Practice Essentials
Hemophilia is the oldest known bleeding disorder and is a disease almost exclusively of males because the defective gene is found on the X chromosome. The deficiency or absence of either of 2 clotting elements—factor VIII or factor IX—leads to the clinical condition described as hemophilia A or hemophilia B, respectively, and these 2 sex-linked disorders become clinically apparent in males. Intra-articular hemarthroses affect approximately 90% of patients with severe hemophilia, with the most frequently involved joints being the ankles, knees, and elbows. Repeated episodes of intra-articular bleeding lead to progressive arthropathy, which is the main cause of morbidity in these patients [1, 2, 3, 4, 5, 6, 7, 8]
Despite the fact that hemophilia diagnosis is primarily based on clinical and laboratory findings, imaging is important in that it is used to evaluate complications, provide diagnostic confirmation, and complement therapeutic follow-up for hemophilic arthropathy. Plain radiographic classification systems can describe clinical progression of arthropathy, but MRI has advantages over radiography because it can visualize soft tissue and cartilage changes in hemophilic joints. Ultrasonography has additional advantages over MRI such as is its ability to differentiate synovium hypertrophy and hemosiderin deposition (see Preferred Examination, below). [9, 10, 7, 11, 12, 13, 14, 15]
Before the widespread availability and adoption of prophylaxis, which seeks to prevent bleeding by routine replacement of FVIII on a regular schedule to prevent bleeding, arthropathy was the most prevalent and costly complication of hemophilia. Hemophilic arthropathy is caused by recurrent hemorrhage into joints and results in an arthritis that is characterized by soft tissue changes of proliferation of hemosiderin-laden synovium and osteochondral changes of subchondral erosions, cyst formation, and cartilage loss. [16]
(See below for images from a father with hemophilia and his affected daughter.)
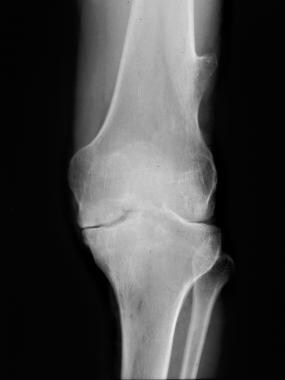 Knee radiograph in a 37-year-old man with moderate factor IX hemophilia. This image shows bony excrescence on the lateral side of the femur is a hemophilic pseudotumor.
Knee radiograph in a 37-year-old man with moderate factor IX hemophilia. This image shows bony excrescence on the lateral side of the femur is a hemophilic pseudotumor.
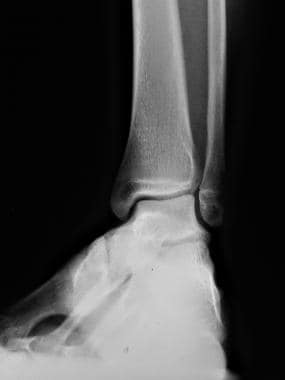 Radiograph of the lower extremity of the 3-year-old daughter of the patient in the previous image. This image shows talar tilt. The girl not only inherited 1 diseased X chromosome with mild factor IX hemophilia from her father, but she also has Turner (XO) syndrome. The child's only X chromosome had the hemophilia gene.
Radiograph of the lower extremity of the 3-year-old daughter of the patient in the previous image. This image shows talar tilt. The girl not only inherited 1 diseased X chromosome with mild factor IX hemophilia from her father, but she also has Turner (XO) syndrome. The child's only X chromosome had the hemophilia gene.
Females are generally asymptomatic carriers; this condition is so rare in females that another cause of unexplained or problematic bleeding should be considered before this condition. For example, a traumatic effusion or pigmented villonodular hyperplasia is more likely than hemophilia as an etiology for a bleeding disorder in a female.
For men with hemophilia, the incidence of postoperative transfusions associated with total hip or knee arthroplasty (1998-2010) was 15.06%, compared with 9.84% in a matched comparison cohort, based on a Nationwide Inpatient Sample. [17]
Pathophysiology
In 1868, the physician Volkmann defined the role of hemorrhage in the pathogenesis of the articular findings in hemophilia. [18] People with one of these bleeding disorders are prone to have recurrent episodes of hemorrhage into the joints. [3] Acute bleeding increases the pressure in the synovial cavity and bone marrow, which possibly leads osteonecrosis or a pseudotumoral mass. Intra-articular bleeding produces a direct chemical effect on the synovium, cartilage, and bone. Over time, the blood becomes deposited in the form of hemosiderin in these tissues. Recurrent hyperemia of the joint in the growing child causes juxta-articular osteoporosis and overgrowth of the epiphysis.
