Practice Essentials
Asbestos is the name given to a group of naturally occurring minerals that are resistant to heat and corrosion. Asbestos includes the mineral fibers chrysotile, amosite, crocidolite, tremolite, anthophyllite, and actinolite, and any of these materials that have been chemically treated or altered. Chrysotile is by far the most common type of asbestos fiber produced in the world and accounts for virtually all asbestos used commercially in the United States. [1]
Exposure to asbestos can cause both benign and malignant, pulmonary and pleural diseases. It is critical to be aware of complications from asbestos exposure, which often arise decades after exposure. Asbestos-related pulmonary complications include asbestosis, pleural plaques, diffuse pleural thickening, benign asbestos-related pleural effusions, and malignant pleural mesothelioma. [2]
The main preventable cause of malignant mesothelioma has been exposure to commercial materials made or contaminated with asbestos. The most common exposure to commercial asbestos is occupational, although workers' families are also at risk from indirect “take-home” exposures transported by contaminated items such as clothing. Contamination of the living environment from asbestos-containing products is another source of exposure. [1]
Asbestos has been used in products such as insulation for pipes, floor tiles, building materials, and vehicle brakes and clutches. Heavy exposures tend to occur in the construction industry and in ship repair, particularly during removal of asbestos materials due to renovation, repairs, or demolition. Workers are also likely to be exposed during the manufacture of asbestos products (eg, textiles, friction products, insulation, other building materials) and during automotive brake and clutch repair work. Exposure to asbestos occurs through inhalation of fibers in air in the working environment, ambient air in the vicinity of factories handling asbestos, or indoor air in housing and buildings containing asbestos materials. Workers in this population are at increased risk despite modest tobacco exposure. Low-dose computed tomography (LDCT) screening is effective for identifying early-stage lung cancer in these individuals. Asbestos with or without tobacco smoke exposure is a major risk factor for lung cancer. [3]
Because the development of asbestosis is dose dependent, symptoms appear only after a latent period of 20 years or longer. This latent period may be shorter after intense exposure.
(Case studies of asbestos-related disease are illustrated in the images below.)
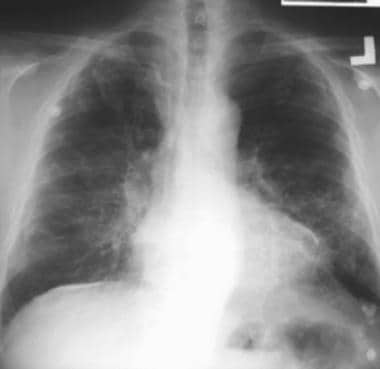 Case 1. Posteroanterior (PA) chest radiograph in a 58-year-old man with a history of occupational exposure to asbestos shows right diaphragmatic pleural plaque calcifications, linear calcification along the left pericardium, and bilateral pleural plaques along upper ribs.
Case 1. Posteroanterior (PA) chest radiograph in a 58-year-old man with a history of occupational exposure to asbestos shows right diaphragmatic pleural plaque calcifications, linear calcification along the left pericardium, and bilateral pleural plaques along upper ribs.
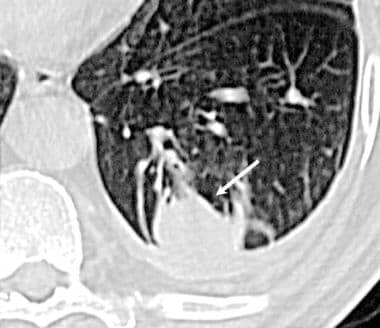 Case 2. An asymptomatic man (>50 y) was noted to have a mass in the left lower lobe after exposure to asbestos. High-resolution computed tomography (HRCT) scan demonstrates a round mass at a site of pleural thickening, with a comet-tail bronchovascular bundle. This is an appearance of a folded lung (round atelectasis). The soft tissue window shows parenchymal enhancement.
Case 2. An asymptomatic man (>50 y) was noted to have a mass in the left lower lobe after exposure to asbestos. High-resolution computed tomography (HRCT) scan demonstrates a round mass at a site of pleural thickening, with a comet-tail bronchovascular bundle. This is an appearance of a folded lung (round atelectasis). The soft tissue window shows parenchymal enhancement.
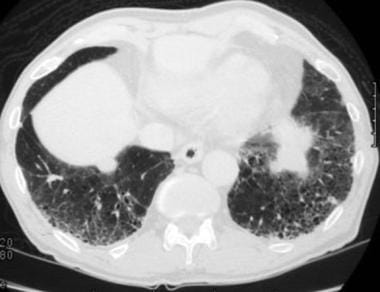 Case 3. A 55-year-old former asbestos worker has been complaining of shortness of breath. High-resolution computed tomography (HRCT) scan obtained at the lung bases shows prominent interstitial septal lines, subpleural cysts, and pleural plaques. This has the appearance of asbestosis.
