Practice Essentials
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is characterized by progressive fibrofatty replacement of the myocardium that predisposes to ventricular tachycardia (see the image below) and sudden death in young individuals and athletes. It primarily affects the right ventricle, and it may also involve the left ventricle. Lesions extend from the epicardium to the endocardium and predominantly involve the area between the anterior part of the pulmonary infundibulum, the apex, and the inferoposterior wall. The presentation of disease is highly variable even within families, and some affected individuals may not meet established clinical criteria. The mean age at diagnosis is 31 years (±13; range, 4-64 years). [1, 2, 3]
Although the estimated prevalence of ARVD/C in the general population is only 1:5000, it represents one of the most common causes of juvenile sudden death. [4] Anatomic changes to the right ventricle consist of mild to severe global dilatation, aneurysms, and segmental hypokinesia. Sites of involvement of the right ventricle are found in the triangle of dysplasia—namely, the right ventricular outflow tract, the apex, and the infundibulum. Mutations have been identified in 5 desmosomal genes. [5, 6, 7, 8, 9, 10, 11, 12, 13]
In a retrospective study, Ghasabeh et al compared epicardial fat in patients with ARVD/C with that in healthy individuals. They found that higher amounts of right ventricular and left ventricular epicardial fat are found in hearts with ARVD/C, particularly adjacent to the left ventricle, which correlates with disease severity and helps differentiate these patients from healthy individuals. [14]
The diagnosis of pediatric-onset ARVD/C might be much more difficult in children. Sudden cardiac death might be prevented in the early period by raising awareness of physicians about the disorder. Prevention of sudden death with an implantable cardioverter-defibrillator is crucial in the management of these patients. It should be kept in mind that children with structurally normal hearts may present at an earlier concealed phase and can be diagnosed with ARVD/C. [15]
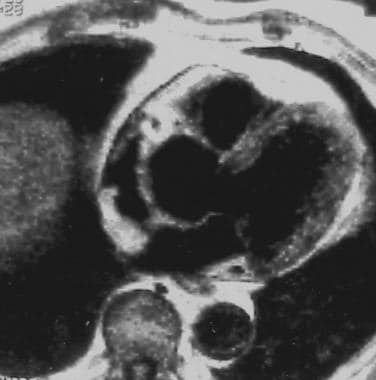 Electrocardiographically gated T1-weighted image shows arrhythmogenic right ventricular dysplasia (ARVD) with prominent epicardial fat in the right ventricular outflow tract.
Electrocardiographically gated T1-weighted image shows arrhythmogenic right ventricular dysplasia (ARVD) with prominent epicardial fat in the right ventricular outflow tract.
Imaging modalities
Recognition of mild forme fruste and localized forms of ARVD/C remains a clinical challenge. Diagnosing ARVD/C in patients with minimal right ventricular abnormalities is difficult to accomplish using echocardiography, ultrafast computed tomography (CT) scanning, radionuclide angiography, multidetector CT, and contrast-enhanced angiography. [16, 17, 18, 19, 20, 21, 22, 23]
Detection of early right ventricular dysfunction in ARVD/C requires a high level of clinical suspicion and an algorithmic approach. A thorough family history of juvenile sudden death, ventricular arrhythmias, and implantable cardioverter-defibrillators should always be sought. Diagnosis usually requires electrocardiographic interpretation as well as cardiac imaging. [3]
Chest radiographs may be normal, or they may demonstrate a wide variety of manifestations not specific for ARVD/C. These range from a completely normal cardiac silhouette to moderate or major cardiomegaly with a convexity between the aortic knob and the left ventricle without pulmonary vascular redistribution. Therefore, chest radiography is not a good tool for diagnosing ARVD/C, but it can help in assessing the patient's prognosis. The cardiothoracic index is less than 0.6 in most patients. In young athletes, this relative cardiomegaly may be incorrectly attributed to training.
Magnetic resonance imaging (MRI) can display the anatomy and function of the right ventricle, as well as characterize the composition of the right ventricle's wall, especially with regard to adipose tissue. However, the diagnostic sensitivity and specificity of MRI are observer dependent. Signal intensity suggesting the presence of fat in the right ventricle may be related to a latent form of the disease or to the dissociation of myocardial tissue by fat. Therefore, only the combination of MRI signs, including size, function, and fat content in the free wall, is necessary to support the diagnosis. [24]
MRI has the advantage of offering methods that specifically eliminate or selectively include signal from only fat, which resonates at a different frequency than that of tissue or water. For example, a spatiospectral pulse sequence can be used to selectively image fat through a gradient-echo technique, and a triple-inversion recovery sequence can be applied to nullify signals from fat. However, wall motion, gating abnormalities, or other artifacts can produce false-positive results, which must be eliminated by means of inspection and/or acquisition of confirmatory views.
