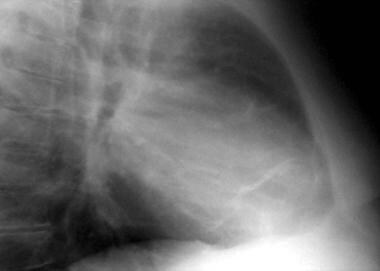 Constrictive pericarditis. Chest radiograph lateral view of the same patient as in the previous image also fails to show pericardial calcifications.
Constrictive pericarditis. Chest radiograph lateral view of the same patient as in the previous image also fails to show pericardial calcifications.
The diagnosis is rarely considered by the referring physician, who usually may think that symptoms are due to another disease process. Patients may present with increasing weight gain, cardiac cirrhosis, and massive ascites. [9, 10, 11] The physician is most likely to refer patients with these symptoms to the radiologist for abdominal ultrasonography, abdominal and pelvic computed tomography (CT), or hepatobiliary scanning, thinking that the patient's symptoms are related to a liver disorder. These imaging techniques can reveal severe diastolic dysfunction, increased pericardial thickness, and calcifications. The gold standard for diagnosis is cardiac catheterization with analysis of intracavitary pressure curves, which are high and, in end diastole, equal in all chambers. Chronic CP is a common cause of recurrent heart failure. [12]
CP diagnosis is often delayed, with a mean time of symptom onset to diagnosis of 24 months; later diagnoses are associated with poorer outcomes. [3]
In a study by Fernandes et al, preoperative cardiac echocardiography and MRI suggested the diagnosis of CP when at least 2 of the following findings compatible with constriction were present: pericardial thickening; septal bounce (paradoxical movement); inspiratory reduction in the mitral E wave (>25%); and inferior vena cava dilatation. MRI had a higher sensitivity for constriction than echocardiography (95.9% vs 53.6%). [4]
Patients respond dramatically to a complete surgical pericardiectomy when it is performed early in the disease process; therefore, it is important to consider CP when making the diagnosis. Anatomic imaging findings such as calcifications (see the images below) and thickening of the pericardium may be present, but the most reliable and the most important findings are related to the filling pattern of the heart.
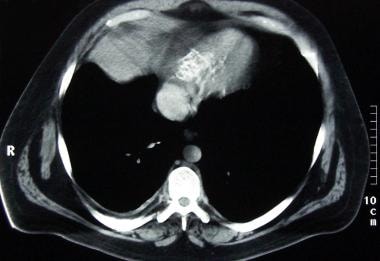 Constrictive pericarditis. Note the pericardial calcification inferior to the heart in this CT scan. Marked dilatation of the inferior vena cava is present despite the absence of cardiac chamber dilatation. The patient's disease responded completely to a total pericardiectomy.
Constrictive pericarditis. Note the pericardial calcification inferior to the heart in this CT scan. Marked dilatation of the inferior vena cava is present despite the absence of cardiac chamber dilatation. The patient's disease responded completely to a total pericardiectomy.
 Constrictive pericarditis. CT image obtained 8 years earlier of the heart in the previous image shows no pericardial calcifications.
Constrictive pericarditis. CT image obtained 8 years earlier of the heart in the previous image shows no pericardial calcifications.
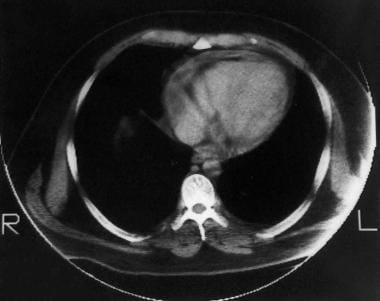
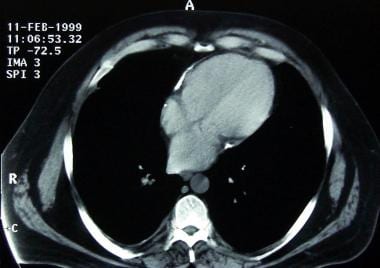 Constrictive pericarditis. The cardiac chambers in this CT scan of the heart are not enlarged and are approximately the same size. Focal pericardial calcifications are noted. The noncalcified portion of the pericardium is not particularly thickened, but it seems tightly adherent to the epicardium.