Case 3. A 55-year-old former asbestos worker has been complaining of shortness of breath. High-resolution computed tomography (HRCT) scan obtained at the lung bases shows prominent interstitial septal lines, subpleural cysts, and pleural plaques. This has the appearance of asbestosis.
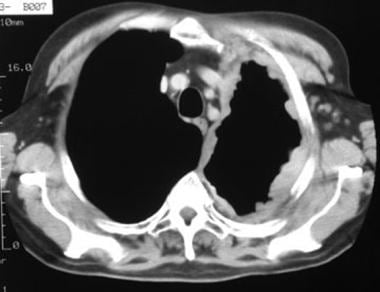 Case 4. The soft tissue window setting of this chest computed tomography (CT) scan shows the envelope-like mass along the pleural surface surrounding the lung. This was a mesothelioma.
Case 4. The soft tissue window setting of this chest computed tomography (CT) scan shows the envelope-like mass along the pleural surface surrounding the lung. This was a mesothelioma.
Knowledge of asbestos-related diseases accumulated for over 100 years as the industrial value of asbestos became recognized for the strength of its fibers and their resistance to destruction, resulting in increasing production and use until multiple health effects became apparent. Deposition in the lung parenchyma results in an inflammatory/progressively fibrotic response, with impaired gas exchange and reduced lung compliance (ie, asbestosis), causing progressive dyspnea and respiratory failure for which only palliation is indicated. Benign pleural effusion, diffuse pleural fibrosis, and pleural plaques are the nonmalignant pleural diseases that occur when fibers reach the pleura. However, the primary issues that led to the ban on asbestos are malignancy and include lung cancer, malignant mesothelioma (MM) of the pleura, and MM of the peritoneum. Risk of bronchogenic carcinoma from asbestos exposure is dose dependent and multiplies patient risk attributable to tobacco smoking. [4]
The spectrum of asbestos-related thoracic diseases includes the following [5] :
-
Benign pleural effusion
-
Pleural plaques [6]
-
Diffuse pleural thickening
-
Rounded atelectasis
-
Asbestosis
-
Mesothelioma
-
Lung cancer
Helsinki guidelines for the diagnosis of asbestos-related disorders define asbestosis as diffuse interstitial fibrosis of the lung as a consequence of exposure to asbestos dust. [7] Mesothelioma is a malignant pleural or peritoneal tumor that rarely occurs in patients who have not been exposed to asbestos. [8, 9, 10, 11, 12, 13]
Mesothelioma is a cancer predominantly of the pleural cavity. Exposure to asbestos has been clearly associated with a dose-dependent risk of mesothelioma. The incidence of mesothelioma in different countries reflects the historical patterns of commercial asbestos utilization over the last century. Modern imaging techniques and advances in immunohistochemical staining have contributed to improved diagnosis of mesothelioma. Advances also include immune checkpoint inhibition; however, mesothelioma remains very challenging to manage, especially because of its limited response to conventional systemic anticancer therapies and the fact that there is no cure. Palliative interventions and support remain paramount; median survival of 9-12 months after diagnosis has been reported. [14, 15]
The diagnostic approach to asbestos-related intrathoracic disease is different from that of other diffuse lung diseases because of the medicolegal implications. [16] The likelihood of asbestos-related disease should be determined, and other possible causes should be eliminated. Assessment of the extent of disease is used to calculate compensation. Therefore, imaging plays a pivotal role in the diagnosis, staging, response assessment, and surveillance of asbestos-related disease. [17]
The key clinical goals of imaging in malignant pleural mesothelioma are early detection of disease; optimized sensitivity and specificity for anatomic involvement of unresectable planes to identify patients who are suitable for surgical resection; improved prognostication; and assessment of response to treatment as a surrogate for therapeutic benefit. [18]
Imaging modalities
High-resolution computed tomography (HRCT) scanning is playing an increasingly important role in the diagnosis of diffuse interstitial lung disease. However, chest radiography remains the initial modality for detection and characterization of pleural and parenchymal disease. Ultrasonography has a role in characterizing pleural effusions and in guiding pleural aspiration and biopsy.
Nuclear medicine study has a limited role in the investigation of asbestos-related intrathoracic disease. Gallium-67 (67Ga) citrate testing has been used to differentiate benign from malignant asbestos-related pleural disease and to obtain a quantitative index of inflammatory activity. [19]
Imaging of mesothelioma typically includes computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) scans; these scans may be acquired during initial tumor diagnosis and staging, treatment response assessment, or patient surveillance.
