Practice Essentials
Mullerian duct anomalies are an uncommon but often treatable cause of infertility. Patients with müllerian duct anomalies are known to have a higher incidence of infertility, repeated first-trimester spontaneous abortions, fetal intrauterine growth retardation, fetal malposition, preterm labor, and retained placenta. The role of imaging is to help detect, diagnose, and distinguish surgically correctable forms of müllerian duct anomalies from inoperable forms (see the images below). In some correctable lesions, the surgical approach is altered based on imaging findings. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]
Imaging modalities
MRI is considered the criterion standard for imaging uterine anomalies. [11, 12, 13, 14, 15] MRI provides high-resolution images of the uterine body, fundus, and internal structure. In addition, it can help evaluate the urinary tract for concomitant anomalies. In the past, intravenous urography was used for this purpose. [16, 17] Most types of uterine anomalies can be diagnosed confidently using pelvic MRI. [18]
MRI provides high-resolution images of the uterine cavity, the configuration of the uterus (body and fundus), and the ovaries. MRI is limited by motion artifact (patient movement, bowel peristalsis) and other features that degrade image quality (eg, metal prostheses, clips, filters) and cannot be performed in some patients (eg, patients who are claustrophobic, have pacemakers, or are obese). A study of 225 genital tract anomalies reported 8.4% were incorrectly diagnosed based on MRI findings. The misdiagnosis was most significant with vaginal and cervical dygenesis. [19]
Once a müllerian anomaly is suggested based on evidence from the patient history and physical examination, the clinician may opt for additional imaging workup. Imaging criteria for distinguishing forms of uterine anomalies are based on the configuration of the endometrial cavity—primarily on the configuration of the uterine fundus. [20, 21]
Typically, the first examination ordered is a pelvic ultrasound (US) with transabdominal and, if feasible, transvaginal imaging. Müllerian duct anomalies may be suggested on transvaginal 2-dimensional (2D) sonographic imaging but may not be excluded on the basis of negative US findings. Three-dimensional (3D) sonographic techniques offer relatively higher sensitivity and specificity. [9, 22, 12, 15]
Ghi et al found 3D transvaginal ultrasonography of the uterine cavity to be extremely accurate in diagnosing and classifying congenital uterine anomalies in nulliparous women who had 3 or more consecutive miscarriages. They were able to identify specific mullerian malformations in 54 women, which were all confirmed by endoscopy. Negative findings were confirmed by hysteroscopy. Concordance between ultrasound and endoscopic findings regarding the type of anomaly was verified in 52 cases, including all patients with septate uterus and 2 of 3 patients with bicornuate uterus. [23]
Although US is often the first imaging modality chosen because of its availability, short scan time, and low cost, several limitations are encountered during imaging. Image quality from transabdominal and transvaginal examinations is operator dependent. Overlying bowel gas can confound transabdominal imaging. Transvaginal imaging, although superior to the transabdominal approach, may not always be possible, as in patients with vaginal septa. Image resolution is a limiting factor. US cannot distinguish between a nonfunctional rudimentary horn and a functional noncommunicating rudimentary horn. [24]
Three-dimensional transvaginal ultrasound is nearly as sensitive as MRI in diagnosing congenital uterine anomalies, but MRI is preferred because MRI T2 sequences clearly delineate the uterine zonal anatomy, ovaries, follicles, vaginal continuity to the uterus, and vaginal septum. [12, 25]
Hysterosalpingography (HSG), performed under fluoroscopy, allows evaluation of the uterine cavity and tubal patency. Anomalies may be suggested but positive findings often are nonspecific for precise diagnosis. [14] HSG is probably the only imaging modality providing high-resolution imaging of the uterine cavity and fallopian tubes, but it is limited to imaging only the endoluminal contour; therefore, characterization of müllerian anomalies can be difficult. For example, visualization of 2 uterine cavities on HSG does not aid the radiologist in distinguishing septate, didelphys, and bicornuate uteri, which are 3 entities with radically different treatments.
Since HSG uses ionizing radiation, ensure that the patient is not pregnant at the time of the examination. In patients who are not pregnant, ovaries receive a small dose of radiation, and the risk to the unfertilized ova is unknown.
(See the images below.)
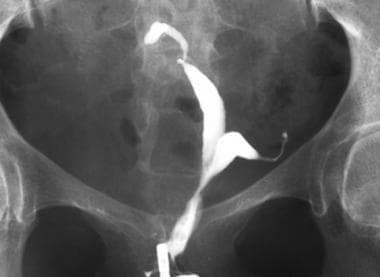 Uterus, müllerian duct abnormalities. This was difficult to differentiate as septate or bicornuate uterus using hysterosalpingography. It was a surgically proven case of bicornuate uterus.