Constrictive pericarditis. The cardiac chambers in this CT scan of the heart are not enlarged and are approximately the same size. Focal pericardial calcifications are noted. The noncalcified portion of the pericardium is not particularly thickened, but it seems tightly adherent to the epicardium.
The severity of clinical symptoms is best correlated with findings from dynamic observation of blood flow and with findings relative to obstruction and poor filling of the right-sided cardiac chambers.
Various imaging methods (echocardiography, magnetic resonance imaging [MRI], computed tomography [CT]) can depict changes in cardiac chamber volumes throughout the cardiac cycle, as well as timing of fluid motion backward into veins and forward into arteries.
Both restrictive and constrictive diseases exhibit an abrupt reduction in filling, increased back pressure, and impaired stroke volume (volume of fluid ejected per heartbeat). Catheterization can be used to observe pressure levels in various chambers during filling. The influence of respiration on filling contributes important diagnostic information, both for imaging and for catheterization.
In CP, the size of the heart is usually normal; flattening of the right ventricle and curving of the intraventricular septum to the left (see the image below) are sometimes found. The azygos vein and the superior vena cava (SVC) are commonly dilated.
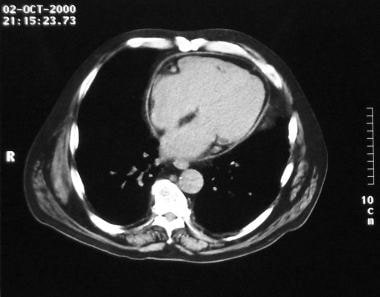 Constrictive pericarditis. Slightly caudal image in this CT scan of the heart shows deviation of the intraventricular septum to the left.
Constrictive pericarditis. Slightly caudal image in this CT scan of the heart shows deviation of the intraventricular septum to the left.
Restrictive disease (stiff muscle) such as that seen in dialysis patients with chronic amyloidosis mimics the physiologic pattern of constrictive disease (stiff muscle). Amyloidosis does not cause CP, but it can cause a restrictive cardiomyopathy that can mimic clinical, imaging, and physiologic alterations of CP.
Symptoms of amyloidosis do not respond to pericardiectomy. Cardiac tamponade, a condition in which fluid accumulates in the pericardial space, is included in the differential diagnosis of impaired filling; however, it is not usually difficult to distinguish tamponade from restrictive or constrictive disease on images.
It is important to recognize the less common effusive-constrictive pericarditis syndrome. About 10% of patients who are initially recognized as having cardiac tamponade present with signs and symptoms of constriction following pericardiocentesis. Causes of effusive-constrictive pericarditis are similar to those of typical constriction, although patients with this syndrome may have a more acute presentation and are more likely to respond to anti-inflammatory therapy. [13]
Echocardiographic findings can also suggest CP. Duplex Doppler waveforms and inflow velocities across mitral and tricuspid valves can be assessed during inspiration and expiration. This assessment is not part of the routine protocol in most echocardiography laboratories, and unless the physician is alerted to the possibility of CP, the diagnosis is almost never suspected. [14]
Dynamic respiratory criteria can also be applied during right- and left-sided heart catheterization when equalization of end-diastolic pressures in the heart chambers and characteristic venous waveforms are present.