Optical imaging using electromagnetic radiation in (or near) the visible light region of the spectrum is now being applied in the intraoperative setting for mesothelioma. Preliminary results reveal the potential for optical imaging to aid surgeons in their attempt to achieve a macroscopic complete resection. [18]
Ultrasonography is useful in characterizing pleural effusions and in evaluating pleural thickening or masses. Ultrasonography also facilitates image-guided pleural intervention.
Ultrasonography has been used to help evaluate round atelectasis and has been shown to reliably demonstrate the mass and adjacent pleural thickening. The presence of a highly echogenic line within the mass, which represents invaginated fibrotic pleura, is described as a useful ancillary finding and is seen in 86% of patients.
Helsinki guidelines for the diagnosis of asbestos-related disorders recommend the use of CT imaging in diagnosis of asbestos-related diseases under the following circumstances [7, 20] :
-
A borderline finding of lung fibrosis (ILO 0/1-1/0) is detected.
-
A discrepancy is evident between the lung function finding of restriction and radiographs interpreted as normal.
-
Widespread pleural changes severely hamper the radiographic visibility of lung parenchyma.
Criteria for diagnosis of asbestosis on CT include the following:
-
Sum grade of ≥2-3 bilateral irregular opacities in lower zones according to the reference film.
OR
-
Bilateral honeycombing (sum grade ≥2) sufficient to represent fibrosis according to the International Classification of HRCT for Occupational and Environmental Respiratory Diseases (ICOERD).
These guidelines further note that subpleural curvilinear lines or dots in HRCT are findings of bronchiolar fibrosis. [7]
According to the Austrian Mesothelioma Interest Group (AMIG) consensus statement for the diagnosis and staging of malignant pleural mesothelioma, after initial imaging with a CT scan and confirmation of disease via video-assisted thoracic surgery (VATS), a potential candidate for surgical treatment should undergo PET-CT scanning to rule out distant metastases and involvement of the abdomen and mediastinal lymph nodes. In some cases of unclear involvement of adjacent structures (eg, chest wall), MRI can be added to discern resectability. [21]
Limitations of techniques
The limitations of chest radiography in the diagnosis and evaluation of asbestos-related disease are well recognized. The quality of the radiograph and the size, shape, position, and degree of calcification determine whether the radiologist can detect pleural plaques on the image. Although identification of bilateral, scattered, calcified, costal, and diaphragmatic pleural plaques is virtually diagnostic of asbestos exposure, studies have shown an 11% false-positive rate with chest radiographs. In particular, extrapleural fat mimics pleural thickening and is a significant cause of false-positive readings. Conversely, a high false-negative rate has been reported.
Computed tomography scans have long been known to be more sensitive and specific than chest radiographs for the diagnosis of asbestos-related pleural disease. [22, 23]
Radiographic-pathologic studies have shown that chest radiographic findings are normal in as many as 20% of patients with asbestosis. High-resolution CT is more sensitive and specific than other studies, particularly when images are obtained with the patient in the prone position, which allows differentiation of mild parenchymal changes from dependent density (increased attenuation of the posterior [usually basal] lung, which is gravity induced and secondary to nonaeration of dependent alveoli). [24]
In addition, it should be noted that mild asbestosis is difficult to distinguish from interstitial fibrosis in heavy smokers. In a report of 24 patients judged radiographically to have absestosis, only 6 had asbestosis histopathologically, and the remaining 18 cases (patients with a mean smoking history of 53 pack-years) had interstitial fibrosis judged to be most consistent with smoking-associated fibrosis. [25]
Tumor contrast enhancement in MRI provides information about tumor vascularity, but the conventional time delay between contrast injection and initiation of image acquisition might be too short for optimal assessment of mesothelioma. A perfusion-based MRI technique, designed to capture early contrast-enhancement features of the pleura characteristic of early-stage mesothelioma, may enhance the role of MRI in tumor detection. [18]
Radiography
Chest radiography remains the initial modality for detection and characterization of pleural and parenchymal disease. Benign pleural plaques may be seen in profile or en face. In profile, plaques appear as focal, smooth opacities, usually less than 1 cm thick, paralleling the chest wall. Appearances en face are of a more ill-defined opacity with irregular margins.
Plaques may occur as isolated abnormalities or in association with lung parenchymal involvement. Most pleural plaques are multiple and bilateral; they often are symmetrical and are located in the midportion of the chest wall between the seventh and tenth ribs, following rib contours, or adjacent to the aponeurotic portion of the diaphragm and the vertebral column. Visceral pleurae, lung apices, and costophrenic angles typically are spared.
