Practice Essentials
Venous vascular malformations, also known as venous angiomas or, more properly, developmental venous anomalies (DVAs), represent congenital anatomically variant pathways in the normal venous drainage of an area of the brain. Once thought to be rare, they are now considered to be the most common vascular malformation in the CNS. [1] They may occur in as many as 2% of individuals.
DVAs are the most common slow-flow venous malformation in the brain. Most DVAs are benign. Uncommonly, DVAs can become symptomatic, leading to a variety of different pathologies. DVAs can vary significantly in size, location, and angioarchitecture, and imaging evaluation of symptomatic DVAs requires a systematic approach. [2]
Although contrast-enhanced computed tomography (CT) scanning and nonenhanced magnetic resonance imaging (MRI) can reveal a DVA, the preferred imaging technique is contrast-enhanced MRI because of its excellent depiction of the small venules and draining vein. The multiplanar capabilities of MRI are especially useful because the typical configuration of a DVA is often best recognized in the coronal plane. [3, 4, 5, 6, 7] Although standard contrast-enhanced MRI is excellent in depicting DVAs, adjacent hemosiderin from associated cavernomas may not be appreciated without the use of gradient-echo or echo-planar imaging, especially with fast spin-echo techniques.
DVAs are intracranial vascular malformations typically characterized by their benign nature, often obviating the need for radiologic follow-up. These anomalies arise from variations in the standard drainage pattern. Although previously deemed congenital, there has been ongoing debate about a developmental component contributing to their etiology. [8]
Symptomatic DVAs are rare. Upon reviewing the pathomechanisms of symptomatic DVAs in 2023, Ahmed et al illustrated their varied clinicoradiologic profiles and contemplated the mechanisms that render these (allegedly) benign entities symptomatic. They concluded that awareness and contextualization of potential flow-related perturbations and mechanical insults that render DVAs symptomatic aid in accurate diagnosis, management, and prognostication. [9]
(See the images of venous vascular malformations below.)
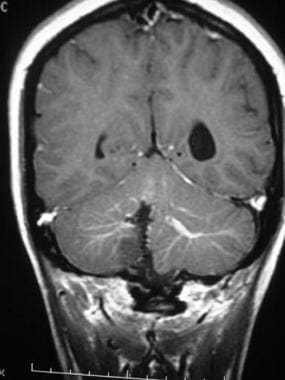 Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image obtained in a patient who had undergone surgery in the past for an arteriovenous malformation (AVM) shows bilateral developmental venous anomalies (DVAs) and the classic caput medusa appearance. Note the signal intensity abnormality in the inferior right cerebellar hemisphere due to the prior surgery.
Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image obtained in a patient who had undergone surgery in the past for an arteriovenous malformation (AVM) shows bilateral developmental venous anomalies (DVAs) and the classic caput medusa appearance. Note the signal intensity abnormality in the inferior right cerebellar hemisphere due to the prior surgery.
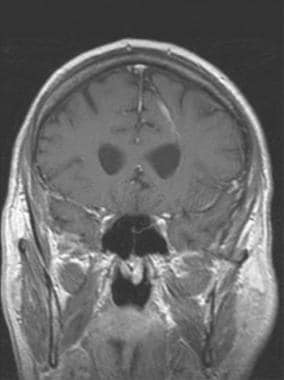 Brain, venous vascular malformation. Coronal T1 postcontrast demonstrates a typical location for a DVA, here within the periventricular white matter. This malformation drained into a cortical vein along the parietal convexity.
Brain, venous vascular malformation. Coronal T1 postcontrast demonstrates a typical location for a DVA, here within the periventricular white matter. This malformation drained into a cortical vein along the parietal convexity.
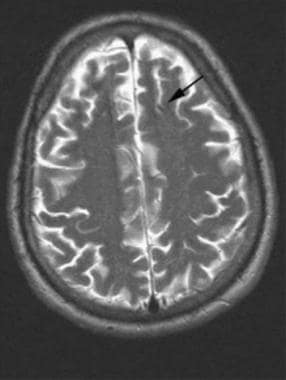 Brain, venous vascular malformation. Axial T2 image shows that the DVA can be subtle. In this patient, the draining vein is large enough to have a flow void on the image. The parenchymal abnormality is typically not visible.
Brain, venous vascular malformation. Axial T2 image shows that the DVA can be subtle. In this patient, the draining vein is large enough to have a flow void on the image. The parenchymal abnormality is typically not visible.