Transient CP is increasingly recognized as a distinct subtype of constrictive pericarditis. The underlying pathophysiology typically relates to impaired pericardial distensibility, associated with acute or subacute inflammation, rather than to the fibrosis or calcification often seen in chronic pericardial constriction. Patients may present with concomitant features of pericarditis and constrictive physiology. Echocardiography remains the mainstay for initial evaluation of the dynamic features of constriction. However, cardiac magnetic resonance imaging (CMR) can provide complementary functional information, with the addition of dedicated sequences to assess for active pericardial edema and inflammation. [15]
Imaging modalities
In the minority of patients for whom the diagnosis of CP is suspected on a clinical basis, the first test should be plain posteroanterior (PA) and lateral chest radiography. Chest radiography in patients with CP may show pleural effusions without significant alveolar edema and biatrial enlargement. Left ventricular and right ventricular and pulmonary vessels are normal in size. [13] If imaging shows characteristic pericardial calcification, the diagnosis of CP is essentially established. Pericardial calcifications are rare, occurring in 20-40% of constrictive cases and, more commonly, in tuberculous pericarditis. [13] The classic and most common distribution is largely over the right ventricle; this appearance can be considered pathognomonic. [16]
If plain radiographic findings are negative and clinical suspicion of CP is present, echocardiography with evaluation of Doppler inflow velocities of mitral and tricuspid valves during inspiration and expiration should be performed. [17, 18, 19, 20, 21] Ventricular interdependence is present in all patients with significant constrictive physiologies.
Transthoracic 2-dimensional and Doppler echocardiography is usually the first diagnostic tool for evaluation of severe diastolic heart failure (HF) and can reliably identify CP in most patients by characteristic real-time motion of the heart and hemodynamic features. CT and MRI provide incremental data for the diagnosis and management of CP and are especially helpful when clinical or echocardiographic findings are inconclusive. [22] Both cardiac MRI and CT can guide surgical planning, but MRI provides both structural and functional information. [23, 24]
No imaging test is definitive for the diagnosis of CP in all patients who have the disease. For a patient in whom clinical findings are suggestive of the disease, noninvasive imaging findings that are consistent with CP are usually sufficient to prompt definitive surgery or invasive evaluation in the catheterization laboratory.
Limitations of techniques
The diagnosis of CP is more difficult in patients without calcification. Pericardial thickening of 4 mm or more on CT or MRI is considered evidence of CP for patients with appropriate symptoms; however, focal thickening of 4 mm or more is commonly seen in patients without physiologic or clinical evidence of constrictive physiology. The converse is also true. Rarely, a constrictive physiology can be present without abnormal thickening of the pericardium.
All noninvasive imaging techniques have limitations for the diagnosis of CP. Ultimately, the diagnosis is made at surgery; however, in the appropriate patient, CT, ultrasonography, conventional radiography, MRI, echocardiography, and right- and left-sided heart catheterization results can be highly suggestive of CP.
A major problem is differentiating restrictive cardiomyopathy from CP. Furthermore, it is difficult to preoperatively predict which patients are likely to respond to total pericardiectomy. Overlapping hemodynamics in CP and restrictive cardiomyopathy often pose difficulties in establishing accurate diagnosis. Echocardiography is the first-line imaging modality used for this purpose, but no single echocardiographic parameter is sufficiently robust for distinguishing between these conditions. [25]
Combining multiple echocardiographic parameters into stepwise algorithms and incorporating myocardial deformation analysis helps improve the diagnostic accuracy of echocardiography for distinguishing between CP and restrictive cardiomyopathy. Use of machine learning may allow easy integration of a wide range of echocardiographic and clinical parameters to permit accurate, automated diagnosis, with less dependence on user expertise. [25]
Radiography
Plain chest radiographs may show pericardial calcification in as many as 50% of CP patients, although anecdotal evidence suggests that this number is decreasing. The cardiac silhouette should be small in a patient with uncomplicated CP.
Constrictive pericarditis can also coexist with cardiomyopathy, and a large heart does not exclude the disease. Other, less reliable plain radiographic findings include an abnormal cardiac contour such as straightening of the right atrial border and, more rarely, straightening of the right and left cardiac borders, with obliteration of the normal curves, on frontal images. On fluoroscopy, diminished cardiac pulsation may be seen.
