Practice Essentials
Choroid plexus papillomas (CPPs) are rare central nervous system tumors. They may be seen at any age but are more common among infants. Their site of occurrence varies according to age. These tumors usually present with increasing head circumference or altered mental status in children; in adults, they present with signs of increased intracranial tension. Imaging shows intraventricular enhancing masses. [1]
Radiologic examination is not sufficient for the diagnosis of CPP; complete excision of the tumor with histologic confirmation is indispensable for accurate diagnosis and treatment. [2]
Patients typically present with a history of gradual deterioration. Signs of raised intracranial tension, such as vomiting, headache, lateral gaze palsies, homonymous visual field defects, reduced mentation, and so on, are usually evident. Patients may develop subarachnoid hemorrhage due to bleeding from the tumor. Infants present with increasing head circumference or poor feeding or both, along with upgaze palsy, reduced activity, and altered consciousness. [1]
Clinical symptoms are predominantly due to hydrocephalus, which results from direct mechanical obstruction to the flow of cerebrospinal fluid (CSF) caused by an arachnoid granulation blockage from hemorrhage or CSF overproduction. Although the condition is benign, studies show rapid growth in tumor size. Sometimes, CSF rhinorrhea or hemifacial spasm may be the only clinical presentation. [1]
Surgery is the therapeutic intervention of choice for these lesions. [3] Postoperative extraventricular drainage has been shown to be effective in lowering the rate of shunt requirement in these patients, probably by releasing the blood-tinged CSF and small tumor particle residue. [4, 5, 6, 7, 8, 9]
Patients have a good prognosis in spite of tumor size, as CPPs are benign. Improvements in surgical techniques and in intensive care management have led to improved survival. [1]
Imaging modalities
Computed tomography (CT) scanning and magnetic resonance imaging (MRI) are the investigative procedures of choice for evaluation of CPPs. Because of the relatively noninvasive nature, ease, widespread availability, high reproducibility, and great contrast resolution of CT scanning and MRI, these examinations have supplanted all other methods of neuroimaging. With the addition of intravenously administered contrast material, the sensitivity of these imaging modalities approaches 100%. The multiplanar capability of MRI further aids characterization and localization of lesions. [10, 11, 12]
If fontanelles are not fused, neurosonography (NSG) through the anterior fontanelle demonstrates an echogenic lesion within the ventricles. This lesion continues to demonstrate bidirectional flow throughout the diastole, indicating blood flow through vessels arranged chaotically. Sometimes, lesions are diagnosed antenatally via ultrasonography. [1]
Although CPPs are readily apparent on most nonenhanced studies, omission of enhanced imaging from the imaging protocol may result in incorrect conclusions about tumor type and extent. In addition, misdiagnosis may result from attempts to classify a choroid plexus tumor as benign or malignant solely on the basis of imaging characteristics.
(See the images below.)
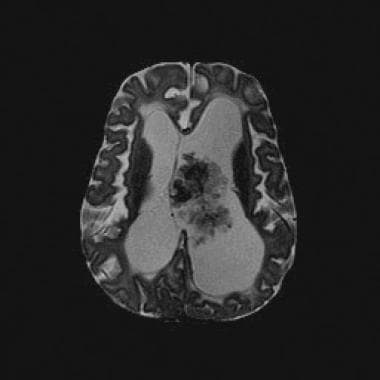 Axial T2-weighted magnetic resonance image (repetition time, 2883 ms; echo time, 100 ms) shows a lobulated mass with frondlike papillary projections in the left lateral ventricle. The mass is isointense relative to the cortex and has internal hypointense foci that likely represent prominent vessels. Note the associated hydrocephalus and transependymal cerebrospinal fluid flow.
Axial T2-weighted magnetic resonance image (repetition time, 2883 ms; echo time, 100 ms) shows a lobulated mass with frondlike papillary projections in the left lateral ventricle. The mass is isointense relative to the cortex and has internal hypointense foci that likely represent prominent vessels. Note the associated hydrocephalus and transependymal cerebrospinal fluid flow.
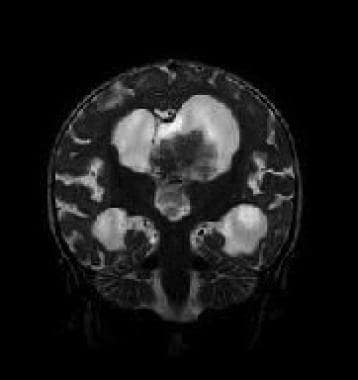 Interventricular extension through the foramen of Munro, cerebral aqueduct, or foramen of Luschka or Magendie can occur with a choroid plexus papilloma; this is an ancillary diagnostic sign that is not described with other interventricular tumors.