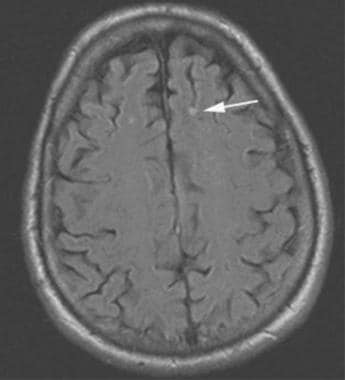 Brain, venous vascular malformation. Axial fluid-attenuated inversion recovery shows some artifactual increased signal within the vessel, which can aid in detection of DVAs on noncontrasted studies.
Brain, venous vascular malformation. Axial fluid-attenuated inversion recovery shows some artifactual increased signal within the vessel, which can aid in detection of DVAs on noncontrasted studies.
Although for many years DVAs were commonly called venous angiomas, the newer term DVA has been recommended as more appropriate because the involved vessels are not abnormally formed, but apparently merely dilated. The majority of DVAs are found incidentally and never cause symptoms, although there are isolated reports of patients with syndromes attributed to DVAs (eg, secondary to hemorrhage or thrombosis).
DVAs are usually incidental and benign, although about 40% are associated with cavernous malformations; it is essential to look for other associated vascular or neurocutaneous anomalies. Thrombosis rarely occurs as a complication of developmental venous anomalies and case reports in the literature suggest that they should be managed conservatively, leaving surgery for other associated complications. As radiologists, we must be aware of the main imaging features so as to be able to make an accurate diagnosis. [10]
The mechanisms of symptomatic DVAs are divided into mechanical (ie, hydrocephalus or nerve compression syndrome), flow-related causes, and idiopathic. Flow-related complications are divided into an increase in inflow and a decrease in outflow. In cases of increases in inflow, such as DVAs with arteriovenous shunts or arteriovenous malformations, patients may initially present with headache, neurologic deficit, seizures, and coma. Hearing loss is an extremely rare presentation that can be attributable to the compression effect on the cranial nerve VII to VIII complex. In the case of compression effect or progression of symptoms, surgical intervention is necessary. A good clinical outcome could be expected postoperatively. [11]
When symptomatic, DVAs may present with seizures; however, little is known about the characteristics of DVA-related epilepsy. Epilepsy can be a complication of DVAs; these DVAs are mostly frontal or parietal, draining via the superior sagittal sinus or the vein of Galen. [12]
Patients with a decrease in outflow are influenced by mechanical or functional causes. The mechanical causes include thrombosis of collecting veins (51.7%), stenosis of the DVA drainage pathway (24.1%), or complete thrombosis of DVA (24.2%). [13]
While some believe that DVAs can hemorrhage on their own, most notably after venous infarction from spontaneous DVA thrombosis, most instances of hemorrhage with DVAs have been in patients with combined vascular malformations. In the vast majority of these cases, the hemorrhage probably originated from the accompanying vascular malformation rather than from the DVA.
Surgical treatment for DVAs has been advocated, but most experts believe that the resulting risk of an iatrogenic venous infarct would far exceed the risk of irreversible damage from the DVA itself during the patient's lifetime. In fact, most patients with DVAs who become symptomatic have an associated cavernous angioma, which suggests that the symptoms are actually caused by the cavernoma. (See the image below.)
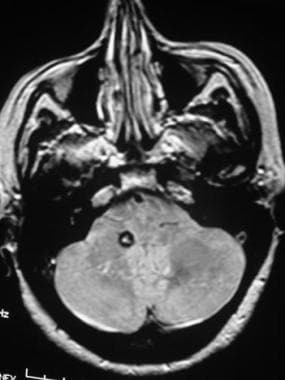 Brain, venous vascular malformation. Axial proton density–weighted image shows an area of marked signal intensity loss in the right cerebellum adjacent to the developmental venous anomaly (DVA). This finding is consistent with a coexistent cavernous angioma.
Brain, venous vascular malformation. Axial proton density–weighted image shows an area of marked signal intensity loss in the right cerebellum adjacent to the developmental venous anomaly (DVA). This finding is consistent with a coexistent cavernous angioma.
