Practice Essentials
Congestive heart failure (CHF) is a clinical syndrome in which the heart fails to pump blood at the rate required by metabolizing tissues or in which the heart can do so only with an elevation in filling pressure. Inability of the heart to pump a sufficient amount of blood to meet the needs of body tissues may be the result of insufficient or defective cardiac filling and/or impaired contraction and emptying. Compensatory mechanisms increase blood volume, as well as cardiac filling pressure, heart rate, and cardiac muscle mass, to maintain the pumping function of the heart and to cause redistribution of blood flow. Despite these compensatory mechanisms, the ability of the heart to contract and relax declines progressively, and heart failure (HF) worsens. Classification of heart failure is based on symptoms and calculated left ventricular ejection fraction (LVEF). Heart failure due to left ventricular dysfunction is categorized into HF with reduced ejection fraction, HF with preserved ejection fraction, and HF with midrange ejection fraction. [1, 2, 3]
Clinical manifestations of HF vary enormously and depend on a variety of factors, including patient age, the extent and rate at which cardiac performance becomes impaired, and which ventricle is initially involved in the disease process. A broad spectrum of severity of impairment of cardiac function is ordinarily included in the definition of HF. Impairment ranges from the mildest forms, which manifest clinically only during stress, to the most advanced forms, in which cardiac pump function is unable to sustain life without external support. [4]
Echocardiography is the preferred imaging modality in patients with CHF, with 2-dimensional echocardiography recommended initially. On plain radiography, findings of CHF include cardiomegaly; grade I, II, or III pulmonary venous hypertension (PVH); and increased central systemic venous volume. Computed tomography is usually not required, but multidetector-row gated CT scanning may provide excellent analysis of the heart. MRI may identify congenital abnormalities and valvular heart disease. Nuclear imaging can be used in the assessment of both heart function and damage in patients with CHF. ECG-gated images are useful for recognizing artifactual defects caused by attenuation (breast and diaphragm) and thus are useful in the quality control of SPECT imaging. ECG-gated SPECT imaging is considered the state of the art of radionuclide myocardial perfusion imaging. [5, 6, 7, 8, 1, 9]
Cardiac catheterization and coronary angiography have been shown to provide a useful role in patients who have CHF, valvular or congenital heart disease, and other cardiac conditions. [6] Lung ultrasonography can be performed easily and rapidly at the bedside. According to Iwakura and Onishi, B-lines with lung sliding represent a specific sign of HF. [7]
Lazarevic et al found that therapeutic thoracentesis assisted by lung ultrasonography induced immediate symptomatic improvement in 462 patients, with a a decrease in NYHA class from 3.84 ± 0.37 before thoracentesis to 2.7 ± 0.55 after the procedure. This improvement was long-lasting (for weeks/months) in 89% of patients. [10]
Echocardiography
Echocardiography is the preferred examination in CHF. Two-dimensional and Doppler echocardiography may be used to determine systolic and diastolic left ventricular (LV) performance, cardiac output (ejection fraction), and pulmonary artery and ventricular filling pressures. [11] Echocardiography also may be used to identify clinically important valvular disease. [8, 12, 13] Although echocardiography is simple and noninvasive, it proves inadequate in 8-10% of cases; in addition, results are difficult to interpret in patients with lung disease.
In a joint report by the American College of Radiology (ACR) and the American College of Cardiology Foundation (ACCF) regarding appropriate use of imaging in HF, the strongest recommendations for newly suspected HF were for echocardiography utilizing 2-dimensional (2D) transthoracic ultrasound and Doppler. [1]
Two-dimensional echocardiography is recommended as an initial part of the evaluation of patients with known or suspected CHF. Ventricular function may be evaluated, and both primary and secondary valvular abnormalities may be accurately assessed. [8, 12, 14] Echocardiography is also used to assess prognosis and can be used serially to evaluate treatment. [9] Appropriate use criteria support the re-evaluation of cardiac function by echocardiography in patients with HF, arrhythmia, and known valvular disease in the event of a change in clinical status without a clear precipitant. [1]
Doppler echocardiography may play a valuable role in determining diastolic function and in establishing the diagnosis of diastolic HF.