Uterus, müllerian duct abnormalities. This was difficult to differentiate as septate or bicornuate uterus using hysterosalpingography. It was a surgically proven case of bicornuate uterus.
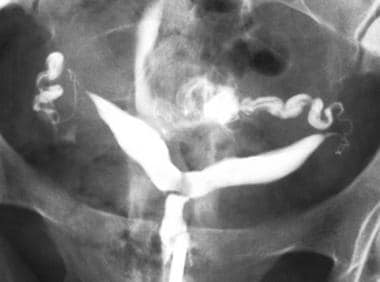 Uterus, müllerian duct abnormalities. Surgically proven case of bicornuate uterus. Correct diagnosis may be suggested based on hysterosalpingography findings, which are, most notably, the widened intercornual distance (>4 cm) and the widened intercornual angle (>60°).
Uterus, müllerian duct abnormalities. Surgically proven case of bicornuate uterus. Correct diagnosis may be suggested based on hysterosalpingography findings, which are, most notably, the widened intercornual distance (>4 cm) and the widened intercornual angle (>60°).
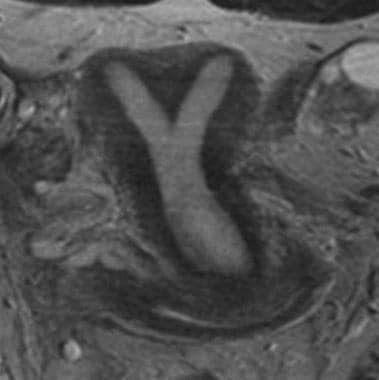 Uterus, müllerian duct abnormalities. T2 fast spin-echo MRI image of septate uterus acquired in the oblique plane along the long axis of the uterus. Note that the outer fundal contour (superior border) is flat or slightly concave, which is sufficient to make the diagnosis of septate uterus.
Uterus, müllerian duct abnormalities. T2 fast spin-echo MRI image of septate uterus acquired in the oblique plane along the long axis of the uterus. Note that the outer fundal contour (superior border) is flat or slightly concave, which is sufficient to make the diagnosis of septate uterus.
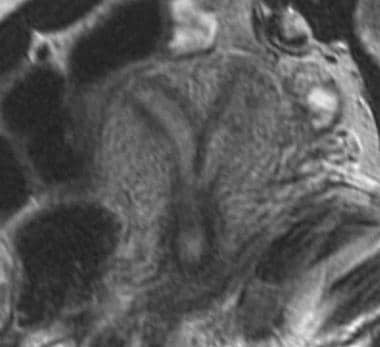 Uterus, müllerian duct abnormalities. Septate uterus. Note that a longer septum divides the uterine cavity. Outer fundal contour is flat.
Uterus, müllerian duct abnormalities. Septate uterus. Note that a longer septum divides the uterine cavity. Outer fundal contour is flat.
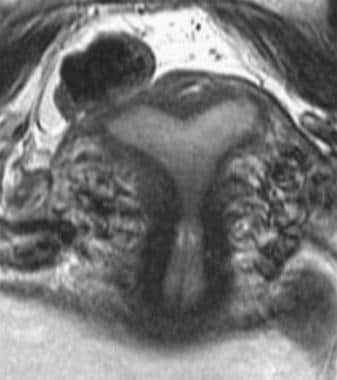 Uterus, müllerian duct abnormalities. MRI image of septate uterus. The patient has a thin, fibrous septum that cannot be resolved distally at the fundus. More importantly, the outer fundal contour remains convex, thus excluding a bicornuate uterus.
Uterus, müllerian duct abnormalities. MRI image of septate uterus. The patient has a thin, fibrous septum that cannot be resolved distally at the fundus. More importantly, the outer fundal contour remains convex, thus excluding a bicornuate uterus.
Embryology
Two paired mullerian ducts ultimately develop into the structures of the female reproductive tract. The structures include the fallopian tubes, uterus, cervix, and upper two thirds of the vagina. The ovaries and lower one third of the vagina have separate embryologic origins not derived from the müllerian system.
Complete formation and differentiation of the müllerian ducts into the segments of the female reproductive tract depend on completion of 3 phases of development as follows:
-
Organogenesis: One or both müllerian ducts may not develop fully, resulting in abnormalities such as uterine agenesis or hypoplasia (bilateral) or unicornuate uterus (unilateral).