Roosendaal and Lafeber found that the articular cartilage is sensitive to the presence of blood and that damage may occur to the cartilage independent of the synovial changes caused by bleeding. [4] However, practically speaking, the imaging changes that appear first are effusion and synovial proliferation. Damage to the bone and articular cartilage appears later.
Bleeding into target joints (the joints that are most commonly affected with repetitive bleeding in an individual patient) starts before the age of 2 years and persists into adolescence. In some patients, bleeding continues into adulthood, even affecting new target joints after age 30 years; however, this occurrence is unusual. The late sequelae of joint hemorrhage appear in adolescence or adulthood as a joint deformity, contracture, and/or degenerative arthritis.
Anatomy
The pathologic-anatomic appearance progresses from joint hemorrhage to joint effusion; synovial hyperplasia; hemosiderin deposition in the synovium, cartilage, and bone; osteoporosis; erosion of subchondral bone; bone cysts; articular cartilage destruction; overgrowth of the epiphysis; joint contracture; and degenerative arthritis.
The disease tends to be asymmetric in its involvement. Approximately 50% of patients with hemophilia develop permanent changes in the joint. These chronic changes include thick synovial deposition, richly laden with hemosiderin. The synovial masses erode the juxta-articular cartilage and the subchondral bone (see the first image below). Invasion into the bone substance produces intraosseous cyst formation. Bleeding into the bone may rarely produce large, vacuolated spaces that are referred to as intraosseous pseudotumors. Similar blood masses may occur in the cortex and the soft tissues (see the second image below).
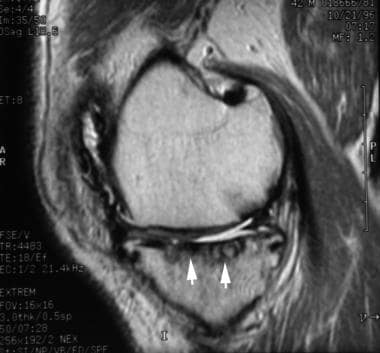 Magnetic resonance image from a patient with hemophilia. This image shows dark, synovial masses that erode the cartilage and produce subchondral cysts (arrows).
Magnetic resonance image from a patient with hemophilia. This image shows dark, synovial masses that erode the cartilage and produce subchondral cysts (arrows).
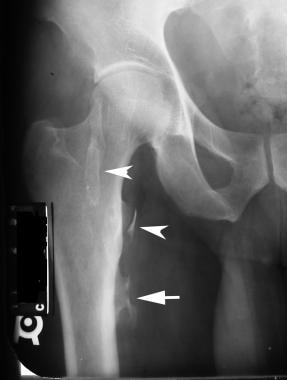 Radiograph in a patient with hemophilia. This image depicts a pseudotumor that is deforming the cortex of the femur (arrow). Other ossified masses in the soft tissues (arrowheads) are probably soft-tissue pseudotumors.
Radiograph in a patient with hemophilia. This image depicts a pseudotumor that is deforming the cortex of the femur (arrow). Other ossified masses in the soft tissues (arrowheads) are probably soft-tissue pseudotumors.
Knee involvement
The knee is the classic target joint. Involvement of this joint is most commonly described in the literature and is the basis for the findings described in the Arnold-Hilgartner classification (see the table below). [5]
The chronic joint effusions in hemophilia may be denser than other effusions because of the presence of iron. Juxta-articular osteoporosis develops, especially in children, secondary to the hyperemic state. The irregular appearance of the subchondral surface is secondary to either blood that directly destroys bone or to synovial intrusion.
Deeper invasion of the synovium and joint fluid leads to multiple subchondral cysts. Chronic hyperemia causes overgrowth of the epiphysis and widening of the intercondylar notch in the growing child. Squaring of the inferior pole of the patella (seen in 20-30% of patients with hemophilia) is another form of overgrowth. A similar effect of overgrowth may be seen in children with juvenile rheumatoid arthritis (JRA).
The Arnold-Hilgartner classification of hemophilic arthropathy is described in the table below.
Table. Arnold-Hilgartner classification [19] (Open Table in a new window)
Stage |
Findings |
0 |
Normal joint |
I |
No skeletal abnormalities, soft-tissue swelling is present |
II |
Osteoporosis and overgrowth of the epiphysis, no cysts, no narrowing of the cartilage space |
III |
Early subchondral bone cysts, squaring of the patella, widened notch of the distal femur or humerus, preservation of the cartilage space |
IV |
Findings of stage III, but more advanced; narrowed cartilage space |
V |
Fibrous joint contracture, loss of the joint cartilage space, extensive enlargement of the epiphysis, substantial disorganization of the joint |
Ankle involvement
Findings similar to those in the knee also develop in the ankle. With increased screening for joint abnormalities as part of an aggressive treatment regimen for patients with hemophilia, investigators have found that the ankle is more commonly involved as a target joint than the knee. The overgrowth pattern in the ankle leads to a condition called talar tilt, which is a tibiotalar slanting that is due to relative undergrowth of the lateral side of the tibial epiphysis and that leads to a pronated foot position. MRI shows the extension of ankle effusion-synovitis into the subtalar joint (see the following image).