DVAs are associated with cavernous angiomas or one of the other types of CNS vascular malformations (ie, arteriovenous malformation [AVM], capillary telangiectasia) in approximately 15–30% of patients. [13] The most frequent conjunction is with cavernous angiomas; indeed, this association is so common that the two may be etiologically related, [14] and the presence of a DVA on an image should prompt a search for a cavernoma, which is more clinically important. [15] DVAs are also associated with head and neck venous malformations and hemangiomas. Rarely, DVAs are associated with varices. [16]
Multiple imaging examples of parenchymal and perfusion abnormalities associated with DVAs have been documented. [17] Larvie et al reported that in a small series of 25 developmental venous anomalies, 72% were associated with the presence of a mild, moderate, or severe degree of DVA-associated hypometabolism (DVAAh). [18] In a follow-up study, 54% of DVAs were associated with a greater than 10% decrease in metabolic activity. The authors surmised that worsening of DVAAh with increasing age could imply that the natural history of DVAs may result in either increasing damage to adjacent brain parenchyma or possibly remodeling of parenchyma away from the region with altered venous drainage. [19]
DVAs have traditionally been defined as nonpathologic congenital lesions. Compared with isolated DVAs, the association of DVAs with arteriovenous shunts seems to have a more adverse clinical connotation. In a 2023 systematic review, Agosti et al described the association between DVA and dural arteriovenous fistula (dAVF) and discussed hemorrhagic risk. Review authors concluded that despite the general opinion that DVAs are benign congenital lesions, increasing epidemiologic and radiologic evidence supports a potential acquired origin, and the venous system seems to play a pivotal role in their postnatal genesis and development. [20]
Computed Tomography
Developmental venous anomalies are typically not visible on nonenhanced CT scans, but they can be visualized after the administration of contrast medium. They appear as a large vascular structure in the brain parenchyma that drains into the deep or superficial venous system. The smaller surrounding veins are usually arranged in a radial pattern around the central vein. DVAs do not have a surrounding mass effect or edema, and the adjacent brain is typically normal.
The typical appearance of a DVA on CT scans is often diagnostic, but MRI may be needed in atypical cases, particularly those involving the posterior fossa, where CT is limited because of streak artifacts.
Although arteriovenous malformations can occasionally be mistaken for DVAs on CT scan and vice versa, differentiation between the two is usually not a problem, because AVMs have large feeding arteries, tortuous vessels, and abnormal adjacent brain parenchyma that are not observed in DVAs.
Magnetic Resonance Imaging
MRI findings are diagnostic in almost all instances; however, in cases with questionable findings, MR venography usually suggests the diagnosis. On contrast-enhanced MRI, the cluster of veins in developmental venous anomalies has a spoke-wheel appearance (see the first image below); the veins are small at the periphery and gradually enlarge as they approach a central draining vein (see the second and third images below). [21, 22] This appearance has been referred to as caput medusa, or the head of Medusa, because of the serpentine appearance of the curvilinear peripheral draining veins. The intervening brain parenchyma is normal; this is a distinguishing characteristic of a DVA. Medusa's head sign, or caput medusae, can be appreciated on contrast-enhanced CT scans and MRIs of the brain. [23] However, 2 studies reported parenchymal abnormalities within the drainage territory of most DVAs. [24, 25]
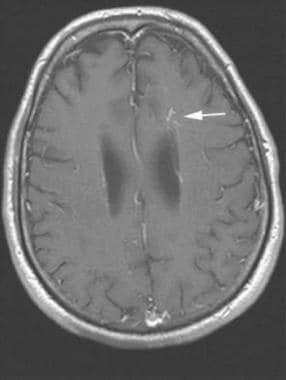 Brain, venous vascular malformation. Axial postcontrast image demonstrates the fine network of feeder veins that converge into the single draining vein.
Brain, venous vascular malformation. Axial postcontrast image demonstrates the fine network of feeder veins that converge into the single draining vein.
 Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image obtained in a patient who had undergone surgery in the past for an arteriovenous malformation (AVM) shows bilateral developmental venous anomalies (DVAs) and the classic caput medusa appearance. Note the signal intensity abnormality in the inferior right cerebellar hemisphere due to the prior surgery.
Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image obtained in a patient who had undergone surgery in the past for an arteriovenous malformation (AVM) shows bilateral developmental venous anomalies (DVAs) and the classic caput medusa appearance. Note the signal intensity abnormality in the inferior right cerebellar hemisphere due to the prior surgery.
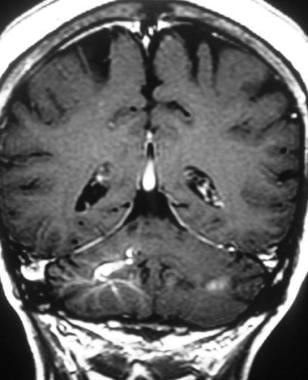 Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image clearly shows the draining vein and associated venous network of a developmental venous anomaly (DVA).
Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image clearly shows the draining vein and associated venous network of a developmental venous anomaly (DVA).
The draining vein has a fairly straight course toward the deep or superficial venous drainage system, depending on the location of the DVA. When it is adjacent to the lateral ventricles, the draining vein usually merges with a subependymal vein, which may be enlarged. Other DVAs may drain into cortical veins or dural sinuses in the supratentorial brain (see the images below). Infratentorial DVAs have a variety of possible drainage pathways without a clearly dominant one.
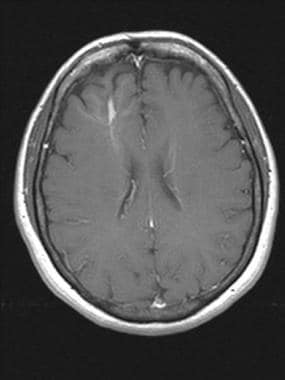 Brain, venous vascular malformation. Axial T1 postcontrast demonstrates a large DVA originating from the frontal lobe white matter. Note the cluster of small vessels that form the large draining vein.
Brain, venous vascular malformation. Axial T1 postcontrast demonstrates a large DVA originating from the frontal lobe white matter. Note the cluster of small vessels that form the large draining vein.
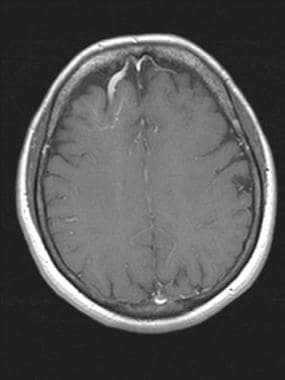 Brain, venous vascular malformation. The large draining vein is noted to drain into the superior sagittal sinus.
Brain, venous vascular malformation. The large draining vein is noted to drain into the superior sagittal sinus.
On T2-weighted and proton density–weighted images, the draining vein may demonstrate increased signal intensity, particularly on standard spin-echo images. This appearance is caused by gradient moment nulling. If the vessel is obliquely oriented, a yin-yang symbol appearance may occur because the high signal intensity is misregistered and a signal void appears next to a similarly shaped area of increased signal intensity. In the absence of an accompanying vascular malformation, the surrounding brain tissue should have normal characteristics on T2-weighted images, although a case in which gliosis surrounded a DVA has been reported. Nonenhanced T1 images may show the draining vein as a flow void, but DVAs are often difficult to visualize without the use of contrast medium. Fluid-attenuated inversion recovery (FLAIR) images may be relatively normal or can show a subtle increased signal. [26] (See the images below.)
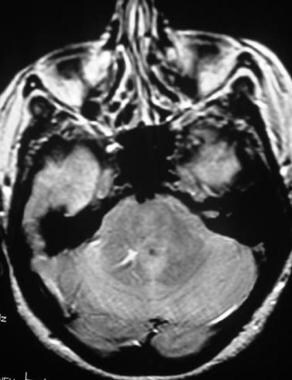 Brain, venous vascular malformation. Axial proton density–weighted image demonstrates the high signal intensity of the draining vein, which is typical on images obtained with this sequence. Note the yin-yang appearance of the vessel with an area of decreased signal intensity adjacent to the area with increased signal intensity.
Brain, venous vascular malformation. Axial proton density–weighted image demonstrates the high signal intensity of the draining vein, which is typical on images obtained with this sequence. Note the yin-yang appearance of the vessel with an area of decreased signal intensity adjacent to the area with increased signal intensity.
 Brain, venous vascular malformation. Axial fluid-attenuated inversion recovery shows some artifactual increased signal within the vessel, which can aid in detection of DVAs on noncontrasted studies.
Brain, venous vascular malformation. Axial fluid-attenuated inversion recovery shows some artifactual increased signal within the vessel, which can aid in detection of DVAs on noncontrasted studies.
Gradient-echo images often show decreased signal intensity in the venous angioma that is not due to hemosiderin but is secondary to the paramagnetic effects of deoxyhemoglobin in the venous blood (see image below). Findings on diffusion images are usually normal.
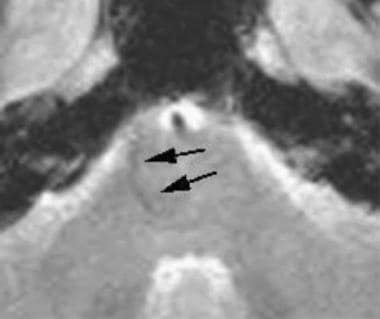 Brain, venous vascular malformation. On fast low-angle shot images, both the venous cluster and the draining vein may have mild susceptibility artifact (although not as much as hemosiderin) secondary to the deoxyhemoglobin within the slow-flowing veins (arrows).