HF in association with normal systolic function but abnormal diastolic relaxation affects 30-40% of patients presenting with CHF. Because treatment for this condition is distinctly different from that for systolic dysfunction, establishing the appropriate etiology and diagnosis is essential. The combination of 2D and Doppler echocardiography is effective for this purpose.
The major limitations of echocardiography include patient-specific factors that lead to suboptimal acoustic windows. [9]
The degree of confidence in echocardiography is high, and rates of false-positive and false-negative findings are low.
Radiography
In cardiogenic cases, radiographs may show cardiomegaly, pulmonary venous hypertension, and pleural effusions. Pulmonary venous hypertension (PVH) may be divided into 3 grades:
-
In grade I PVH, upright examination reveals redistribution of blood flow to nondependent portions of the lungs and upper lobes.
-
In grade II PVH, evidence shows interstitial edema with ill-defined vessels and peribronchial cuffing, as well as interlobular septal thickening.
-
In grade III PVH, perihilar and lower lobe airspace filling is evident, with features typical of consolidation (eg, confluent opacities, air bronchogram, inability to see pulmonary vessels in the area of abnormality). Airspace edema tends to spare the periphery in the mid and upper lungs.
In noncardiogenic cases, cardiomegaly and pleural effusions are usually absent. Edema may be interstitial but is more often consolidative. No cephalization of flow is noted, although shifting of blood flow to less affected areas may be evident. Edema is diffuse and does not spare the periphery of the mid or upper lungs.
In cases of large, acute myocardial infarction (MI) and infarction of the mitral valve, support apparatus may produce atypical patterns of pulmonary edema and may mimic noncardiogenic edema in patients who in fact have cardiogenic edema.
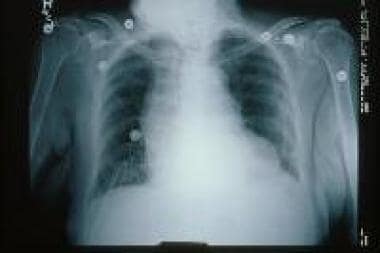
(Signs of CHF are shown in the image below.)
Two principal features of the chest radiograph are useful in evaluation of patients with CHF: (1) size and shape of the cardiac silhouette, and (2) edema at the lung bases (see the image below). [13]
The size and shape of the cardiac silhouette provide important information concerning the precise nature of underlying heart disease. Cardiothoracic ratio and heart volume, as determined on plain film, are relatively specific but insensitive indicators of increased LV end-diastolic volume. There is a weak inverse correlation between the cardiothoracic ratio and the LV ejection fraction (LVEF) in patients with HF; this relationship is not clinically useful in the individual patient.
In the presence of normal pulmonary capillary and venous pressures, the lung bases are better perfused than the apices when the patient is in the erect position, and vessels supplying the lower lobes are significantly larger than those supplying the upper lobes. With elevation of left atrial and pulmonary capillary pressures, interstitial and perivascular edema develops; such edema is most prominent at the lung bases because hydrostatic pressure is greater there.
When pulmonary capillary pressure is slightly elevated (13-17 mm Hg), resultant compression of pulmonary vessels in the lower lobes causes equalization in the size of vessels at the apices and bases (early grade I PVH). With greater pressure elevation (18-23 mm Hg), actual pulmonary vascular redistribution into nondependent portions of the lung occurs (ie, in an upright patient, further constriction is seen in vessels leading to the lower lobes, and there is dilatation of vessels leading to the upper lobes).
When pulmonary capillary pressures exceed 20-25 mm Hg, interstitial pulmonary edema occurs (grade II PVH). With grade II PVH, there is evidence of interstitial edema, with ill-defined vessels and peribronchial cuffing, as well as interlobular septal thickening. This interlobular septal thickening is referred to as Kerley B lines. Early blunting of lateral and posterior costophrenic angles may occur; such blunting indicates the presence of pleural fluid.