Interventricular extension through the foramen of Munro, cerebral aqueduct, or foramen of Luschka or Magendie can occur with a choroid plexus papilloma; this is an ancillary diagnostic sign that is not described with other interventricular tumors.
Plain radiography is not used to investigate CPP because of its low sensitivity and specificity; computed tomography (CT) scanning and magnetic resonance imaging (MRI) are the imaging studies of choice. [13] However, evidence of increased intracranial pressure may be noted on plain films. Faint intracranial calcifications in appropriate locations, which are observed in 4.1% of patients, may suggest the diagnosis.
Angiographic signs of CPP may include small spiral arteries; a meningioma-type blush with early tumoral circulation and persistent enhancement into the venous phase; displacement of vessels such as the internal cerebral veins; and evidence of ventricular dilatation. [14]
Pathophysiology
Studies have shown that Simian virus (SV) 40 is associated with the occurrence of choroid plexus tumors. BK virus and John Cunningham (JC) viruses have also been implicated. Binding of the large T antigen with both p53 and pRb tumor suppressor proteins, forming complexes, has been demonstrated in humans harboring choroid plexus tumors. Current data do not support a causative role. [1]
The blood supply to CPPs is derived from the choroid plexus. An enlarged anterior choroidal artery supplies tumors within the temporal horns of the lateral ventricles; the posterior choroidal arteries supply masses in the atria or in the posterior horn. Branches of the posterior inferior cerebellar artery may supply tumors in the fourth ventricle.
According to the World Health Organization, choroid plexus tumors are classified as papillomas (CPPs) (grade I), atypical tumors (grade II), and carcinomas (grade III). CPPs have fewer than 2 mitotic figures per 10 high power fields, atypical tumors have 2 to 5 mitotic figures per 10 high power fields, and carcinomas have more than 5 mitotic figures per 10 high power fields. Grossly, these tumors are soft, globular, friable pink masses with irregular projections and high vascularity. [1]
Epidemiology
Choroid plexus papilloma is a rare tumor without specific clinical patterns or imaging findings. [15]
Choroid plexus papillomas are of neuroectodermal origin. In the pediatric population, they are the third most common congenital brain tumors, after teratomas and gliomas, accounting for 0.4-0.6% of all intracranial neoplasms. They represent approximately 1% of all brain tumors, 2-6% of pediatric brain tumors, and 0.5% of adult brain tumors. [15]
Choroid plexus papilloma is generally a disease of childhood, with median age at diagnosis of 3.5 years. Typically, the location in infants is supratentorial, within the lateral ventricles (most commonly in the atrium); in adults, the preferred site is the fourth ventricle. [1]
The pigmented subtype of CPP is observed very rarely. [16] Bilateral lateral ventricle CPP is extremely uncommon. [3]
Atypical CPP can metastasize in the form of leptomeningeal seeding. Symptoms of food refusal, apathy, speechlessness, and low mood have been reported. [15]
Multifocal concomitant CPPs are rare. These may occur secondary to biologic seeding of CSF from a primary papilloma, or as a mere coincidental tumor occurrence. [17]
Computed Tomography
Computed tomography (CT) scanning has resulted in improved detection and characterization of all intracranial masses, including CPPs. Choroid plexus papillomas appear as well-marginated round or lobulated solid masses that are isoattenuating or hyperattenuating relative to normal brain parenchyma on nonenhanced scans. In as many as 24% of patients, these tumors may contain foci of calcification, which can be readily demonstrated on CT scans when compared to MRI. Choroid plexus papillomas are strongly enhancing after intravenous administration of contrast material. [18]
In children, CPPs can be heterogeneous in appearance because of accumulation of CSF, blood, and blood products between fronds and papillae. A heterogeneous appearance may be a sign of malignancy. In adults, most CPPs are heterogeneous secondary to cystic and/or calcific degeneration. Associated findings include hydrocephalus, which may involve the lateral, third, and fourth ventricles to varying degrees. [19, 20]
Lesions are lobulated with slightly irregular margins. They also show intense and somewhat heterogeneous contrast enhancement. Angiographic and cross-sectional imaging shows enlargement of the choroidal artery. [1]
The presence of irregular margins should raise concerns about malignancy. Choroid plexus papillomas may have limited parenchymal invasion, which makes distinction of the benign tumor from its malignant counterpart difficult.