Pleural calcification
On chest radiographs, the prevalence of calcification in pleural plaques is reported to be 10-15%. In profile, calcified plaques appear as opaque lines that lie parallel to the chest wall, mediastinum, pericardium, and diaphragm. Viewed en face, calcified plaques are seen as irregular, heterogeneous densities, the so-called holly leaf. The presence of bilateral superior diaphragmatic surface calcifications with clear costophrenic angles is virtually pathognomonic for asbestos-related pleural disease. (See the image below.)
 Case 1. Posteroanterior (PA) chest radiograph in a 58-year-old man with a history of occupational exposure to asbestos shows right diaphragmatic pleural plaque calcifications, linear calcification along the left pericardium, and bilateral pleural plaques along upper ribs.
Case 1. Posteroanterior (PA) chest radiograph in a 58-year-old man with a history of occupational exposure to asbestos shows right diaphragmatic pleural plaque calcifications, linear calcification along the left pericardium, and bilateral pleural plaques along upper ribs.
Diffuse pleural thickening
Asbestos-related diffuse pleural thickening is defined as a smooth, uninterrupted pleural opacity extending over at least one quarter of the chest wall, with or without obliteration of the costophrenic angles.
Pleural thickening may be difficult to diagnose using conventional chest radiographs, and differentiation between diffuse thickening and focal pleural plaques may be problematic. However, diffuse pleural thickening due to asbestos exposure rarely calcifies, tends to involve costophrenic angles (unlike plaques), tends to be ill defined rather than sharply marginated, and is more extensive than focal plaques. Involvement of interlobar fissures is not uncommon. Diffuse pleural thickening is more often associated with radiographically detectable asbestosis than are pleural plaques.
Benign asbestos-related pleural effusions
Benign asbestos-related effusions have the same radiographic appearance as effusions due to other causes; the diagnosis is usually one of exclusion. (See the image below.)
 Case 4. A 67-year-old man with a history of occupational exposure to asbestos for decades began experiencing a nagging, left-sided chest pain. Posteroanterior (PA) chest radiograph shows a left pleural effusion and peripheral, left-sided nodules.
Case 4. A 67-year-old man with a history of occupational exposure to asbestos for decades began experiencing a nagging, left-sided chest pain. Posteroanterior (PA) chest radiograph shows a left pleural effusion and peripheral, left-sided nodules.
Effusions usually are small and may be unilateral or bilateral; they tend to resolve spontaneously over 3-4 months, although as many as 30% recur. Some effusions are associated with pleural plaques.
Effusions tend to resolve over a period ranging from 1 month to 1 year, with residual blunting of costophrenic angles due to pleural thickening in 50% of patients.
Asbestosis
Asbestosis is defined as interstitial pulmonary fibrosis secondary to the presence of intrapulmonary asbestos bodies or asbestos fibers. Fibrosis is usually most severe in the subpleural lower zones.
Chest radiographic findings include fine reticular opacities and septal lines that progress toward a coarser linear pattern of honeycombing.
With advanced disease, the heart border and diaphragmatic contours become ill defined—the so-called shaggy heart.
Round atelectasis
Round atelectasis appears as a well-defined, rounded, focal, subpleural soft tissue mass 2-7 cm in diameter that abuts an area of pleural thickening. Most atelectases are located in the posterolateral or posteromedial parts of the lower lobes. Occasionally, bilateral masses are seen. Mild volume loss may be associated with the condition.
Appearances usually remain stable over time, but occasionally, masses may increase or decrease in size.
Bronchogenic carcinoma associated with asbestos exposure
Appearances of bronchogenic carcinoma are those of non–asbestos-related lung cancers. The most common radiographic finding is a pulmonary mass with associated mediastinal lymphadenopathy.
Lung cancer in asbestos-related disease tends to occur at the lung bases but can arise in any location if also associated with smoking.
Malignant mesothelioma
Mesothelioma typically appears as irregular, nodular, diffuse pleural thickening, occasionally associated with pleural effusion. Less commonly, an isolated effusion or pleural mass is seen.
The tumor progresses to encase the entire hemithorax, including the lung. The mediastinum usually is fixed or is shifted toward the side of the tumor.
Evidence of other asbestos-related disease such as plaque is seen in only approximately 20-25% of patients with malignant mesothelioma. Patients rarely may present with spontaneous pneumothorax.
Degree of confidence
The presence of bilateral calcified pleural plaques can be considered diagnostic of asbestos exposure. [26, 27]
Unfortunately, the value of chest radiography is limited. Upon reviewing a series of 200 radiographs, Epstein and colleagues found that images in 18% of patients showed small opacities. [28] This finding was consistent with pneumoconiosis, according to the International Labour Office (ILO) classification. Among these patients, 61% had no known occupational exposure to asbestos. Furthermore, as many as 20% of patients with histologically proven asbestos-related lung disease had normal chest radiographic findings, and 80% with radiographic findings of mild disease had histologic results of moderate or severe fibrosis.