-
Fusion: The process during which the lower segments of the paired müllerian ducts fuse to form the uterus, cervix, and upper vagina is termed lateral fusion. Failure of fusion results in anomalies such as bicornuate or didelphys uterus. The term vertical fusion occasionally is used to refer to fusion of the ascending sinovaginal bulb with the descending müllerian system (ie, fusion of the lower one third and upper two thirds of the vagina). Complete vertical fusion forms a normal patent vagina, while incomplete vertical fusion results in an imperforate hymen.
-
Septal resorption: After the lower müllerian ducts fuse, a central septum is present, which subsequently must be resorbed to form a single uterine cavity and cervix. Failure of resorption is the cause of septate uterus.
Ovaries and the lower vagina are not derived from the müllerian system. The ovaries are derived from germ cells that migrate from the primitive yolk sac into the mesenchyme of the peritoneal cavity and subsequently develop into ova and supporting cells. The lower vagina arises from the sinovaginal bulb, which fuses with the müllerian-derived upper two thirds to form the complete vagina.
Mullerian duct anomalies
Suggestion of a müllerian duct anomaly may arise in different clinical situations. In the newborn or infant, the initial presentation may be an obstructed system discovered as a palpable abdominal, pelvic, or vaginal mass (mucocolpos). [26, 27]
Similarly, an adolescent girl may present to a clinician because of delayed menarche or because of an obstructed system presenting as an intra-abdominal mass (hematocolpos). Many patients also have cyclical pain.
Women of childbearing age often present with various problems of infertility, repeated spontaneous abortions, or premature delivery. As part of an infertility workup, routine imaging often detects the anomaly. Occasionally, the anomaly is discovered incidentally during imaging evaluation for another condition or during surgery such as elective sterilization.
American Society for Reproductive Medicine (ASRM) Classification
Class I (hypoplasia/agenesis) abnormalities include entities such as uterine/cervical agenesis or hypoplasia. The most common form is the Mayer-Rokitansky-Kuster-Hauser syndrome, which is combined agenesis of the uterus, cervix, and upper portion of the vagina. [25, 28, 29, 30, 31] Patients have no reproductive potential aside from medical intervention in the form of in vitro fertilization of harvested ova and implantation in a host uterus.
Class II (unicornuate uterus) is the result of complete, or almost complete, arrest of development of 1 mullerian duct (see the image below). If the arrest is incomplete, as in 90% of patients, a rudimentary horn with or without functioning endometrium is present. If the rudimentary horn is obstructed, it may come to surgical attention when presenting as an enlarging pelvic mass. If the contralateral healthy horn is almost fully developed, a full-term pregnancy is believed to be possible (see didelphys uterus). [25, 32]
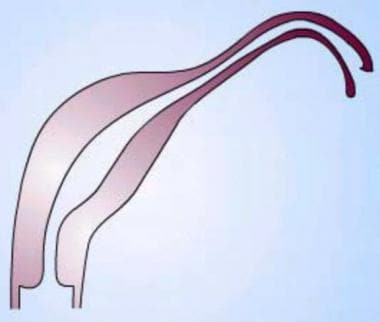 Uterus, müllerian duct abnormalities. Unicornuate uterus. Note the failure of the development of one half of the uterus. This form may be associated with a rudimentary horn arising from the contralateral müllerian duct.
Uterus, müllerian duct abnormalities. Unicornuate uterus. Note the failure of the development of one half of the uterus. This form may be associated with a rudimentary horn arising from the contralateral müllerian duct.
Class III (didelphys uterus) results from complete nonfusion of both müllerian ducts (see the image below). The individual horns are fully developed and almost normal in size. Two cervices are inevitably present. A longitudinal or transverse vaginal septum may be noted as well. Didelphys uteri have the highest association with transverse vaginal septa, but septa also may be observed in other anomalies. Consider metroplasty; however, since each horn is almost a fully developed uterus, patients have been known to carry pregnancies to full term. [25, 33]
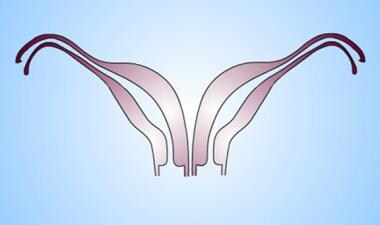 Uterus, müllerian duct abnormalities. Didelphys uterus. Note the complete separation but full development of each müllerian duct.
Uterus, müllerian duct abnormalities. Didelphys uterus. Note the complete separation but full development of each müllerian duct.