For a patient with diffuse pericardial calcification on radiographs and appropriate clinical symptoms of constrictive physiology, the diagnosis of CP can be reliably made. Absence of calcification does not exclude the disease, and further testing should include an extensive workup in the echocardiography laboratory, with assessment of Doppler velocities across mitral and tricuspid valves during inspiration and expiration. (Calcification does not appear in the images shown below.)
 Constrictive pericarditis. A man presented with an 8-year history of slow worsening of exercise intolerance, weight gain, and dyspnea. His chest radiograph shows a normal-sized heart. Calcifications are not visible in the pericardium on the frontal view. The azygos vein is moderately distended.
Constrictive pericarditis. A man presented with an 8-year history of slow worsening of exercise intolerance, weight gain, and dyspnea. His chest radiograph shows a normal-sized heart. Calcifications are not visible in the pericardium on the frontal view. The azygos vein is moderately distended.
 Constrictive pericarditis. Chest radiograph lateral view of the same patient as in the previous image also fails to show pericardial calcifications.
Constrictive pericarditis. Chest radiograph lateral view of the same patient as in the previous image also fails to show pericardial calcifications.
Because complete surgical pericardiectomy is usually effective (but not without risks of morbidity and mortality), most patients also undergo simultaneous right- and left-sided heart catheterization, with measurement of various pressures during inspiration and expiration.
If the radiograph is positive for pericardial calcifications and the patient's symptoms are consistent with CP, false-positive findings should not occur. The diagnosis is difficult to make in patients with CP but without pericardial calcifications. For these patients, normal findings on plain radiographs are false negatives.
Computed Tomography
Computed tomography (CT) is recognized as an excellent tool for determining pericardial thickness and is the most sensitive technique for identifying pericardial calcification. [23] Abdominal CT most often is performed in patients for whom the diagnosis of CP is not being considered on the basis of clinical findings. Symptoms in these patients usually are thought to be associated with a liver disorder. The radiologist can be of great service to the patient if CP is considered in the presence of appropriate imaging findings. [26, 27]
(See the CT scans of constrictive pericarditis below.)
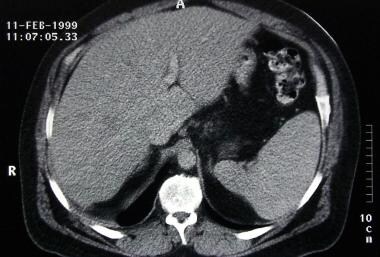 Constrictive pericarditis. The liver in this CT scan is poorly enhanced, congested, and enlarged. The aorta is poorly opacified, and passage of the contrast-agent bolus is mildly delayed.
Constrictive pericarditis. The liver in this CT scan is poorly enhanced, congested, and enlarged. The aorta is poorly opacified, and passage of the contrast-agent bolus is mildly delayed.
 Constrictive pericarditis. The cardiac chambers in this CT scan of the heart are not enlarged and are approximately the same size. Focal pericardial calcifications are noted. The noncalcified portion of the pericardium is not particularly thickened, but it seems tightly adherent to the epicardium.
Constrictive pericarditis. The cardiac chambers in this CT scan of the heart are not enlarged and are approximately the same size. Focal pericardial calcifications are noted. The noncalcified portion of the pericardium is not particularly thickened, but it seems tightly adherent to the epicardium.
The pericardium should be diffusely thicker than 3 mm; however, many patients do not present with this finding, and the diagnosis of CP should not be discarded if thickening is not present. The size of all 4 heart chambers should be within the normal range; however, CP can coexist with other diseases, and global or focal dilatation of the cardiac chambers does not exclude CP.