The extent of malignant mesothelioma is frequently underestimated with chest radiography.
False-positive, false-negative, and interobserver variability rates are relatively high when chest radiographs are evaluated for pleural plaques. Prominent subpleural fat or normal rib companion shadows may mimic focal or diffuse pleural thickening, leading to false-positive diagnoses in as many as 20% of patients.
Plaques may be difficult to differentiate radiographically from diffuse pleural thickening. Plaques usually spare the costophrenic angles and apices and rarely extend over more than 4 rib interspaces; however, diffuse pleural thickening rarely calcifies and usually is more irregular and ill defined.
Extensive pleural calcification usually suggests other etiologies such as talc exposure, hemothorax, empyema, and therapeutic pneumothorax. Bilateral diaphragmatic calcification with costophrenic angle sparing is considered pathognomonic for asbestos-related disease.
The differential diagnosis of predominantly basal subpleural fibrosis includes idiopathic pulmonary fibrosis (cryptogenic fibrosing alveolitis or usual interstitial pneumonitis), drug reactions, and connective tissue disease.
The most important differential diagnosis of round atelectasis is bronchogenic carcinoma. Biopsy may be necessary. Round atelectasis is not specific for asbestos exposure and may be preceded by pulmonary infarction, Dressler syndrome, heart failure, and nonspecific pleural effusions.
No definitive evidence links benign asbestos-related pleural effusion to the development of malignant mesothelioma; however, malignant mesothelioma is an important differential diagnosis for benign asbestos-related effusion. Specific criteria for the diagnosis of a benign asbestos-related effusion include (1) history of asbestos exposure, (2) elimination of other etiologies of an effusion, and (3) absence of malignancy for 3 years after onset of the effusion.
Although benign asbestos-related effusion and mesothelioma are related to asbestos exposure, effusion tends to occur earlier with benign asbestos-related effusion than with mesothelioma, with onset approximately 10 years after exposure for the former and 20-40 years after exposure for the latter.
Computed Tomography
Computed tomography (CT) scanning has been established as the criterion standard in the evaluation of pleural disease (see the image below). [7] Developments in HRCT scanning have made it an invaluable tool for assessment of asbestosis. [29, 30, 31, 32, 33, 34] Scanning the patient in the prone and supine positions increases sensitivity and specificity. [24]
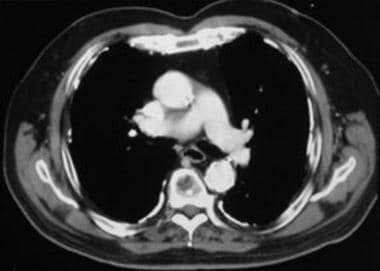 Case 1. Contrast-enhanced computed tomography (CT) scan of the chest at the level of the pulmonary artery bifurcation shows calcified pleural plaques along the posterior, lateral, and anterior pleural surfaces.
Case 1. Contrast-enhanced computed tomography (CT) scan of the chest at the level of the pulmonary artery bifurcation shows calcified pleural plaques along the posterior, lateral, and anterior pleural surfaces.
Focal pleural plaques
Plaques appear as discrete, well-defined areas of localized pleural thickening. They usually are multiple, bilateral, and located adjacent to rigid structures such as the ribs, the midportion of the chest, the aponeurotic portion of the diaphragm, the mediastinum, and paravertebral regions. Lung apices and costophrenic angles typically are spared. Rarely, the visceral pleura within fissures is involved, or plaques may be pedunculated. (See the image below.)
 Case 3. A 55-year-old former asbestos worker has been complaining of shortness of breath. High-resolution computed tomography (HRCT) scan obtained at the lung bases shows prominent interstitial septal lines, subpleural cysts, and pleural plaques. This has the appearance of asbestosis.
Case 3. A 55-year-old former asbestos worker has been complaining of shortness of breath. High-resolution computed tomography (HRCT) scan obtained at the lung bases shows prominent interstitial septal lines, subpleural cysts, and pleural plaques. This has the appearance of asbestosis.
Computed tomography is reported to be 50% more sensitive than chest radiography for detection of pleural calcification.
Pleural plaques occur in isolation in 48% of patients and in association with parenchymal disease in 41%. Plaques remain stable over time.
Occasionally, pleural plaques are associated with short lines in the adjacent parenchyma. These hairy plaques (so called because of their fuzzy appearance) are of undetermined significance, although they should be distinguished from the more generalized fibrosis of asbestosis.
Diffuse pleural thickening
Diffuse pleural thickening is defined as an uninterrupted sheet at least 5 cm wide, 8-10 cm long craniocaudally, and 3 mm thick. Proliferation of extrapleural fat is a frequent finding; this presumably occurs as a response to pleural retraction. (See the image below.)