When pulmonary capillary pressure exceeds 25 mm Hg, images may show large pleural effusions and grade III PVH, with consolidative alveolar edema in a perihilar and lower lobe distribution.
With elevation of systemic venous pressure, the azygos vein, brachiocephalic veins, and the superior vena cava may become enlarged.
In patients with chronic LV failure, higher pulmonary capillary pressures may be accompanied by fewer clinical and radiologic signs, presumably because of enhanced lymphatic drainage.
In summary, typical findings of CHF on plain radiography include cardiomegaly; grade I, II, or III PVH; and increased central systemic venous volume, with enlargement of mediastinal veins (including the azygos vein) and pleural effusions.
The degree of confidence in radiography is low. Weak negative correlation between the cardiothoracic ratio and the ejection fraction does not permit accurate determination of systolic function in the absence of radiographic evidence of PVH or pleural effusion in individual patients with HF. For this reason, chest radiography may not be very useful for determining the type of LV dysfunction. During the treatment phase of CHF, chest radiographic findings often lag behind clinical improvement. False-negative findings are frequent.
Electrocardiography
In cardiogenic cases, the electrocardiogram (ECG) may show evidence of MI or ischemia. In noncardiogenic cases, the ECG is usually normal.
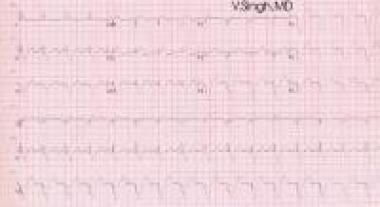
The ECG image below depicts biventricular pacing.
Computed Tomography
Computed tomography (CT) scanning of the heart usually is not required in routine diagnosis and management of CHF.
Multichannel CT scanning is useful in delineating congenital and valvular abnormalities; however, echocardiography and magnetic resonance imaging (MRI) may provide similar information without exposing the patient to ionizing radiation.
In cases that are clinically troublesome, multidetector-row gated CT scanning may provide excellent analysis of the heart and may reveal the nature of pulmonary edema. [15]
The degree of confidence in CT scanning is moderate, and rates of false-positive and false-negative findings with this modality are low.
Magnetic Resonance Imaging
Because of widespread acceptance of echocardiography, magnetic resonance imaging (MRI) is used only infrequently in the workup of patients with CHF. Its main use involves delineation of congenital cardiac abnormalities and assessment of valvular heart disease; MRI is also used in patients with other conditions. [16]
In newly suspected or potential HF, appropriate use criteria support cardiovascular magnetic resonance imaging (CMR). Although LV volume and ejection fraction (EF) measurements are as accurate as those obtained with echocardiography, viability and fibrosis imaging can assist in identification of etiology and in assessment of prognosis. LV mass quantitation by CMR predicts future risk in patients with HF. [1]
Delayed enhancement cardiovascular magnetic resonance imaging (DE-CMR) may be used to identify the underlying etiology of acute chest pain. A subendocardial or transmural hyperenhancement pattern is most frequently observed in ischemic injury, based on the wave front phenomenon of ischemic myocardial injury, whereas a mid to epicardial hyperenhancement pattern suggests nonischemic pathology (eg, myocarditis). CRM is highly accurate for detecting structural heart disease that may be responsible for stable and mildly abnormal high-sensitivity cardiac troponin (hs-cTn) levels (eg, dilated, hypertrophic, or infiltrative cardiomyopathy). Although it is generally considered less accurate than computed tomography angiography (CTA), CMR may be useful for excluding acute aortic dissection (AAD) or pulmonary embolism (PE). Disadvantages of CMR include limited availability, the need for specialized equipment and personnel, and use of time-consuming scanning protocols that can be strenuous for patients. [17]
The degree of confidence in MRI is high, and rates of false-positive and false-negative findings are low.