Class IV (bicornuate uterus) results from partial nonfusion of the müllerian ducts (see the image below). The central myometrium may extend to the level of the internal cervical os (bicornuate unicollis) or external cervical os (bicornuate bicollis). The latter is distinguished from didelphys uterus because it demonstrates some degree of fusion between the 2 horns, while in classic didelphys uterus, the 2 horns and cervices are separated completely. In addition, the horns of the bicornuate uteri are not fully developed; typically, they are smaller than those of didelphys uteri. Some patients are surgical candidates for metroplasty. [25]
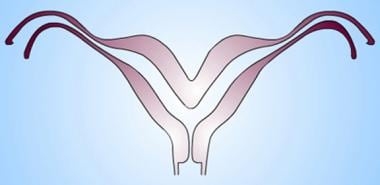 Uterus, müllerian duct abnormalities. Bicornuate uterus. Note the partial fusion of the lower uterine segment and persistently separated upper uterine segments. Of key importance is the prominent fundal cleft (>1 cm), which distinguishes the anomaly from septate uterus.
Uterus, müllerian duct abnormalities. Bicornuate uterus. Note the partial fusion of the lower uterine segment and persistently separated upper uterine segments. Of key importance is the prominent fundal cleft (>1 cm), which distinguishes the anomaly from septate uterus.
Class V (septate uterus) results from failure of resorption of the septum between the 2 uterine horns. The septum can be partial or complete, in which case it extends to the internal cervical os (see the images below). Histologically, the septum may be composed of myometrium or fibrous tissue. The uterine fundus is typically convex but may be flat or slightly concave (< 1 cm fundal cleft). Women with septate uterus have the highest incidence of reproductive complications. Differentiation between a septate and a bicornuate uterus is important because septate uteri are treated by using transvaginal hysteroscopic resection of the septum, whereas if surgery is possible and/or indicated for the bicornuate uterus, an abdominal approach is required to perform metroplasty. [25]
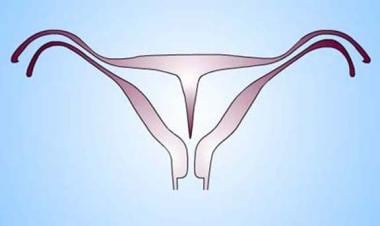 Uterus, müllerian duct abnormalities. Septate uterus. Midline septum can be of variable length and can be muscular or fibrous. In the diagram, the septum is shown as an extension of the uterine myometrium.
Uterus, müllerian duct abnormalities. Septate uterus. Midline septum can be of variable length and can be muscular or fibrous. In the diagram, the septum is shown as an extension of the uterine myometrium.
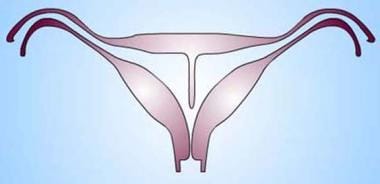 Uterus, müllerian duct abnormalities. Septate uterus. The midline septum can extend for a variable length and can be muscular or fibrous. In the diagram, the septum is thin and linear as expected in the fibrous type. Since the composition of the septum varies, whether it is composed of muscle or fibrous tissue is not a means to distinguish septate from other forms of uterine anomalies.
Uterus, müllerian duct abnormalities. Septate uterus. The midline septum can extend for a variable length and can be muscular or fibrous. In the diagram, the septum is thin and linear as expected in the fibrous type. Since the composition of the septum varies, whether it is composed of muscle or fibrous tissue is not a means to distinguish septate from other forms of uterine anomalies.
Class VI (arcuate uterus) has a single uterine cavity with a convex or flat uterine fundus, the endometrial cavity, which demonstrates a small fundal cleft or impression (>1.5 cm). The outer contour of the uterus is convex or flat (see the image below). This form is often considered a normal variant because it is not significantly associated with the increased risks of pregnancy loss and the complications found in other subtypes. [25]
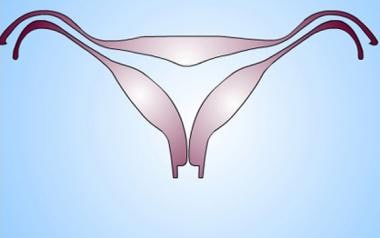 Uterus, müllerian duct abnormalities. Arcuate uterus. Mild thickening of the midline fundal myometrium resulting in fundal cavity indentation but normal outer fundal contour. Some authors consider it a normal variant. It is not associated with an increased risk of obstetric/gynecologic complications.