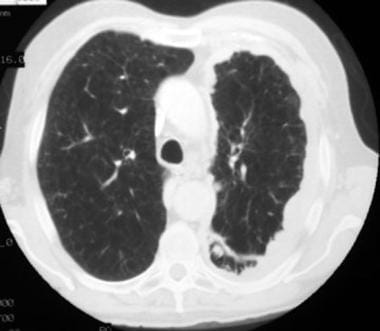 Case 4. The pulmonary window setting of this chest computed tomography (CT) scan shows an irregular, nodular pleural surface, not lung parenchymal nodules. Nodularity is also present along the fissure.
Case 4. The pulmonary window setting of this chest computed tomography (CT) scan shows an irregular, nodular pleural surface, not lung parenchymal nodules. Nodularity is also present along the fissure.
Disease may be bilateral and tends to involve the posterolateral surfaces of the lower thorax.
Differentiation of diffuse pleural thickening from discrete pleural plaques is important because the former may be associated with a significant reduction in pulmonary function.
Benign asbestos-related pleural effusion
Effusions may be unilateral or bilateral and usually are small. Benign asbestos-related effusions greater than 500 mL are uncommon. Effusions are associated with subsequent development of diffuse pleural thickening in just over 50% of patients, and an association with subsequent development of round atelectasis has been noted.
Computed tomography is useful for helping to exclude an underlying cause or an associated pleural mass. [35]
Rounded atelectasis
As on chest radiographs, round atelectasis appears on CT as a well-defined, rounded or wedge-shaped mass forming an acute angle with the adjacent pleura, which is always thickened. The mass usually is separated from the diaphragm by interposed lung. (See the image below.)
 Case 2. An asymptomatic man (>50 y) was noted to have a mass in the left lower lobe after exposure to asbestos. High-resolution computed tomography (HRCT) scan demonstrates a round mass at a site of pleural thickening, with a comet-tail bronchovascular bundle. This is an appearance of a folded lung (round atelectasis). The soft tissue window shows parenchymal enhancement.
Case 2. An asymptomatic man (>50 y) was noted to have a mass in the left lower lobe after exposure to asbestos. High-resolution computed tomography (HRCT) scan demonstrates a round mass at a site of pleural thickening, with a comet-tail bronchovascular bundle. This is an appearance of a folded lung (round atelectasis). The soft tissue window shows parenchymal enhancement.
Additional features identifiable on CT scans may include crowding of air bronchograms and the presence of a comet-tail sign, or a hurricane sign, which is a curvilinear bronchovascular bundle leading into the mass. [36]
Volume loss, focal emphysema, and calcification may be seen.
Because a round atelectasis represents a collapsed lung, it enhances significantly after intravenous administration of contrast material, usually with a minimum attenuation increase of 200%. Enhancement is usually uniform.
Pleuro-parenchymal fibrous bands may be seen radiating from the thickened pleura. These crows' feet are believed to represent contiguous, thickened interlobular septa.
Asbestosis
The earliest abnormal HRCT scan finding in patients with asbestosis is subpleural micronodularity, which represents early, mild peribronchial and centrilobular fibrosis.
As disease progresses, intralobular and interlobular septal thickening are seen, eventually resulting in architectural distortion, traction bronchiectasis, and honeycombing.
Ground-glass attenuation (increased lung attenuation without obscuration of the underlying pulmonary architecture) is a relatively unusual feature of asbestosis. When present, it may represent edema or fine intralobular fibrosis.
Ancillary findings of curvilinear subpleural lines and parenchymal bands, which are linear opacities 2-5 cm long extending from or paralleling the pleural surface, may be seen. These are characteristic (occurring in 60-80% of patients with asbestosis), although not specific (occurring in 10-20% of patients with non–asbestos-related diseases). The lines and bands represent contiguous, thickened interlobular septa, which are areas of subsegmental atelectasis or fibrosis along bronchovascular bundles.
Mild mediastinal lymphadenopathy, which is believed to represent a form of reactive hyperplasia, is found frequently in patients with uncomplicated asbestosis.
Malignant mesothelioma
Computed tomography findings include irregular, nodular pleural thickening (92%), which may involve interlobar fissures (86%), pleural effusion (74%), loss of volume (42%), pleural calcification (20%), and chest wall invasion (18%). (See the image below.)
 Case 4. The soft tissue window setting of this chest computed tomography (CT) scan shows the envelope-like mass along the pleural surface surrounding the lung. This was a mesothelioma.
Case 4. The soft tissue window setting of this chest computed tomography (CT) scan shows the envelope-like mass along the pleural surface surrounding the lung. This was a mesothelioma.
Pleural thickening typically is nodular and usually measures more than 1 cm, circumferentially involving the parietal and visceral costal and mediastinal pleurae.