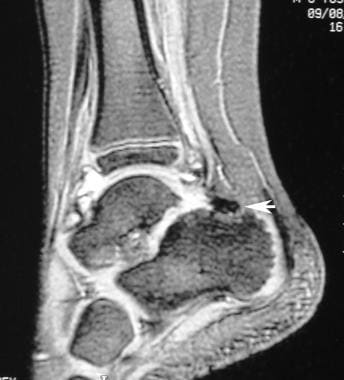 Sagittal magnetic resonance image of the ankle joint in a patient with hemophilia. This image shows extension of abnormal joint fluid from the ankle joint into the subtalar joint. Note the dark hemosiderin posterior to the subtalar fluid (arrow).
Sagittal magnetic resonance image of the ankle joint in a patient with hemophilia. This image shows extension of abnormal joint fluid from the ankle joint into the subtalar joint. Note the dark hemosiderin posterior to the subtalar fluid (arrow).
Elbow involvement
A radiopaque effusion is more easily seen on radiographs in the elbow than in the knee or ankle (see the image below). The trochlear notch may be widened. Enlargement of the radial head and limited elbow motion are present in advanced cases.
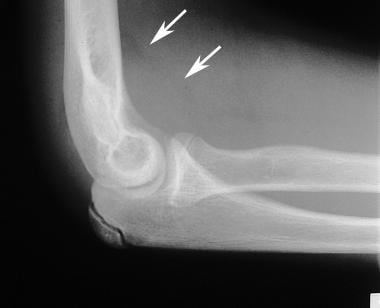 Lateral elbow radiograph in a patient with hemophilia. This image shows an opaque joint effusion due to the presence of iron in the synovium (arrows).
Lateral elbow radiograph in a patient with hemophilia. This image shows an opaque joint effusion due to the presence of iron in the synovium (arrows).
Hip involvement
The hip is not commonly a target joint, but because of the way the blood supply reaches the femoral head, osteonecrosis may occur after intra-articular bleeding does. Osteonecrosis may also occur in the shoulder or ankle (see the image below). Severe osteoporosis and spontaneous hip dislocation may develop. This distribution in the hips, shoulders, and ankles is probably related to the way the vascular supply enters the joint capsule and nourishes the underlying epiphysis.
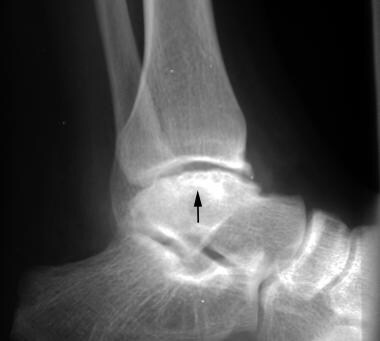 Radiograph of the ankle in a 20-year-old patient with hemophilia. This image shows the development of osteonecrosis (arrow) in the talar dome.
Radiograph of the ankle in a 20-year-old patient with hemophilia. This image shows the development of osteonecrosis (arrow) in the talar dome.
Wrist involvement
Wrist-joint deformity may occur from synovitis, but clinical wrist involvement is more common in juvenile rheumatoid arthritis than in hemophilia.
Shoulder involvement
As in the hip joint, osteonecrosis may occur in the shoulder joint (see the following image).
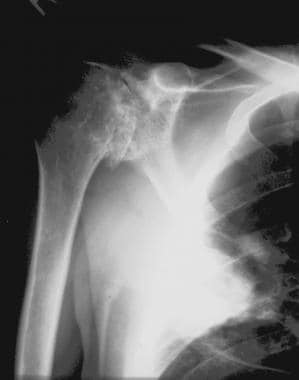 Radiograph of the shoulder of a 35-year-old man with hemophilia. This image shows advanced degenerative joint disease. The infiltrate in the lung is due to a fungal infection as a complication of the patient's positive human immunodeficiency virus (HIV) status.
Radiograph of the shoulder of a 35-year-old man with hemophilia. This image shows advanced degenerative joint disease. The infiltrate in the lung is due to a fungal infection as a complication of the patient's positive human immunodeficiency virus (HIV) status.
Preferred examination
Imaging tests such as plain radiography or magnetic resonance imaging (MRI) are helpful in defining the degree of joint destruction. MRI and US have been shown to provide information that is complementary to clinical evaluation, especially in patients with normal radiographs. [20, 21] The stages of altered anatomy are defined by classification systems that have been developed for plain radiographs and MRI. [19, 22, 23] Plain radiographic classification systems for describing the clinical progression of arthropathy were developed in the 1970s. However, radiographs may lead to the underestimation of soft tissue changes that precede bone destruction. Treatment of patients with hemophilia at an early age may prevent progression to the later, destructive changes seen on radiographs.