Uterus, müllerian duct abnormalities. Arcuate uterus. Mild thickening of the midline fundal myometrium resulting in fundal cavity indentation but normal outer fundal contour. Some authors consider it a normal variant. It is not associated with an increased risk of obstetric/gynecologic complications.
Class VII (diethylstilbestrol-related anomaly) has occurred in several million women who were treated with diethylstilbestrol (DES), an estrogen analogue prescribed to prevent miscarriage from 1945-1971. The drug was withdrawn once its teratogenic effects on the reproductive tracts of male and female fetuses were understood. The uterine anomaly is seen in the female offspring of as many as 15% of women exposed to DES during pregnancy. Affected female fetuses have a variety of abnormal findings that include uterine hypoplasia and a T-shaped uterine cavity. Patients also may have abnormal transverse ridges, hoods, stenoses of the cervix, and adenosis of the vagina with increased risk of vaginal clear cell carcinoma. Imaging findings are pathognomonic for this anomaly (see the image below). [25, 34]
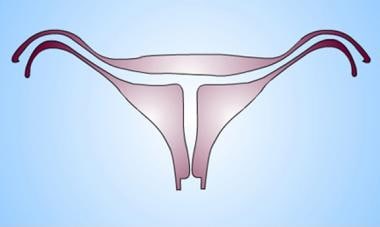 Uterus, müllerian duct abnormalities. Diethylstilbestrol-exposed uterus. Myometrial hypertrophy results in a T-shaped uterine cavity and cavity irregularity, which is pathognomonic for the anomaly. Typically, the uteri are hypoplastic.
Uterus, müllerian duct abnormalities. Diethylstilbestrol-exposed uterus. Myometrial hypertrophy results in a T-shaped uterine cavity and cavity irregularity, which is pathognomonic for the anomaly. Typically, the uteri are hypoplastic.
Other classification systems
Rock and Adam
Other systems (eg, in the surgical literature) classify the anomalies differently. [35] Rock and Adam modified the ASRM classification to include a broader collection of anomalies [36] :
-
Class 1 is identical to AFS class I (agenesis/hypoplasia).
-
Class 2 pertains to disorders compromising patency of the reproductive tract. This class includes all anomalies in which incomplete fusion occurs between the descending müllerian ducts and the ascending urogenital sinus (sinovaginal bulb). Milder forms may present with only a thin membrane at the junction, while more severe forms result in a thick atretic tissue involving up to one half of the vaginal length. Class 2 anomalies also include obstructive or nonobstructive transverse vaginal septa (although not believed to be a disorder of vertical fusion) and cervical agenesis and dysgenesis with or without obstruction.
-
Class 3 describes anomalies in a patent but often duplicated or partially duplicated reproductive tract and includes disorders of lateral fusion such as didelphys, unicornuate, bicornuate, and septate uteri (AFS classes II, III, IV, and V).
The disorders arise from impaired fusion and/or septal resorption of fusing müllerian ducts attempting to form the uterus, cervix, and upper vagina. The disorders may present in 1 of 2 forms: a symmetric unobstructed form or an asymmetric obstructed form.
The distinguishing feature of the asymmetric obstructed form is observed in the obstructed side, which is always associated with ipsilateral renal agenesis. [37] This likely explains why the symmetric obstructed form rarely is encountered, since bilateral renal agenesis is not viable. The symmetric unobstructed form is seen in 5 types, including septate, bicornuate, didelphys, DES, and unicornuate uterus with or without rudimentary horn.
The asymmetric obstructed form is seen in 3 types, including unicornuate with noncommunicating obstructed horn, unilaterally obstructed double uterus, and unilateral vaginal obstruction.
Class 4 includes all other unusual configurations of vertical-lateral fusion defects to varying degrees as well as anomalies that are difficult to classify.
European Society of Human Reproduction and Embryology (ESHRE) and the European Society for Gynaecological Endoscopy (ESGE)
The ESHRE/ESGE classifies anomalies according to the morphology of the uterus, cervix, and vagina independently. [12, 25, 38] Uterine anatomic anomalies are classified as normal uterus (U0), dysmorphic uterus (U1), septate uterus (U2), bicorporeal uterus (U3), hemiuterus (U4), aplastic uterus (U5), and still-unclassified cases (U6).
Radiography
Before the advent of MRI and ultrasonography, the primary imaging modality for evaluating uterine anomalies was limited to fluoroscopy or hysterosalpingography (HSG). Fluoroscopic spot films are obtained after the cervix is cannulated and radiopaque contrast is injected into the uterine cavity.