Brain, venous vascular malformation. On fast low-angle shot images, both the venous cluster and the draining vein may have mild susceptibility artifact (although not as much as hemosiderin) secondary to the deoxyhemoglobin within the slow-flowing veins (arrows).
Magnetic resonance venography is almost never necessary. If obtained, venograms show the draining vein with some of the surrounding radially arranged veins. Because the DVA provides the venous drainage for a section of brain, anatomically normal venous drainage is not present in that area.
Because DVAs are often associated with other CNS vascular lesions (particularly cavernous angiomas), when a DVA is identified, carefully evaluate the brain and obtain gradient-echo (GRE) images. Cavernomas typically appear as focal areas of blood products that often show different stages of evolution (ie, hemosiderin with extracellular methemoglobin). Capillary telangiectasias are small areas of lacelike enhancement that are dark on GRE images without signal intensity abnormality on T2-weighted images. AVMs have enlarged feeding arteries and tortuous vessels with surrounding gliosis.
In 2023, Yoo et al compared and analyzed arterial spin-labeling findings in patients with DVAs or venous-predominant AVMs, using digital subtraction angiography (DSA) as the criterion standard. These researchers noted that arterial spin-labeling may predict the presence and amount of arteriovenous shunting in venous-predominant AVMs, and that use of arterial spin-labeling enables confirmation of typical venous-predominant AVMs without DSA. However, lesions with an intermediate amount of shunting suggest a spectrum of vascular malformations ranging from purely vein-draining developmental venous anomalies to venous-predominant AVMs with overt arteriovenous shunting. [27]
Angiography
Developmental venous anomalies found on angiograms are almost invariably incidental findings, as they are with newer MRIs, and the diagnosis can be made in almost every instance. When observed, the DVA appears as a blush of contrast enhancement during the venous phase of the study and drains into a large anatomically anomalous vein. In its most frequent location (adjacent to the lateral ventricles), the vein usually drains into a subependymal vein, although superficial drainage also occurs. [28, 29] A DVA has a characteristic angiographic appearance and should not be confused with an AVM; no early filling occurs with a DVA.
-
Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image obtained in a patient who had undergone surgery in the past for an arteriovenous malformation (AVM) shows bilateral developmental venous anomalies (DVAs) and the classic caput medusa appearance. Note the signal intensity abnormality in the inferior right cerebellar hemisphere due to the prior surgery.
-
Brain, venous vascular malformation. Coronal T1-weighted contrast-enhanced image clearly shows the draining vein and associated venous network of a developmental venous anomaly (DVA).
-
Brain, venous vascular malformation. Axial proton density–weighted image demonstrates the high signal intensity of the draining vein, which is typical on images obtained with this sequence. Note the yin-yang appearance of the vessel with an area of decreased signal intensity adjacent to the area with increased signal intensity.
-
Brain, venous vascular malformation. Axial proton density–weighted image shows an area of marked signal intensity loss in the right cerebellum adjacent to the developmental venous anomaly (DVA). This finding is consistent with a coexistent cavernous angioma.
-
Brain, venous vascular malformation. Coronal T1 postcontrast demonstrates a typical location for a DVA, here within the periventricular white matter. This malformation drained into a cortical vein along the parietal convexity.
-
Brain, venous vascular malformation. Axial postcontrast image demonstrates the fine network of feeder veins that converge into the single draining vein.
-
Brain, venous vascular malformation. Axial T2 image shows that the DVA can be subtle. In this patient, the draining vein is large enough to have a flow void on the image. The parenchymal abnormality is typically not visible.
-
Brain, venous vascular malformation. Axial fluid-attenuated inversion recovery shows some artifactual increased signal within the vessel, which can aid in detection of DVAs on noncontrasted studies.
-
Brain, venous vascular malformation. On fast low-angle shot images, both the venous cluster and the draining vein may have mild susceptibility artifact (although not as much as hemosiderin) secondary to the deoxyhemoglobin within the slow-flowing veins (arrows).
-
Brain, venous vascular malformation. Axial T1 postcontrast demonstrates a large DVA originating from the frontal lobe white matter. Note the cluster of small vessels that form the large draining vein.
-
Brain, venous vascular malformation. The large draining vein is noted to drain into the superior sagittal sinus.