Tumor often spreads to involve the underlying lung, causing thickening of interlobular septa and parenchymal nodules.
Chest wall and mediastinal invasion spreading to the contralateral hemithorax and through the diaphragm may be identified.
Mediastinal lymphadenopathy occurs, although distant metastases, including hematogenous spread to the contralateral lung, are uncommon.
Rarely, malignant mesotheliomas may present as localized masses.
Degree of confidence
Compared to chest radiographs, CT scans are more sensitive and specific for detection of diffuse pleural thickening. [22, 23] Furthermore, interobserver agreement in assessing pleural disease is greater with CT than with chest radiography.
The positive predictive value of chest radiographic findings for diagnosis of pleural disease in asbestos-exposed individuals is reported to be 79%, compared to 100% for HRCT scan findings.
High-resolution CT scans can depict subtle parenchymal abnormalities in approximately 33% of patients with asbestos-related disease with no chest radiographic evidence of interstitial disease and isolated borderline diminished lung capacity. However, no HRCT scan finding is specific for asbestosis. Thus, although HRCT is more sensitive than chest radiography for diagnosis of asbestos-related disease, its specificity has been questioned.
Diagnosis is based on a combination of features, including bilateral pulmonary fibrosis and bilateral pleural plaques or diffuse pleural thickening in an individual with an appropriate history of exposure. The specificity of the diagnosis increases with the number of features identified; however, although asbestosis rarely occurs in the absence of plaques, plaques are not invariably present. [6]
A small study correlating HRCT features and histopathologic findings revealed the presence of interstitial lines (84%), parenchymal bands (76%), architectural distortion (56%), subpleural lines (44%), and honeycombing (32%) in patients with proven asbestos-related lung disease. Specificity was increased from 60% for patients in whom 1 feature was identified to 80% for patients with 2 features and 100% for those with 3 or more features.
In addition, HRCT scanning is valuable in excluding disease in individuals with equivocal chest radiographic findings. Computed tomography is of value in differentiating benign disease from malignant pleural disease. The presence of a contiguous sheet or a pleural rind, pleural nodularity, and thickening greater than 1 cm, as well as involvement of the mediastinal pleura, is regarded as suggestive of malignancy. However, case reports describe a variant of asbestos-related, diffuse pleural thickening that appears nodular and is radiologically indistinguishable from mesothelioma. In equivocal cases, biopsy is recommended.
False positives/negatives
Normal extrapleural fat can be seen on HRCT internal to the ribs, particularly posterolaterally from the fourth to eighth ribs extending into the costophrenic angles. Fat can be several millimeters thick. Fat may be difficult to distinguish from pleural plaques or thickening, particularly when extended window settings are used, but it is more easily recognizable on soft tissue window settings because of very low attenuation.
The transversus thoracis and subcostalis muscles, segments of intercostal veins, visceral pleural thickening, and confluent subpleural nodules (pseudoplaques) mimic pleural thickening. The transversus thoracis and subcostalis muscles usually are smooth, of uniform thickness, and bilaterally symmetrical. The subcostalis muscle is present in only a minority of individuals and is seen as 1- to 2-mm-thick lines internal to 1 or more lower ribs. The transversus thoracis muscle is almost always visible internal to the costochondral junctions anterior to the heart and adjacent to the sternum.
Intercostal vessels are seen commonly in paravertebral regions and may cause the spurious appearance of focal pleural thickening. Intercostal vessels occasionally can be traced to the azygos or hemi-azygos vein; this observation allows correct interpretation of findings. Moreover, extrapleural fat should be visible between the vessel and the pleura. When images are read on the lung window setting, intercostal segments do not indent adjacent lung but pleural plaques invariably indent. Furthermore, pleural plaques usually are visible over several contiguous intercostal segments and may contain calcification.
Bilateral calcified pleural plaques usually are considered asbestos related, although rare causes such as radiation exposure, hyperparathyroidism, pulmonary infarction, and pancreatitis have been reported. Unilateral pleural calcification rarely is related to asbestos exposure and should prompt consideration of old tuberculosis, empyema, or hemothorax. Unilateral noncalcified pleural plaques are rare in asbestos-related disease.
Coal miner's pneumoconiosis, silicosis, and sarcoidosis occasionally can give rise to confluent subpleural nodules (pseudoplaques) that may mimic pleural plaques; however, these are associated with pulmonary nodules rather than with pulmonary fibrosis. [37] Interstitial fibrosis seen in asbestosis is indistinguishable from idiopathic pulmonary fibrosis noted on radiographic examination, HRCT scan, and pathologic examination; the only distinguishing feature is the presence of asbestos bodies.