Although nuclear ventriculoscintigraphy is accurate in the quantitative evaluation of right ventricular function, it does not provide specific information. Nuclear ventriculoscintigraphy provides data concerning size, ejection fraction, and contraction pattern of the ventricles. In most cases of ARVD/C, the size of the right ventricle is increased, but it can be within normal limits. Wall motion during contraction is not uniform as a result of dyskinetic areas of fat replacement in the free wall of the right ventricle. Specific isotopic markers have demonstrated regional sympathetic denervation of these areas. [25] With nuclear ventriculoscintigraphy, ARVD/C should be in the differential diagnosis for any condition that results in a large right ventricle, a diminished ejection fraction, or an alternating contraction pattern in the right ventricle.
Because MRI and 3D echocardiography are noninvasive and repeatable examinations, they are preferred over angiography for evaluation of structure and function of the right ventricle. Angiographic features of ARVD/C include global and/or regional function and morphologic abnormalities of the right ventricle. Such features include localized akinetic or dyskinetic bulges, outpouchings, dilatation of the infundibulum, trabecular hypertrophy, and/or disarray with deep fissures. [18]
In some cases, endomyocardial biopsy may be necessary to confirm the diagnosis.
An international task force facilitated recognition and interpretation of the frequently nonspecific clinical features of ARVD/C. The task force elaborated on newer imaging techniques such as contrast-enhanced echocardiography, 3-dimensional echocardiography, cardiovascular magnetic resonance with late enhancement, and electroanatomic voltage mapping. [26]
Zghaib et al described left ventricular (LV) findings on cardiac magnetic resonance (CMR) in 73 patients with ARVD/C. CMR evidence of LV abnormalities was seen in half of the patients, typically manifesting as fibrofatty infiltration in the subepicardium of the apicolateral wall, and no association was found with arrhythmic outcomes. [27]
Magnetic Resonance Imaging
The role of magnetic resonance imaging (MRI) in the diagnosis of ARVD/C has been established. In conjunction with electrophysiologic, electrocardiographic, and familial indicators, MRI results are specific. MRI is considered highly sensitive and highly specific and can serve as an effective noninvasive examination for fatty infiltration of the myocardium. However, because fat is a normal component of the right ventricle in humans, it is necessary to interpret MRI results in light of all findings in the clinical context. MRI can assess biventricular morphology, volumes, thickness, and mass, as well as regional motion and myocardial fibrous or adipose content, edema, and flow, with high spatial and temporal resolution [1, 28] .
Magnetic resonance imaging enables clear visualization of morphology of the right ventricle and permits characterization of the composition of the wall, especially with regard to fatty tissue. [29] Typical MRI ARVD/C protocols include (1) bright-blood gradient-echo imaging in cine mode to assess wall motion, (2) dark-blood T1-weighted imaging to evaluate wall thickness, and (3) specific fat-sensitive or fat-suppressive imaging to confirm fatty infiltration or transdifferentiation on short-axis views spanning the entire right ventricle. [30, 31, 32, 33]
Use of orthogonal views, attention to gating, and/or alternative fat-sensitive methods help eliminate a false-positive signal dropout. [16, 17, 19, 34, 35]
(Images derived from MRI techniques appear below.)
 Electrocardiographically gated T1-weighted image shows arrhythmogenic right ventricular dysplasia (ARVD) with prominent epicardial fat in the right ventricular outflow tract.
Electrocardiographically gated T1-weighted image shows arrhythmogenic right ventricular dysplasia (ARVD) with prominent epicardial fat in the right ventricular outflow tract.
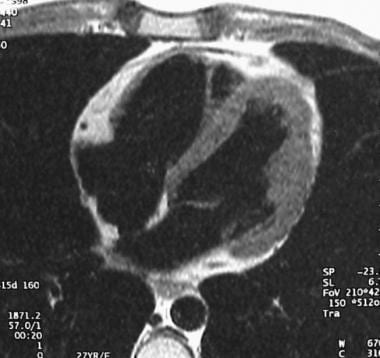 Electrocardiographically gated axial spin-echo T1-weighted image demonstrates diffuse high signal intensity in the myocardium of the right ventricular anterior free wall. This finding corresponds to fatty infiltration.
Electrocardiographically gated axial spin-echo T1-weighted image demonstrates diffuse high signal intensity in the myocardium of the right ventricular anterior free wall. This finding corresponds to fatty infiltration.
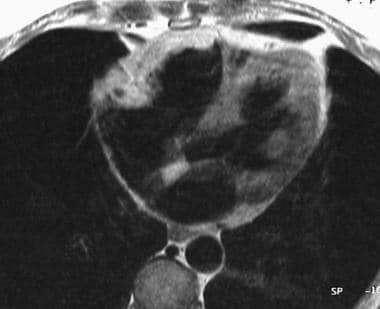 Electrocardiographically gated axial spin-echo T1-weighted image shows the high signal intensity due to fatty infiltration of the right ventricular free wall.
Electrocardiographically gated axial spin-echo T1-weighted image shows the high signal intensity due to fatty infiltration of the right ventricular free wall.