The inflow veins to the right atrium, including the SVC, the inferior vena cava (IVC), and the hepatic veins, should be dilated. This finding is necessary but is not sufficient to make the diagnosis of CP because it commonly occurs in the setting of congestive heart failure brought on by a variety of causes. Most often, when the hepatic veins and the IVC are dilated for reasons other than CP, dilation of one or all cardiac chambers is present and is caused by systolic dysfunction or valve disease. If significant cirrhosis has already occurred, the hepatic veins may not be dilated.
There should be no progression of the contrast agent bolus through the vascular system, and evidence of significant systolic dysfunction should be absent. For example, if the injection protocol involves a 60-second delay from time of injection to the start of scanning, contrast enhancement in the portal veins and waning of that enhancement in the abdominal aorta are usually seen.
In CP, there should be poor opacification of liver parenchyma due to congestion, and there should be no contrast enhancement in the portal vein.
In the appropriate patient, CT findings can be highly suggestive of CP; however, because the preferred treatment is total pericardiectomy, which has significant morbidity and mortality risks, almost all patients should be referred for cardiac echocardiography and/or simultaneous right- and left-sided heart catheterization.
Focal or diffuse thickening of the pericardium can occur in the absence of constrictive physiology. An apparently delayed bolus of contrast material can be caused by technical factors during acquisition of the CT scan. Dilated veins can be caused by right-sided heart failure. Liver cirrhosis can mimic CT findings of CP.
Magnetic Resonance Imaging
Diffuse thickening of the pericardium greater than 3 mm can be observed on multiplanar magnetic resonance imaging (MRI). [28]
Electrocardiography (ECG)-triggered MRI is sensitive to constrictive disease of the pericardium because the fibrous layers are bordered by fat, which produces a distinct MRI signal. Magnetic resonance imaging can be used to measure pericardial thickness; ideal views for measuring pericardial thickness are oriented perpendicular to the long axis of the left ventricle. MRI can also be used to measure chamber sizes at successive 50-msec delays after the R wave and to determine whether or not a filling plateau is present.
Similar to echocardiography and/or Doppler imaging, velocity-encoded (VENC) MRI can be used to assess volumetric flow and regurgitant flow to the pulmonary veins and the hepatic vein. Magnetic resonance imaging can reveal focal abnormalities and can cover the heart to show whether the disease encapsulates its entirety.
Fast imaging can be performed during deep respiration to establish whether filling is concordant or discordant. Constrictive pericarditis restriction creates discordance with reduced left ventricular filling, which corresponds to increased right ventricular filling.
Magnetic resonance imaging dynamically and clearly shows a reversed curvature of the intraventricular septum. [29]
Magnetic resonance imaging does not depict pericardial calcifications, but otherwise, MRI is highly sensitive and specific. The main limitation with MRI is its greater cost relative to echocardiography. Furthermore, most patients with CP who are referred for imaging are not suspected of having the disease; therefore, MRI is rarely ordered to rule out CP.
A patient may have focal or even diffuse thickening on MRI without a constrictive physiology. Conversely, a patient may have a constrictive physiology with subtle, diffuse thickening of the pericardium measuring less than 3 mm (which is the upper limit of the normal range); however, the physiologic alteration created by CP can be evaluated by performing VENC MRI of the pulmonary and hepatic veins and of velocities across the mitral and tricuspid valves. This technique is usually reliable in detecting constrictive physiology.
Ultrasonography
Transthoracic echocardiography has limited accuracy for assessing pericardial thickness; transesophageal echocardiography is superior but is rarely performed for this indication alone. Pericardial adhesion may be evident as thickened, parallel, adherent pericardial layers that are pulled together during systole. Pericardial tethering and restricted posterior wall motion are commonly reported in patients with CP. [23, 14]
Cardiac echograms show normal contraction and systolic function. Special procedures, including assessment of Doppler velocities across mitral and tricuspid valves during inspiration and expiration, are needed to detect ventricular interdependence. Unless staff in the echocardiography laboratory is alerted to the clinical suspicion of CP, this diagnosis often is not considered and therefore is missed. [19, 20, 21]
Echocardiographic procedures such as evaluation of early diastolic Doppler myocardial velocity gradients at the posterior wall, echocardiographic tissue Doppler imaging (TDI), and color M mode flow propagation have been reported to enhance the differentiation between CP and restrictive cardiomyopathy. [30, 31]
Liver sonograms show dilated hepatic veins and abnormal pulse Doppler waveforms in the portal and hepatic veins due to outflow obstruction. Budd-Chiari syndrome, cirrhosis, and right-sided heart failure can mimic some of the findings of CP at liver ultrasonography. Abdominal ultrasonographic findings are nonspecific and must be confirmed with echocardiography and cardiac catheterization.