MRI has played a major role in defining such destructive soft-tissue changes and has led to early, aggressive treatment of affected patients. [22] Overall, the best imaging modality is MRI, because this technique combines excellent spatial resolution with the ability to detect soft-tissue bleeding at an early stage. In a given case, all of the other mentioned imaging modalities may play a role, but the first choice is MRI. Early arthropathic changes may be identified by T2 mapping MRI of cartilage before clinical symptoms become apparent. Cross-sectional imaging modalities such as CT, ultrasonography, and MRI may be useful in assessing bleeding-related musculoskeletal complications, such as pseudotumors. [24, 25]
MRI scales for measuring joint status in patients with hemophilia include the extended magnetic resonance imaging (eMRI) scale, the International Prophylaxis Study Group (IPSG) MRI scale, and the Colorado Adult Joint Assessment Scale (CAJAS). [26, 27, 28, 29, 30]
Ultrasonography offers an appealing alternative for assessing the joint fluid and synovial proliferation. [31, 9] Musculoskeletal US is valuable for point-of-care imaging to determine the presence of soft-tissue accumulation in discrete areas. However, musculoskeletal US may be unable to discriminate between coagulated blood, synovium, intrasynovial or extrasynovial fat tissue, or hemosiderin deposits because of wide variations in echogenicity. Because of the limitations of musculoskeletal US in discriminating the nature of pathologic soft tissues and detecting hemosiderin, MRI will be required if such discrimination is clinically important. [10, 25, 32, 33]
Development of the ultrasound protocol HEAD-US (Haemophilia Early Arthropathy Detection with Ultrasound ) by Martinoli et al in 2013 to detect early changes in hemophilic arthropathy provided a rapid way to perform and enable full screening of the 6 most commonly affected joints in one study. [7, 11, 34, 35, 36, 14, 37]
Differential diagnosis and other problems to be considered
Conditions in the differential diagnosis include ankle fractures, femoral head avascular necrosis, elbow trauma, juvenile rheumatoid arthritis, and pigmented villonodular synovitis. Trauma, scurvy, myeloproliferative disease, and anticoagulant overdose may cause acute hemarthrosis. Over time, juvenile rheumatoid arthritis causes joint deformities in a pattern that is similar to that of hemophilia, and pigmented villonodular synovitis produces hemosiderin deposition in the synovium similar to that of hemophilia. In addition, synovial hemangiomas may look like hemorrhagic synovitis. [18]
Radiologic intervention
MRI is used to detect early hemophilic disease and to help direct the appropriate therapy.
Radioactive injections into joints (radiosynoviorthesis) can control hemorrhage. This treatment was initially used in cases of juvenile rheumatoid arthritis. Subsequently, radiosynoviorthesis was shown to be effective in reducing bleeding and effusion in selected cases of hemophilic arthropathy.
Imaging may aid in needle placement for surgical total joint replacement, which is a valuable treatment for end-stage hemophilic disease. Soft-tissue pseudocysts may be drained percutaneously (with adequate factor replacement during the procedure).
According to data from 130 hemophilia treatment centers enrolled in the Universal Data Collection (UDC) program from 2000 to 2010, there was a 5.6% decrease in invasive orthopedic interventions in patients with hemophilia. [2]
Special concerns
Radionuclide synovectomy has a good record for low complication rates; however, there remains concern that the radiation exposure in young children could lead to radiation necrosis or tumor induction.
Both radiosynovectomy and arthroscopic synovectomy are effective in reducing the degree of synovitis and the number of hemarthroses, although there is little evidence that either prevents progression to end-stage arthritis. [38, 39]
Radiography
In 1977, Arnold and Hilgartner published a classification scheme for staging hemophilic arthropathy that has been widely used. [19, 6] The radiographic progression of disease is divided into 5 stages. The early changes of effusion and synovitis in hemophilic arthropathy are poorly seen on radiographs, and early soft-tissue abnormalities are not well depicted on radiographs. Radiographic findings of other diseases may mimic hemophilia in a given joint. (See the Table, above, as well as the images below).
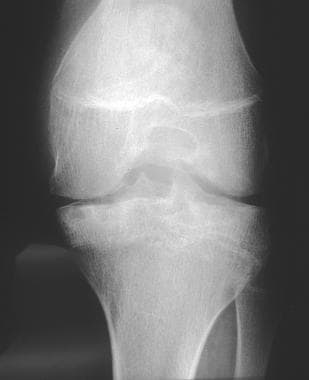 Radiograph of the leg in a patient with hemophilia. This image depicts stage III joint disease, as determined by the Arnold-Hilgartner classification.