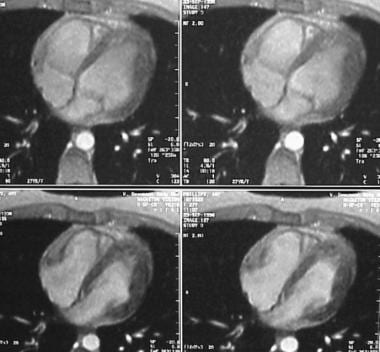 Four-chamber electrocardiographically gated cine gradient-echo images show an abnormality in right ventricular wall motion during systole or a systolic aneurysm.
Four-chamber electrocardiographically gated cine gradient-echo images show an abnormality in right ventricular wall motion during systole or a systolic aneurysm.
In ARVD/C, MRI findings consist of abnormalities in signal intensity, which usually affect the right ventricular free wall and/or the previously mentioned triangle of dysplasia. Fibrofatty deposition in these regions of the right ventricle may be evident on T1- and T2-weighted images, where they appear as areas of focal or diffuse high signal intensity. Other relatively nonspecific signal intensity abnormalities in the right ventricle may be due to fibrosis or inflammation.
In a cross-sectional study, Motevali et al compared cardiac MRI with echocardiography in patients with ARVD/C. The average ejection fraction was 43 ± 9.4% and 42.8 ± 8.5% for MRI and echocardiography, respectively. Additionally, 46% of patients given an MRI and 35.8% who underwent echocardiography had hypokinesia in the right ventricle. The right ventricular aneurysm was found in 20.5% of MRI patients and 5.1% of echocardiographic patients. [36]
Regardless of the MRI findings obtained, myocardial biopsy is often warranted. In terms of morphology, diffuse or focal dilatation of the chamber of the right ventricle is a key finding in ARVD/C. Often, the free wall of the right ventricle is noticeably thinned. Motion abnormalities depend on the extent of underlying fibrofatty infiltration and vary from a focal bulge to a low ejection fraction due to disease that is more diffuse.
MRI may be indicated for (1) young athletes with frequent simple arrhythmias, even in the absence of echocardiographic abnormalities; (2) patients with ventricular tachyarrhythmias in a left bundle-branch-block pattern; (3) patients with palpitations, syncopal episodes, or echocardiographic abnormalities of the right ventricle; and (4) patients with a familial occurrence of ARVD/C or sudden death syndrome.
Ultrasonography
Two-dimensional echocardiography is the most widely available imaging method for the evaluation of patients with known or suspected ARVC/D. New echocardiographic techniques, such as 3D echocardiography and tissue deformation imaging, may increase the performance of echocardiography, especially in thevearly stages of disease. Three-dimensional echocardiography allows the measurement of RV volumes and the RV ejection fraction. [1, 2]
Echocardiography is sensitive for ARVD/C, but it is not specific because it does not allow for assessment of the adipose substitution of the myocardium, which is a hallmark of ARVD/C. Echocardiography is less sensitive than MRI because it may fail to depict focal right ventricular wall thickening, and it does not reliably help distinguish fat from muscle. The last limitation also decreases the specificity of echocardiography. Right ventricular hypertrophy, right ventricular infarct, and prominent pericardial fat contribute to false-positive results. Limitation of views and focality on thickening of the right ventricular wall contribute to false-negative findings.
Echocardiography may show morphologic abnormalities such as dilatation of the right ventricle and outflow tract, segmental wall bulging or aneurysms in the predominantly affected portions during diastole, and hypokinetic or dyskinetic areas in the inferobasal region. [37, 38] However, in most cases of ARVD/C, structural abnormalities are moderate, and they may be difficult to detect. The posterior and inferior walls of the right ventricular inflow tract under the tricuspid valve must be visualized because they are most frequently affected.
Contrast-enhanced echocardiography with injections of sodium chloride solution may aid in evaluating the outline of the right ventricle to permit an analysis of right ventricular volume.
The use of three-dimensional echocardiography (3D echo), particularly in combination with the transesophageal approach, is being investigated as a means to enhance the diagnostic accuracy of this technique.
Two-dimensional echocardiography (2D echo) is a major tool for the diagnosis of ARVD/C. Transesophageal echocardiography and intracardiac echocardiography can provide additional information on small, focal, structural abnormalities in patients with ARVD/C, such as bulges, sacculations, aneurysms with or without associated thrombus, partial or complete loss of trabeculations, and hypertrophy of the moderator band. These changes are particularly important in cases with a concealed form of the disease in which no morphologic abnormalities are evident on transthoracic echocardiography. [39]
Angiography
-
Electrocardiographically gated T1-weighted image shows arrhythmogenic right ventricular dysplasia (ARVD) with prominent epicardial fat in the right ventricular outflow tract.
-
Electrocardiographically gated axial spin-echo T1-weighted image demonstrates diffuse high signal intensity in the myocardium of the right ventricular anterior free wall. This finding corresponds to fatty infiltration.
-
Electrocardiographically gated axial spin-echo T1-weighted image shows the high signal intensity due to fatty infiltration of the right ventricular free wall.
-
Four-chamber electrocardiographically gated cine gradient-echo images show an abnormality in right ventricular wall motion during systole or a systolic aneurysm.
-
Triangle of dysplasia.