Nuclear Imaging
Nuclear imaging can be used in assessment of heart function and damage in CHF. [5]
ECG-gated myocardial perfusion imaging
The high photon flux of compounds labeled with technetium-99m (99mTC) makes it feasible to acquire myocardial perfusion images in an ECG-gated mode. ECG-gated myocardial perfusion images may be displayed as an endless-loop cine on the computer screen. ECG-gated single-photon emission CT (SPECT) images allow assessment of global LVEF, regional wall motion, and regional wall thickening.
Regardless of whether injection of a radiopharmaceutical was performed during peak stress or at rest, because acquisition is performed at rest, ECG-gated SPECT images reveal resting global function and wall motion and resting wall thickening in areas with defects of exercise-induced myocardial perfusion.
On ECG-gated SPECT images, regional wall thickening may be quantified as a percentage of wall thickening in comparison with end-diastole. Commercially available and validated software packages are available for automatic calculation of resting global LVEF, LV volume, and regional wall thickening derived from ECG-gated SPECT sections.
In general, LVEF derived from gated SPECT agrees well with resting LVEF as determined by other modalities. Quality assurance is important. Determination of LVEF with gated SPECT may be less accurate, even invalidated, in the presence of an irregular heart rate, low count density, intense extracardiac radiotracer uptake adjacent to the LV, or a small LV.
Combined assessment of perfusion and function on ECG-gated images substantially increases confidence in interpretation. ECG-gated images are useful for recognizing artifactual defects caused by attenuation (breast and diaphragm) and thus are helpful in quality control of SPECT imaging. ECG-gated SPECT imaging is considered the state of the art of radionuclide myocardial perfusion imaging.
Ma et al compared myocardial blood flow (MBF) using rapid-rotating gantry (RRG) and cadmium-zinc-telluride (CZT) SPECT cameras and concluded that physical corrections along with other image corrections can provide comparable MBF quantitations in both CHF and non-CHF patients, regardless of the type of SPECT system used. [18]
Assessment of myocardial viability
For patients with angina, known coronary artery disease (CAD), previous infarction, and LV dysfunction, a reliable method for assessing the presence, extent, and location of viable myocardium is of considerable clinical importance. It is well established that global or regional ischemic LV dysfunction is not always an irreversible condition. Approximately 25-40% of patients may experience improved function after adequate revascularization.
Two important practical issues must be addressed when patients with presumed ischemic dysfunction are evaluated: (1) assessment of relative regional myocardial uptake of thallium-201 (201Tl; often after rest reinjection), 99mTc-sestamibi, or 99mTc-tetrofosmin (often after rest administration of nitroglycerin) (when resting uptake of radiotracer is greater than 50% of normal, recovery of function may be expected after revascularization); and (2) assessment of the presence of demonstrable ischemia (eg, partially reversible defect) in a myocardial segment with decreased uptake (even when resting uptake is less than 50%).
Equilibrium radionuclide angiocardiography
Equilibrium radionuclide angiocardiography (ERNA) uses ECG events to define the temporal relationship between acquisition of nuclear data and volumetric components of the cardiac cycle.
Sampling is performed repetitively over several hundred heartbeats, with physiologic segregation of nuclear data in accordance with their occurrence within the cardiac cycle.
Data are quantified and displayed in an endless-loop, cinegraphic format for additional qualitative visual interpretation and analysis. Equilibrium blood-pool labeling is achieved with the use of 99mTc. The intravascular label is affixed to the patient's own red blood cells by an in vitro or modified in vitro technique. Unlabeled stannous pyrophosphate is used to facilitate this reaction. Conventional Anger scintillation cameras are used for these studies. Data are analyzed with the use of a computer, generally with some operator interaction.
Analysis may be conducted in the frame or list mode. Radionuclide data are collected and segregated temporally. This process generally requires 3 to 10 minutes for completion of each view. Following data acquisition, data from the several hundred individual beats are summed, processed, and displayed as a single representative cardiac cycle.