Radiograph of the leg in a patient with hemophilia. This image depicts stage III joint disease, as determined by the Arnold-Hilgartner classification.
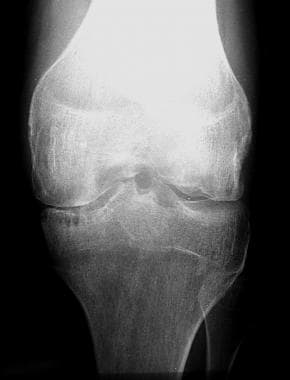 Radiograph of the leg in a patient with hemophilia. This image depicts stage IV joint disease, as determined by the Arnold-Hilgartner classification.
Radiograph of the leg in a patient with hemophilia. This image depicts stage IV joint disease, as determined by the Arnold-Hilgartner classification.
Other radiographic images are shown below
 Knee radiograph in a 37-year-old man with moderate factor IX hemophilia. This image shows bony excrescence on the lateral side of the femur is a hemophilic pseudotumor.
Knee radiograph in a 37-year-old man with moderate factor IX hemophilia. This image shows bony excrescence on the lateral side of the femur is a hemophilic pseudotumor.
 Radiograph of the lower extremity of the 3-year-old daughter of the patient in the previous image. This image shows talar tilt. The girl not only inherited 1 diseased X chromosome with mild factor IX hemophilia from her father, but she also has Turner (XO) syndrome. The child's only X chromosome had the hemophilia gene.
Radiograph of the lower extremity of the 3-year-old daughter of the patient in the previous image. This image shows talar tilt. The girl not only inherited 1 diseased X chromosome with mild factor IX hemophilia from her father, but she also has Turner (XO) syndrome. The child's only X chromosome had the hemophilia gene.
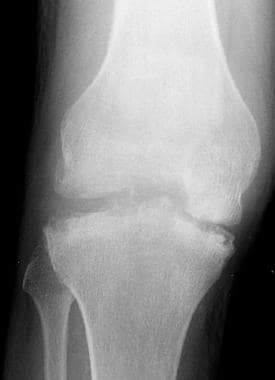 Anteroposterior radiograph from a patient with hemophilia. This image demonstrates hemarthrosis with hemosiderin deposition. Note the irregularity of the articular surfaces and the presence of subchondral sclerosis with cysts. Image courtesy of Javier Beltran, MD.
Anteroposterior radiograph from a patient with hemophilia. This image demonstrates hemarthrosis with hemosiderin deposition. Note the irregularity of the articular surfaces and the presence of subchondral sclerosis with cysts. Image courtesy of Javier Beltran, MD.
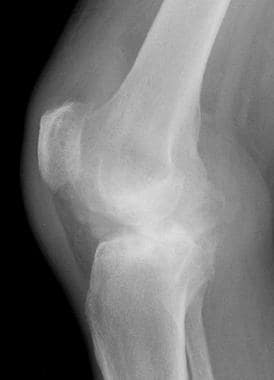 Lateral radiograph from the same patient as in the previous image. This image shows a large hemarthrosis that is distending the suprapatellar recess. Image courtesy of Javier Beltran, MD.
Lateral radiograph from the same patient as in the previous image. This image shows a large hemarthrosis that is distending the suprapatellar recess. Image courtesy of Javier Beltran, MD.
 Lateral elbow radiograph in a patient with hemophilia. This image shows an opaque joint effusion due to the presence of iron in the synovium (arrows).
Lateral elbow radiograph in a patient with hemophilia. This image shows an opaque joint effusion due to the presence of iron in the synovium (arrows).
 Radiograph of the ankle in a 20-year-old patient with hemophilia. This image shows the development of osteonecrosis (arrow) in the talar dome.
Radiograph of the ankle in a 20-year-old patient with hemophilia. This image shows the development of osteonecrosis (arrow) in the talar dome.
 Radiograph of the shoulder of a 35-year-old man with hemophilia. This image shows advanced degenerative joint disease. The infiltrate in the lung is due to a fungal infection as a complication of the patient's positive human immunodeficiency virus (HIV) status.
Radiograph of the shoulder of a 35-year-old man with hemophilia. This image shows advanced degenerative joint disease. The infiltrate in the lung is due to a fungal infection as a complication of the patient's positive human immunodeficiency virus (HIV) status.
Computed Tomography
Masses that develop in a patient with hemophilia can be evaluated by computed tomography (CT) scanning, which is good for defining pseudotumors, whether they occur in soft tissues, in cortical bone, or in the medullary cavity (see the following image). Soft-tissue hemorrhage that causes neurovascular compromise can also be evaluated with this modality. In particular, pathology in bone, such as an osseous pseudotumor, is well seen with CT scanning. Soft-tissue changes are better seen with MRI than with CT scanning.