For most patients, CT scan appearances of round atelectasis are sufficiently characteristic to obviate intervention; however, differentiating benign fibrotic masses from bronchial carcinoma is essential; some patients require close follow-up monitoring or biopsy. Although bronchial neoplasms can opacify to some degree after intravenous administration of contrast material, uniform enhancement has not been reported as a feature of lung cancer. On radiographs, malignant mesothelioma cannot be reliably distinguished from pleural metastases resulting from an adenocarcinoma. Focal mesothelioma may be mistaken for a benign fibrous tumor.
Magnetic Resonance Imaging
No consistent pattern of signal intensity has been described for round atelectasis on magnetic resonance imaging (MRI). [38] However, mesothelioma typically shows high signal intensity on T1-weighted images and moderately high signal intensity on T2-weighted images.
Magnetic resonance imaging and CT scanning have been shown to be similar in terms of accuracy in the diagnosis of malignant mesothelioma, although MRI is superior to CT in depicting isolated foci of the chest wall and diaphragmatic invasion. However, this difference has not been shown to confer any benefit in terms of overall staging.
Magnetic resonance imaging provides a certain advantage because the thorax can be directly imaged in various planes. Normal pleural space cannot be depicted on MRI. T2-weighted sequences may offer tissue-specific information concerning pleural effusions and chest wall invasion by malignant processes due to increased tumor-to-muscle contrast.
Nuclear Imaging
Lung uptake of gallium-67 (67Ga) has been used to create a quantitative index of inflammatory activity in patients with asbestosis. [19] Combined with evidence of serum markers indicating inflammation-associated pulmonary collagen formation, these findings may provide a clinically useful algorithmic approach permitting early diagnosis of asbestosis. Single photon-emission computed tomography (SPECT) improves sensitivity for detecting the presence and extent of interstitial occupational lung disease.
Gallium-67 has been used to differentiate malignant from benign asbestos-related pleural disease. Although studies in 86% of patients with asbestos-related mesothelioma show radionuclide uptake, only 19% of patients with benign asbestos effusions have positive findings.
Positron emission tomography (PET) scanning with [fluorine-18]-fluorodeoxy-glucose (18F-FDG) has been suggested to aid in the differentiation of round atelectasis from bronchogenic carcinoma. To date, limited studies have shown round atelectasis to be metabolically inactive. [39] In a study conducted to evaluate the role of 18F-FDG PET/CT integrated imaging in differentiating malignant from benign pleural effusion, 18F-FDG PET/CT integrated imaging was found to be a more reliable modality than 18F-FDG PET imaging and CT imaging alone. The sensitivities of CT imaging, 18F-FDG PET imaging, and 18F-FDG PET/CT integrated imaging in detecting malignant effusion were 75.0%, 91.7%, and 93.5%, respectively, and were 69.8%, 91.9%, and 93.0% in distinguishing metastatic effusion. [40]
Although experience is limited, the combination of HRCT scanning, 67Ga scanning, and inflammatory serum marker testing may allow earlier diagnosis of asbestosis. Mesothelioma unrelated to asbestos exposure is known to give rise to positive findings on 67Ga scans.
-
Case 1. Posteroanterior (PA) chest radiograph in a 58-year-old man with a history of occupational exposure to asbestos shows right diaphragmatic pleural plaque calcifications, linear calcification along the left pericardium, and bilateral pleural plaques along upper ribs.
-
Case 1. Contrast-enhanced computed tomography (CT) scan of the chest at the level of the pulmonary artery bifurcation shows calcified pleural plaques along the posterior, lateral, and anterior pleural surfaces.
-
Case 2. An asymptomatic man (>50 y) was noted to have a mass in the left lower lobe after exposure to asbestos. High-resolution computed tomography (HRCT) scan demonstrates a round mass at a site of pleural thickening, with a comet-tail bronchovascular bundle. This is an appearance of a folded lung (round atelectasis). The soft tissue window shows parenchymal enhancement.
-
Case 3. A 55-year-old former asbestos worker has been complaining of shortness of breath. High-resolution computed tomography (HRCT) scan obtained at the lung bases shows prominent interstitial septal lines, subpleural cysts, and pleural plaques. This has the appearance of asbestosis.
-
Case 4. A 67-year-old man with a history of occupational exposure to asbestos for decades began experiencing a nagging, left-sided chest pain. Posteroanterior (PA) chest radiograph shows a left pleural effusion and peripheral, left-sided nodules.
-
Case 4. The pulmonary window setting of this chest computed tomography (CT) scan shows an irregular, nodular pleural surface, not lung parenchymal nodules. Nodularity is also present along the fissure.
-
Case 4. The soft tissue window setting of this chest computed tomography (CT) scan shows the envelope-like mass along the pleural surface surrounding the lung. This was a mesothelioma.