Ossification does not occur in CPP, although it has been described in a case of papilloma within the fourth ventricle in a 21-year-old man. [21]
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is the test of choice for the diagnosis of CPP for several reasons. [22, 23] Multiplanar imaging, which is possible with MRI, can be used to precisely localize and determine the extent of CPP, thereby facilitating surgical planning. MRI also offers the advantage of eliminating artifacts of the posterior fossa, which occasionally can be problematic with CT scanning. In addition, MRI easily depicts local parenchymal invasion, which is occasionally present, and may be useful in distinguishing benign tumors from more aggressive or malignant choroid plexus tumors.
Magnetic resonance imaging reveals well-defined, frondlike intraventricular lobulated masses, which are hypointense on T1-weighted imaging (T1WI) and hyperintense on T2WI. Flow voids are seen, indicating active blood flow. Lesions are brilliantly enhancing because of rich vascularity. Use of arterial spin labeling can help distinguish CPP from choroid plexus carcinoma. [1]
Occasionally, CPPs can be bilateral or multiple, and interventricular extension—an ancillary diagnostic sign of CPP—is readily identified in the coronal plane. This sign is not described with other interventricular tumors. Although uncommon, interventricular extension through the foramen of Munro, the cerebral aqueduct, or the foramen of Luschka or Magendie is another helpful diagnostic sign. [24]
(See the following image.)
 Interventricular extension through the foramen of Munro, cerebral aqueduct, or foramen of Luschka or Magendie can occur with a choroid plexus papilloma; this is an ancillary diagnostic sign that is not described with other interventricular tumors.
Interventricular extension through the foramen of Munro, cerebral aqueduct, or foramen of Luschka or Magendie can occur with a choroid plexus papilloma; this is an ancillary diagnostic sign that is not described with other interventricular tumors.
Choroid plexus papillomas appear as heterogeneous masses, with or without cystic components; they may have intratumoral signal voids that correspond to a rich vascular supply or low-intensity areas that correspond to calcifications. On T1WI, CPPs are slightly hypointense relative to gray matter in adults (see the images below). In children, these masses are isointense. Small areas of high signal intensity are compatible with localized hemorrhagic components.
 Axial T2-weighted magnetic resonance image (repetition time, 2883 ms; echo time, 100 ms) shows a lobulated mass with frondlike papillary projections in the left lateral ventricle. The mass is isointense relative to the cortex and has internal hypointense foci that likely represent prominent vessels. Note the associated hydrocephalus and transependymal cerebrospinal fluid flow.
Axial T2-weighted magnetic resonance image (repetition time, 2883 ms; echo time, 100 ms) shows a lobulated mass with frondlike papillary projections in the left lateral ventricle. The mass is isointense relative to the cortex and has internal hypointense foci that likely represent prominent vessels. Note the associated hydrocephalus and transependymal cerebrospinal fluid flow.
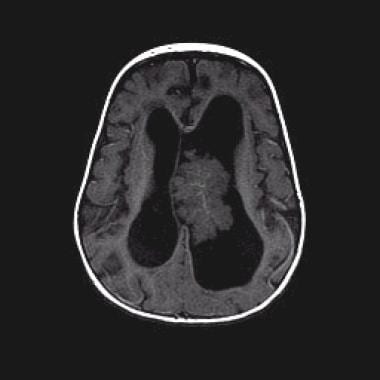 Axial T1-weighted nonenhanced magnetic resonance image (repetition time, 450 ms; echo time, 20 ms) shows that the mass is predominantly isointense relative to the cortex.
Axial T1-weighted nonenhanced magnetic resonance image (repetition time, 450 ms; echo time, 20 ms) shows that the mass is predominantly isointense relative to the cortex.
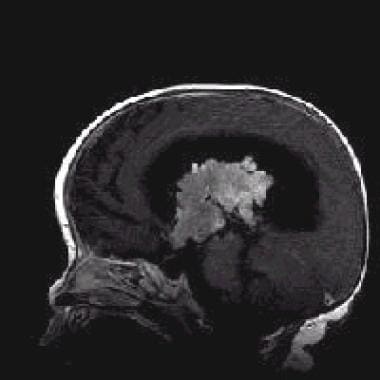 Sagittal T1-weighted contrast-enhanced magnetic resonance image (repetition time, 500 ms; echo time, 12 ms) shows intense heterogeneous enhancement. Note the extension into the third ventricle.
Sagittal T1-weighted contrast-enhanced magnetic resonance image (repetition time, 500 ms; echo time, 12 ms) shows intense heterogeneous enhancement. Note the extension into the third ventricle.