Using high-resolution peripheral quantitative computed tomography (HR-pQCT), Lee et al demonstrated the presence of low trabecular and cortical bone density contributing to lower volumetric bone mineral density at both the distal radius and tibia in patients with hemophilia, as compared with age- and sex-matched controls. The authors noted that low bone density is a growing concern in aging men with hemophilia and may result in high-morbidity fragility fractures. [40]
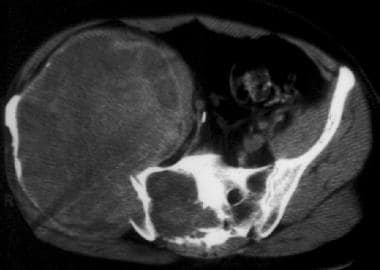 Computed tomography scan of the pelvis in a patient with hemophilia. This image demonstrates a giant pseudotumor (a large expansile lytic lesion that involves the right iliac bone and extends into the sacrum, with inhomogeneous internal attenuation). Image courtesy of Javier Beltran, MD.
Computed tomography scan of the pelvis in a patient with hemophilia. This image demonstrates a giant pseudotumor (a large expansile lytic lesion that involves the right iliac bone and extends into the sacrum, with inhomogeneous internal attenuation). Image courtesy of Javier Beltran, MD.
Magnetic Resonance Imaging
Soft-tissue changes are depicted well on MRI, including joint effusion, hemarthrosis, synovitis, and hemosiderin deposition (see the images below). MRI offers excellent soft-tissue contrast resolution and spatial resolution, and special techniques have been developed to improve the visualization of articular cartilage. MRI scales for measuring joint status in patients with hemophilia include the extended magnetic resonance imaging (eMRI) scale, the International Prophylaxis Study Group (IPSG) MRI scale, and the Colorado Adult Joint Assessment Scale (CAJAS). [22, 24, 25, 26, 27, 28, 29, 30]
 Magnetic resonance image from a patient with hemophilia. This image shows dark, synovial masses that erode the cartilage and produce subchondral cysts (arrows).
Magnetic resonance image from a patient with hemophilia. This image shows dark, synovial masses that erode the cartilage and produce subchondral cysts (arrows).
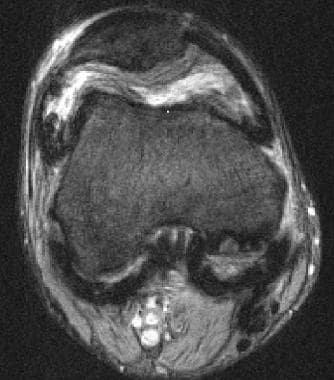 Axial gradient-echo magnetic resonance image from a patient with hemophilia. This image demonstrates hemosiderin deposition in the synovium as hypointense material that outlines the joint capsule. Image courtesy of Javier Beltran, MD.
Axial gradient-echo magnetic resonance image from a patient with hemophilia. This image demonstrates hemosiderin deposition in the synovium as hypointense material that outlines the joint capsule. Image courtesy of Javier Beltran, MD.
 Sagittal magnetic resonance image of the ankle joint in a patient with hemophilia. This image shows extension of abnormal joint fluid from the ankle joint into the subtalar joint. Note the dark hemosiderin posterior to the subtalar fluid (arrow).
Sagittal magnetic resonance image of the ankle joint in a patient with hemophilia. This image shows extension of abnormal joint fluid from the ankle joint into the subtalar joint. Note the dark hemosiderin posterior to the subtalar fluid (arrow).
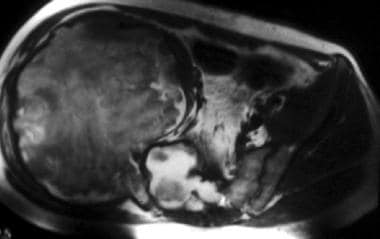 Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates a large pseudotumor of the pelvis with sacral extension. Image courtesy of Javier Beltran, MD.
Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates a large pseudotumor of the pelvis with sacral extension. Image courtesy of Javier Beltran, MD.
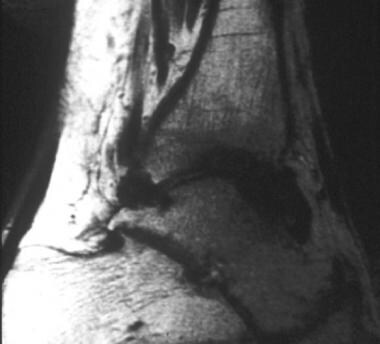 Sagittal T2-weighted magnetic resonance image of the lower extremity in a patient with hemophilia. This image demonstrates hemosiderin-laden synovium with low signal intensity that outlines the capsule of the tibiotalar joint. Image courtesy of Javier Beltran, MD.