HSG provides high-resolution images of the contour of the uterine cavity and fallopian tubes and remains the key imaging test for assessing tubal abnormalities that may cause infertility. Typically, the question of müllerian duct anomaly arises during HSG examination if the typical trigone configuration of the cavity is not demonstrated.
A common finding is separation of the uterine cavity into right and left compartments. A divided uterine cavity can result from septate, bicornuate, or didelphys uterus. Certain criteria are used to increase confidence in diagnosing 1 of the 3 entities.
Intercornual distance is the distance between the distal ends of the horns (ends that are continuous with fallopian tubes). When it is less than 2 cm, the likelihood of septate uterus is increased. If the distance is greater than 4 cm, the likelihood of didelphys uterus is increased. Measurements of 2-4 cm (typical distance in a normal uterus) are indeterminate in an abnormal cavity configuration.
Intercornual angle is the angle formed by the most medial aspects of the 2 uterine hemicavities. If the angle is less than 60°, septate uterus is more likely. For larger angles, the anomaly is more likely to be a bicornuate uterus (see the image below).
 Uterus, müllerian duct abnormalities. Surgically proven case of bicornuate uterus. Correct diagnosis may be suggested based on hysterosalpingography findings, which are, most notably, the widened intercornual distance (>4 cm) and the widened intercornual angle (>60°).
Uterus, müllerian duct abnormalities. Surgically proven case of bicornuate uterus. Correct diagnosis may be suggested based on hysterosalpingography findings, which are, most notably, the widened intercornual distance (>4 cm) and the widened intercornual angle (>60°).
A hypoplastic, irregular, T-shaped uterine cavity is pathognomonic for in utero DES exposure. The uterus typically is much smaller than average, and many forms of cavity appearance exist with varying, but usually symmetric, regions of narrowing or dilatation in the segments of the T. MRI and US examinations typically demonstrate uterine hypoplasia only (see the image below).
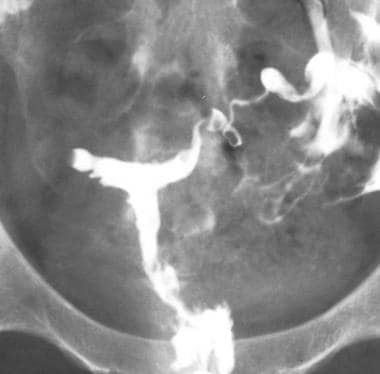 Uterus, müllerian duct abnormalities. T-shaped uterus. Classic configuration of the uterine cavity in a typical diethylstilbestrol-exposed uterus (American Fertility Society class VII). Uteri are typically hypoplastic. In this patient, no maternal history of diethylstilbestrol exposure was found.
Uterus, müllerian duct abnormalities. T-shaped uterus. Classic configuration of the uterine cavity in a typical diethylstilbestrol-exposed uterus (American Fertility Society class VII). Uteri are typically hypoplastic. In this patient, no maternal history of diethylstilbestrol exposure was found.
Degree of confidence
A large overlap exists between the subtypes when comparing uterine cavitary configuration, intercornual distance, and intercornual angle. In several studies, HSG had significantly less accuracy for diagnostic precision than MRI or US. In the studies, much of the final pathology was based on laparoscopic or surgical findings, primarily of the appearance of the uterine fundus, which HSG was not able to assess.
Since HSG techniques did not provide diagnoses with high degrees of confidence, US and MRI soon began to play a larger role in assessment and treatment of patients. HSG has been all but abandoned as a primary modality for workup of potential congenital uterine anomalies. Anomalies incidentally discovered on HSG are referred for further evaluation using MRI or US.
The only anomaly in which HSG plays a significant role in diagnosis is DES uterus (AFS class VII). The abnormal uterine cavity can be depicted clearly on HSG but often is visualized as only uterine hypoplasia on US or MRI.
HSG findings commonly allow misdiagnoses of partial septate versus bicornuate uteri or complete septate versus bicornuate bicollis versus didelphys uteri because of the large degree of overlap in the intercornual distances and angles in the entities. While the specific diagnosis may be uncertain, an abnormality usually is clearly present.
Magnetic Resonance Imaging
Examination protocol
MRI of the uterus (in benign conditions such as congenital anomalies or fibroid evaluation) is performed at the author's institution following administration of 1.0 mg IM of glucagon to decrease motion artifacts associated with bowel peristalsis.