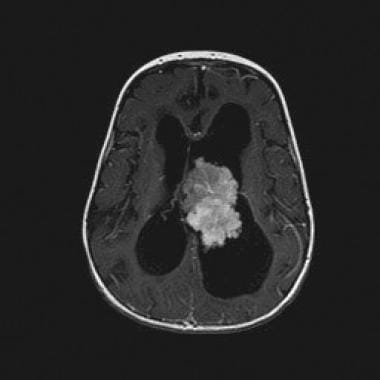 Axial T1-weighted contrast-enhanced magnetic resonance image (repetition time, 500 ms; echo time, 20 ms) demonstrates the strongly enhancing lateral ventricular mass.
Axial T1-weighted contrast-enhanced magnetic resonance image (repetition time, 500 ms; echo time, 20 ms) demonstrates the strongly enhancing lateral ventricular mass.
On T2WI, tumors in both adults and children have high signal intensity, which may approximate the signal intensity of CSF. After injection of a paramagnetic contrast agent such as gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA), intense enhancement is observed. [25]
The superior imaging capability of MRI for examination of the spinal canal may reveal evidence of seeding to the spinal subarachnoid space on rare occasions, particularly with posterior fossa tumors.
Choroid plexus tumors can present in baseline MRI with lesions compatible with leptomeningeal dissemination. Therapeutic strategy in this condition is controversial. In these challenging cases, several aspects, such as histopathologic/molecular diagnosis and close radiologic follow-up, should be taken into account to avoid unnecessary treatments. [26]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). NSF/NFD has occurred in patients with moderate to end-stage renal disease after they were given a gadolinium-based contrast agent to enhance MRI or magnetic resonance angiography (MRA) scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Ultrasonography
Ultrasonography may be useful in special circumstances, as when neonates are assessed. [27] Its availability, portability, accuracy, low cost, and lack of requirement for sedation make ultrasonography a valuable tool. In many instances, ultrasonography is the examination of choice for initial screening. Ultrasonographic evaluation may also be valuable for postoperative assessment of the neonatal brain.
In cases of CPP, real-time ultrasonography demonstrates the presence of hydrocephalus. Tumors themselves appear as heterogeneous, highly echogenic intraventricular masses with irregular borders. Doppler ultrasonographic studies have shown pulsatile intratumoral vascular channels with biphasic flow. Occasionally, intratumoral cysts are identified; these appear as hypoechoic areas and are believed to represent areas of hydropic degeneration.
Prognosis
Recent advances in imaging, surgical approaches, and quality of intensive care have improved surgical outcomes, with the chance of cure reaching almost 100%. In the pediatric population, significant (12%) perioperative mortality, mainly from catastrophic blood loss, has been reported. Preoperative embolization can minimize this risk and can optimize the ability of the surgeon to resect the tumor completely. Radiosurgery may be a treatment option, but further studies are required to determine its efficacy. Percutaneous stereotactic intratumoral embolization with a sclerosing agent has been attempted, to reduce blood flow and improve tumor resectability. [1]
Adjuvant chemotherapy, although limited in use, can prevent recurrence and can prolong survival. A growing residual CPP may be treated by irradiation, followed by subtotal resection, providing an even higher chance of success. Adjuvant therapy is also necessary for malignant tumors and those that have shown leptomeningeal spread. Bevacizumab is used increasingly for treatment of disseminated CPP. [1]
Postoperative chemotherapy is the recommended first-line treatment when gross total removal is not achieved, or in cases of disseminated disease. MRI findings show diffuse leptomeningeal enhancement at diagnosis, with spontaneous resolution of leptomeningeal involvement after removal of primary lesions. [28]
-
Axial T2-weighted magnetic resonance image (repetition time, 2883 ms; echo time, 100 ms) shows a lobulated mass with frondlike papillary projections in the left lateral ventricle. The mass is isointense relative to the cortex and has internal hypointense foci that likely represent prominent vessels. Note the associated hydrocephalus and transependymal cerebrospinal fluid flow.
-
Axial T1-weighted nonenhanced magnetic resonance image (repetition time, 450 ms; echo time, 20 ms) shows that the mass is predominantly isointense relative to the cortex.
-
Sagittal T1-weighted contrast-enhanced magnetic resonance image (repetition time, 500 ms; echo time, 12 ms) shows intense heterogeneous enhancement. Note the extension into the third ventricle.
-
Axial T1-weighted contrast-enhanced magnetic resonance image (repetition time, 500 ms; echo time, 20 ms) demonstrates the strongly enhancing lateral ventricular mass.
-
Interventricular extension through the foramen of Munro, cerebral aqueduct, or foramen of Luschka or Magendie can occur with a choroid plexus papilloma; this is an ancillary diagnostic sign that is not described with other interventricular tumors.