Sagittal T2-weighted magnetic resonance image of the lower extremity in a patient with hemophilia. This image demonstrates hemosiderin-laden synovium with low signal intensity that outlines the capsule of the tibiotalar joint. Image courtesy of Javier Beltran, MD.
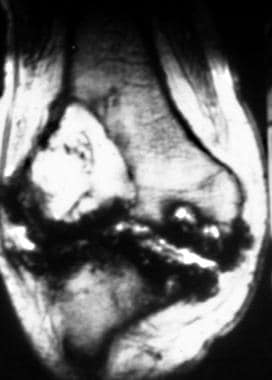 Coronal T2-weighted magnetic resonance image of the knee in a patient with hemophilia. This image demonstrates pseudotumor of the knee, a lytic lesion in the lateral femoral condyle, as well as the characteristic manifestations of hemosiderin deposition in the knee joint and the subchondral cystic changes in the medial femoral condyle. Image courtesy of Javier Beltran, MD.
Coronal T2-weighted magnetic resonance image of the knee in a patient with hemophilia. This image demonstrates pseudotumor of the knee, a lytic lesion in the lateral femoral condyle, as well as the characteristic manifestations of hemosiderin deposition in the knee joint and the subchondral cystic changes in the medial femoral condyle. Image courtesy of Javier Beltran, MD.
Effusion and synovitis may develop from causes other than hemophilia, such as trauma or infection. In patients with pigmented villonodular synovitis, hemosiderin deposition may look exactly like that which is caused by hemophilia in a given joint. Juvenile rheumatoid arthritis can cause knee deformity in a way that is similar to that in hemophilia, and bleeding may cause ectopic ossification in the soft tissues that looks like posttraumatic myositis ossificans.
Masses are especially common in the pelvis. Starker described these masses as pseudotumors in 1918; they are seen in 2% of patients with hemophilia. [18]
The 3 forms of pseudotumor are intraosseous, subperiosteal (or cortical), and soft tissue. The intraosseous form is most common in the femur, pelvic bones, tibia, and hand bones; the lesions can be variably sized. The pseudotumor is usually well demarcated, but it may also be bubbly and destructive. The lesion may simulate malignancy such as that from Ewing sarcoma, metastasis, or infection because of the pseudotumor's aggressive appearance. The subperiosteal type leads to cortical atrophy, subperiosteal new-bone formation, and soft-tissue extension. This is seen most commonly in the fibula. The soft-tissue form of the mass is surrounded by a fibrous capsule and may cause deformity of the adjacent bone.
Regarding other findings, calcification in hemosiderin may simulate other calcified masses on MRIs. Chondrocalcinosis occurs more commonly in calcium pyrophosphate deposition disease or primary hyperparathyroidism than in hemophilia. Septic arthritis can develop in a child with hemophilia; this may be a problem in early diagnosis. Contracture of soft tissues around joints may cause impingement on blood vessels or nerves.
Ultrasonography
Soft tissues are seen well with ultrasonography. [41] A mass that is suspected of being a joint effusion or a soft-tissue pseudotumor can be demonstrated quickly with this modality. [9] If the acoustic window is adequate, the finding of a sonolucent mass on ultrasonography is highly suggestive of fluid. Mixed signal intensity is likely due to a soft-tissue mass, such as a hematoma. Ultrasound has excellent spatial resolution and can depict the small, superficial structures of the musculoskeletal system present in the early stages of hemophilic arthropathy. [11]
Development of the ultrasound protocol HEAD-US (Haemophilia Early Arthropathy Detection with Ultrasound ) by Martinoli et al in 2013 to detect early changes in hemophilic arthropathy provided a rapid way to perform and enable full screening of the 6 most commonly affected joints in one study. [7, 11, 34, 35, 36, 14, 37]
The inability to place the ultrasound transducer over the area of interest precludes scanning. In addition, not only does bone block the sound waves and limit the ability to see subchondral cysts and erosions, but cartilage cannot be adequately differentiated from bone.
Nuclear Imaging
Bone scans are highly sensitive for detecting areas of increased osteoblastic activity; the scans are useful for surveying the entire skeleton for disease. Follow-up bone scans can be used to determine the effectiveness of a patient's treatment. Radioisotopes, such as phosphorus-32 (32P), can be injected therapeutically into a joint to decrease the amount of bleeding/effusion. [42]
Bone scans combine high sensitivity with low specificity. Therefore, a negative bone scan should exclude acute joint hemorrhage or synovitis.
The lack of specificity of bone scans makes it difficult to determine the exact cause of a positive bone scan. The findings are more helpful in the acute phase of the disease than at other times.