For the diagnosis of most anomalies, 5 main sequences are sufficient:
-
Coronal single-shot fast spin-echo (FSE) images of the kidneys, ureters, and pelvis provide good localized and survey views of kidneys and ureters. This sequence is important because of the high incidence of associated renal anomalies (up to 50%), including agenesis, kidney duplication, or pelvic kidney.
-
Axial T1 spin-echo (without fat-saturation pulse) images. Fat signal is useful to delineate pelvic structures. An anterior saturation band is used to attenuate ghosting artifact from respiration.
-
Sagittal T2 FSE (without fat-saturation pulse) images. Fat signal is useful to delineate pelvic structures. An anterior saturation band is recommended. This sequence is useful for determining anterior versus retroverted uterus and to set up subsequent oblique scans along the long and short axes of the uterine fundus.
-
Oblique long-axis T2 FSE (without fat-saturation pulse) images are similar to sagittal T2 FSE sequences but obtained along the long axis of the uterus (perpendicular to the sagittal plane, usually a slightly oblique coronal). This plane is ideal for visualization of the uterine cavity and uterine fundal contour.
-
Oblique short-axis T2 FSE (without fat-saturation pulse) images are perpendicular to the long axis and sagittal planes (usually oblique axial), providing short-axis (target) views of the uterine cavity useful for visualizing a transverse septum if present.
MRI appearance of müllerian anomalies (AFS classification system)
Class I (hypoplasia/agenesis) includes absence of the uterus, cervix, and/or upper two thirds of the vagina. In uterine agenesis, no identifiable uterine tissue is noted. Partial agenesis of müllerian duct derivatives also can be visualized. In uterine hypoplasia, the endometrial cavity is small, with a reduced intercornual distance (< 2 cm). When uterine hypoplasia is associated with hormonal dysfunction (infantile uterus), not only is the uterus small, but the zonal anatomy is differentiated poorly on T2-weighted images.
Class II (unicornuate uterus) appears banana shaped without the usual rounded fundal contour and triangular appearance of the fundal cavity (see the image below). Uterine zonal anatomy is normal. If present, a rudimentary horn can be observed as a soft-tissue mass with signal intensity similar to that of myometrium. If obstructed, a rudimentary horn with functioning endometrium may be distended by blood or blood products.
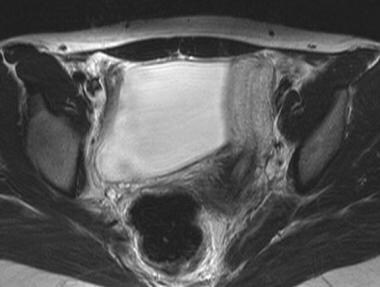 Uterus, müllerian duct abnormalities. Unicornuate uterus. Patient has full development of a single uterine horn and a normal-appearing cervix. This anomaly was one of many in this patient with Goldenhar syndrome.
Uterus, müllerian duct abnormalities. Unicornuate uterus. Patient has full development of a single uterine horn and a normal-appearing cervix. This anomaly was one of many in this patient with Goldenhar syndrome.
In class III (didelphys uterus), 2 separate normal-sized uteri and cervices are seen (see the image below). A septum may be visualized extending into the upper vagina. The 2 uterine horns are usually widely splayed, and endometrial and myometrial zonal widths are preserved. Vaginal septa are most commonly associated with this type but can be seen in the other anomalies.
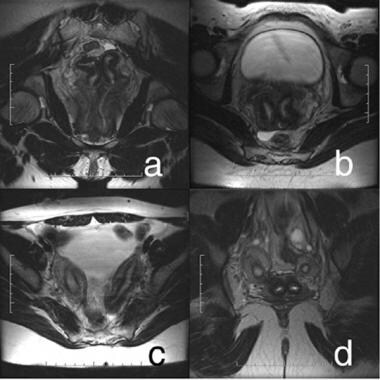 Uterus, müllerian duct abnormalities. Didelphys uterus. Complete separation and full development of both müllerian ducts is noted. (a) Two vaginas and 2 cervices; (b) 2 distinct cervices; (c) 2 uterine horns are widely splayed; (d) cross section of uterine bodies and cervices.
Uterus, müllerian duct abnormalities. Didelphys uterus. Complete separation and full development of both müllerian ducts is noted. (a) Two vaginas and 2 cervices; (b) 2 distinct cervices; (c) 2 uterine horns are widely splayed; (d) cross section of uterine bodies and cervices.