Data from the left anterior oblique (LAO) view are also used for qualitative analysis of global LV function. On this view, overlap of the 2 ventricles is minimal. With a count-based approach, LVEF and other indices of filling and ejection are calculated from LV radioactivity preset at various points throughout the cardiac cycle.
Right ventricular function is best evaluated by first-pass techniques. The LAO view provides qualitative information concerning contraction of septal, inferoapical, and lateral walls. The anterior view provides data concerning regional motion of anterior and apical segments. The left lateral or left posterior oblique view yields optimal qualitative information concerning contraction of the inferior wall and the posterobasal segment.
Ventricular aneurysm may be best assessed in lateral views as well. Each segment is generally graded on a 5-point scale, and specific numeric grades are assigned for dyskinesis, akinesis, mild and severe hypokinesis, and normal function.
ERNA may be combined easily with additional physiologic stress testing or provocation, which may involve physiologic stress such as that seen with exercise; pharmacologic stress, as occurs with the use of positive inotropic agents such as dobutamine or isoproterenol; or psychological stress.
Angiography
Cardiac catheterization and coronary angiography have a useful role in patients with CHF, in those with valvular heart disease or congenital heart disease, and in patients with other conditions. [6]
CHF
For patients with CHF, cardiac catheterization and coronary angiography are clearly indicated in the following situations:
-
CHF caused by systolic dysfunction in association with angina or regional wall motion abnormalities and/or scintigraphic evidence of reversible myocardial ischemia when revascularization is considered.
-
Before cardiac transplantation.
-
CHF secondary to postinfarction ventricular aneurysm or other mechanical complications of MI.
For such patients, these procedures are frequently indicated when systolic dysfunction of unexplained cause is present on noninvasive testing or when normal systolic function with episodic HF suggests ischemically mediated LV dysfunction.
Valvular heart disease
In patients with valvular heart disease, cardiac catheterization and coronary angiography are clearly indicated in the following situations:
-
Before valve surgery or balloon valvotomy in an adult with chest discomfort, ischemia by noninvasive imaging, or both.
-
Before valve surgery in an adult who is free of chest pain but has many risk factors for CAD.
-
Infective endocarditis with evidence of coronary embolization.
Congenital heart disease
In patients with congenital heart disease, cardiac catheterization and coronary angiography are clearly indicated in the following situations:
-
Before surgical correction of congenital heart disease when chest discomfort or noninvasive evidence is suggestive of associated CAD.
-
With a form of congenital heart disease that is associated with coronary artery anomalies that may complicate surgical management.
-
When unexplained cardiac arrest has occurred in a young patient.
These procedures are frequently indicated before corrective open heart surgery for congenital heart disease in an adult whose risk profile is associated with increased risk of coexisting CAD.
Other conditions
In patients with other conditions, cardiac catheterization and coronary angiography are clearly indicated in the following situations:
-
Disease affecting the aorta when knowledge of the presence or extent of coronary artery involvement is necessary for management (eg, aortic dissection, aneurysm with known CAD).
-
Hypertrophic cardiomyopathy with angina despite medical therapy when knowledge of coronary anatomy might affect therapy.
-
Hypertrophic cardiomyopathy with angina when heart surgery is planned.
For such patients, these procedures are frequently indicated in the following situations:
-
High risk of CAD when other cardiac surgical procedures are planned (eg, pericardiectomy, removal of chronic pulmonary emboli).
-
Prospective immediate cardiac transplant donor with a risk profile that increases the likelihood of CAD.
-
Asymptomatic patient with Kawasaki disease who has coronary artery aneurysm evident on echocardiography.
-
Before surgery for aortic aneurysm/dissection in a patient without known CAD.
-
Recent blunt chest trauma and suspicion of acute MI, without evidence of preexisting CAD.
-
Chest radiograph showing signs of congestive heart failure (CHF).
-
ECG showing biventricular pacing (double ventricular pacing spikes).