Questions & Answers
Overview
What is the pathophysiology of hemophilia?
What is the pathologic-anatomic appearance characteristic of hemophilia?
Which imaging findings of the knee are characteristic of hemophilia?
What is the Arnold-Hilgartner classification of hemophilic arthropathy?
Which imaging findings of the ankle are characteristic of hemophilia?
Which imaging findings of the elbow are characteristic of hemophilia?
Which imaging findings of the hip are characteristic of hemophilia?
Which imaging findings of the wrist are characteristic of hemophilia?
Which imaging findings of the shoulder are characteristic of hemophilia?
Which imaging modalities are used in the workup of hemophilia?
What are limitations of imaging in the workup of hemophilia?
Which conditions are included in the differential diagnoses of hemophilia?
What is the role of radiologic interventions in the treatment of hemophilia?
What is the role of radiography in the workup of hemophilia?
What is the role of CT scanning in the workup of hemophilia?
What is the role of MRI in the workup of hemophilia?
What is the role of ultrasonography in the workup of hemophilia?
What is the role of nuclear imaging in the workup of hemophilia?
What is the role of angiography in the workup of hemophilia?
-
Knee radiograph in a 37-year-old man with moderate factor IX hemophilia. This image shows bony excrescence on the lateral side of the femur is a hemophilic pseudotumor.
-
Radiograph of the lower extremity of the 3-year-old daughter of the patient in the previous image. This image shows talar tilt. The girl not only inherited 1 diseased X chromosome with mild factor IX hemophilia from her father, but she also has Turner (XO) syndrome. The child's only X chromosome had the hemophilia gene.
-
Magnetic resonance image from a patient with hemophilia. This image shows dark, synovial masses that erode the cartilage and produce subchondral cysts (arrows).
-
Anteroposterior radiograph from a patient with hemophilia. This image demonstrates hemarthrosis with hemosiderin deposition. Note the irregularity of the articular surfaces and the presence of subchondral sclerosis with cysts. Image courtesy of Javier Beltran, MD.
-
Lateral radiograph from the same patient as in the previous image. This image shows a large hemarthrosis that is distending the suprapatellar recess. Image courtesy of Javier Beltran, MD.
-
Axial gradient-echo magnetic resonance image from a patient with hemophilia. This image demonstrates hemosiderin deposition in the synovium as hypointense material that outlines the joint capsule. Image courtesy of Javier Beltran, MD.
-
Radiograph in a patient with hemophilia. This image depicts a pseudotumor that is deforming the cortex of the femur (arrow). Other ossified masses in the soft tissues (arrowheads) are probably soft-tissue pseudotumors.
-
Sagittal magnetic resonance image of the ankle joint in a patient with hemophilia. This image shows extension of abnormal joint fluid from the ankle joint into the subtalar joint. Note the dark hemosiderin posterior to the subtalar fluid (arrow).
-
Computed tomography scan of the pelvis in a patient with hemophilia. This image demonstrates a giant pseudotumor (a large expansile lytic lesion that involves the right iliac bone and extends into the sacrum, with inhomogeneous internal attenuation). Image courtesy of Javier Beltran, MD.
-
Axial T2-weighted magnetic resonance image of the pelvis. This image demonstrates a large pseudotumor of the pelvis with sacral extension. Image courtesy of Javier Beltran, MD.
-
Lateral elbow radiograph in a patient with hemophilia. This image shows an opaque joint effusion due to the presence of iron in the synovium (arrows).
-
Radiograph of the ankle in a 20-year-old patient with hemophilia. This image shows the development of osteonecrosis (arrow) in the talar dome.
-
Sagittal T2-weighted magnetic resonance image of the lower extremity in a patient with hemophilia. This image demonstrates hemosiderin-laden synovium with low signal intensity that outlines the capsule of the tibiotalar joint. Image courtesy of Javier Beltran, MD.
-
Radiograph of the shoulder of a 35-year-old man with hemophilia. This image shows advanced degenerative joint disease. The infiltrate in the lung is due to a fungal infection as a complication of the patient's positive human immunodeficiency virus (HIV) status.
-
Radiograph of the leg in a patient with hemophilia. This image depicts stage III joint disease, as determined by the Arnold-Hilgartner classification.
-
Radiograph of the leg in a patient with hemophilia. This image depicts stage IV joint disease, as determined by the Arnold-Hilgartner classification.
-
Coronal T2-weighted magnetic resonance image of the knee in a patient with hemophilia. This image demonstrates pseudotumor of the knee, a lytic lesion in the lateral femoral condyle, as well as the characteristic manifestations of hemosiderin deposition in the knee joint and the subchondral cystic changes in the medial femoral condyle. Image courtesy of Javier Beltran, MD.