In a study of 34 patients with CP and 26 patients with restrictive cardiomyopathy caused by cardiac amyloidosis, Butz et al concluded that echocardiographic TDI is an effective modality for differentiating CP from restrictive cardiomyopathy. [30] Employing receiver operating characteristic (ROC) analysis, Butz and coworkers found that determining a combination of systolic longitudinal velocity (S') and early diastolic longitudinal velocity (E'), each with a cutoff value less than 8 cm per second, at the lateral and septal mitral annulus resulted in diagnostic specificity and sensitivity of 88% and 93%, respectively, for restrictive cardiomyopathy. [30]
Lu et al concluded that use of a combination of 2-dimensional (2-D) echocardiography and quantitative TDI (QTDI) provides an effective method of diagnosing pericardial adhesion in patients with CP. Study authors used quantitative tissue displacement curves to measure systolic peak displacements of the pericardium (D[1]), the outer-layer myocardium (D[2]), and the inner-layer myocardium (D[3]) in 20 patients and 20 controls. They found that for patients with CP, the ratio (D[3] - D[2])/(D[2] - D[1]) was significantly higher than it was for control patients. [32]
In a study by Welch et al regarding the echocardiographic diagnosis of constrictive pericarditis, their analysis focused on 5 variables: (1) respiration-related ventricular septal shift; (2) variation in mitral inflow E velocity; (3) medial mitral annular e' (early diastolic) velocity; (4) ratio of medial mitral annular e' to lateral e'; and (5) hepatic vein expiratory diastolic reversal ratio. Of the 5 criteria studied, the 3 most important for the diagnosis of constrictive pericarditis, according to the authors, were the presence of respiration-related ventricular septal shift, preserved or increased medial mitral annular e' velocity, and prominent hepatic vein expiratory diastolic flow reversals. [14]
Nuclear Imaging
Because of liver function abnormalities caused by hepatic congestion, hepatobiliary scanning (see the image below) is often ordered for patients in whom CP is not suspected. Hepatobiliary scan findings include impaired hepatic clearance of the agent from the blood pool and severely impaired excretion of the radiopharmaceutical agent into the biliary tree.
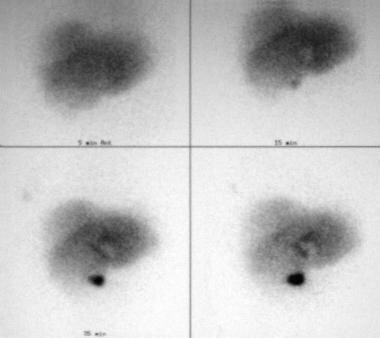 Constrictive pericarditis. Hepatobiliary scans were obtained for this patient because of liver function abnormalities. The radiopharmaceutical slowly clears from the hepatic parenchyma despite prompt visualization of the common bile duct and gallbladder.
Constrictive pericarditis. Hepatobiliary scans were obtained for this patient because of liver function abnormalities. The radiopharmaceutical slowly clears from the hepatic parenchyma despite prompt visualization of the common bile duct and gallbladder.
Gated nuclear ventriculography may show rapid ventricular filling in CP. Reportedly, these findings can be used to differentiate CP from restrictive cardiomyopathy.
Many conditions that are more common than CP can impair hepatic uptake and excretion of the radiopharmaceutical agent. If the physician is perceptive enough to consider the diagnosis of CP, the diagnosis must be confirmed with echocardiography and heart catheterization.
Hepatitis, drug-induced cholestatic liver disease, severe cirrhosis, and severe right-sided heart failure can lead to findings similar to those seen in CP.
Angiography
Angiography usually has no role in the evaluation of CP. Simultaneous right- and left-sided heart catheterization and measurement of cardiac chamber pressures during inspiration and expiration are the most useful confirmatory tests.
Measurements obtained in the catheterization laboratory can be highly suggestive of CP. Traditional hemodynamic criteria used in the catheterization laboratory for the diagnosis of CP are neither sensitive nor specific, and they significantly overlap with those of restrictive diseases that can also alter diastolic filling properties. These criteria are as follows:
-
End-diastolic pressure equalization is present: The difference between left and right ventricular end-diastolic pressures is 5 mm Hg or less.
-
Pulmonary arterial pressure is less than 55 mm Hg.
-
Right ventricular end-diastolic pressure divided by right ventricular end-systolic pressure is greater than 1/3.
-
A dip-and-plateau diastolic pressure morphology, as reflected by height of the left ventricular rapid filling wave (>7 mm Hg), is present.
-
The Kussmaul sign is present: Mean right atrial pressure does not decrease during inspiration.
Significant information can also be gained from echocardiography. Ultimately, the diagnosis must be confirmed surgically at the time of complete pericardiectomy. Only 50% of patients respond to surgery, but for many patients, symptoms are dramatically resolved.
Restrictive heart disease can mimic some manifestations of CP at catheterization or on echocardiography.
-
Constrictive pericarditis. A man presented with an 8-year history of slow worsening of exercise intolerance, weight gain, and dyspnea. His chest radiograph shows a normal-sized heart. Calcifications are not visible in the pericardium on the frontal view. The azygos vein is moderately distended.
-
Constrictive pericarditis. Chest radiograph lateral view of the same patient as in the previous image also fails to show pericardial calcifications.
-
Constrictive pericarditis. Hepatobiliary scans were obtained for this patient because of liver function abnormalities. The radiopharmaceutical slowly clears from the hepatic parenchyma despite prompt visualization of the common bile duct and gallbladder.
-
Constrictive pericarditis. The liver in this CT scan is poorly enhanced, congested, and enlarged. The aorta is poorly opacified, and passage of the contrast-agent bolus is mildly delayed.
-
Constrictive pericarditis. The cardiac chambers in this CT scan of the heart are not enlarged and are approximately the same size. Focal pericardial calcifications are noted. The noncalcified portion of the pericardium is not particularly thickened, but it seems tightly adherent to the epicardium.
-
Constrictive pericarditis. The interventricular septum in this CT scan of the heart is slightly straightened or bowed to the left. Note the delayed opacification of the aorta.
-
Constrictive pericarditis. Note the pericardial calcification inferior to the heart in this CT scan. Marked dilatation of the inferior vena cava is present despite the absence of cardiac chamber dilatation. The patient's disease responded completely to a total pericardiectomy.
-
Constrictive pericarditis. CT image obtained 8 years earlier of the heart in the previous image shows no pericardial calcifications.
-
Constrictive pericarditis. Image obtained in a patient who presented with an increasing serum creatinine level, peripheral edema, and signs of low cardiac output. This nonenhanced abdominal CT scan of the heart (obtained because of renal failure) shows mild but diffuse and circumferential pericardial thickening.
-
Constrictive pericarditis. Slightly caudal image in this CT scan of the heart shows deviation of the intraventricular septum to the left.
-
Constrictive pericarditis. The interatrial septum in this CT scan of the heart is bowed to the left because of an elevated right atrial filling pressure.
-
Constrictive pericarditis.
-
Constrictive pericarditis.
-
Constrictive pericarditis.