In class IV (bicornuate uterus), 2 uterine cavities are seen with normal endometrium (see the image below). The most important imaging finding is a concave fundus with a fundal cleft greater than 1 cm. This has been shown to be a reliable means of distinguishing bicornuate uterus from septate uterus. An increased intercornual distance (>4 cm) may be observed. The cleft is visualized best on oblique coronal images in the plane of the long axis of the uterus. The tissue separating the 2 horns demonstrates signal intensity identical to myometrium on all pulse sequences. The inferior portion of the septum (extending for a variable length inferiorly) may be fibrous, with low T1 and T2 signal intensity.
In class V (septate uterus), the outer fundal contour is convex, flattened, or mildly concave (fundal cleft < 1 cm; see the images below). The intercornual distance is usually normal (< 4 cm), and each uterine cavity is usually small. The septum may be composed of muscle or fibrous tissue and is not a reliable means of distinguishing septate uterus from bicornuate uterus. A more reliable means for differentiating the 2 is to examine the fundal contour (see class IV). Differentiation between a septate and a bicornuate uterus is important because septate uteri are treated with transvaginal hysteroscopic resection of the septum, whereas if surgery is possible or indicated for the bicornuate uterus, an abdominal approach is required for metroplasty.
 Uterus, müllerian duct abnormalities. T2 fast spin-echo MRI image of septate uterus acquired in the oblique plane along the long axis of the uterus. Note that the outer fundal contour (superior border) is flat or slightly concave, which is sufficient to make the diagnosis of septate uterus.
Uterus, müllerian duct abnormalities. T2 fast spin-echo MRI image of septate uterus acquired in the oblique plane along the long axis of the uterus. Note that the outer fundal contour (superior border) is flat or slightly concave, which is sufficient to make the diagnosis of septate uterus.
 Uterus, müllerian duct abnormalities. Septate uterus. Note that a longer septum divides the uterine cavity. Outer fundal contour is flat.
Uterus, müllerian duct abnormalities. Septate uterus. Note that a longer septum divides the uterine cavity. Outer fundal contour is flat.
 Uterus, müllerian duct abnormalities. MRI image of septate uterus. The patient has a thin, fibrous septum that cannot be resolved distally at the fundus. More importantly, the outer fundal contour remains convex, thus excluding a bicornuate uterus.
Uterus, müllerian duct abnormalities. MRI image of septate uterus. The patient has a thin, fibrous septum that cannot be resolved distally at the fundus. More importantly, the outer fundal contour remains convex, thus excluding a bicornuate uterus.
Class VI (arcuate uterus) abnormality may be detected by MRI, but typically, it is not clinically significant because arcuate uterus has no significant negative effects on pregnancy outcome.
Class VII (DES related) may be detected as a hypoplastic uterus on MRI. Typically, the DES-related anomaly is diagnosed confidently using HSG.
Degree of confidence
MRI has consistently demonstrated high sensitivity and specificity for evaluation of uterine anomalies. Pellerito et al found MRI capable of helping correctly diagnose all of 24 anomalies (100% accuracy), compared to 11 of 12 anomalies (92%) detected on endovaginal sonography (EVS). [39] For anomalies requiring surgery (unicornuate or bicornuate uteri), MRI demonstrated 100% sensitivity and specificity, compared to 67% sensitivity and 100% specificity for EVS. For nonsurgical lesions, both MRI and EVS had 100% sensitivity and specificity.
Pellerito et al also noted that MRI had the added advantage of detecting other incidental abnormalities, including a dermoid and submucosal leiomyoma, found on EVS to be indeterminate and nonvisualized, respectively. [39]
Data suggest very low false-negative and false-positive rates. [18]
Ultrasonography
Examination protocol
Most commonly, 2-dimensional (2D) endovaginal sonography (EVS) is used to help evaluate uterine anatomy. Transabdominal 2D imaging may be performed, ideally through a distended bladder, but offers reduced sensitivity and specificity because of increased distance from the uterus and, often, intervening bowel.
Preliminary studies indicate that 3D techniques in sonography may offer improved sensitivity and specificity for detection of uterine anomalies. Image quality for all techniques is highly operator dependent.
US appearance of mullerian duct anomalies (AFS classification system)
For class I (hypoplasia/agenesis) anomalies, findings include absence of the cervix and/or uterus with a blind-ending vagina. In uterine agenesis, no identifiable uterine tissue is present. Partial agenesis of müllerian duct derivatives also can be observed. In uterine hypoplasia, the endometrial cavity is small with a reduced intercornual distance (< 2 cm).
In class II (unicornuate uterus) anomalies, the unicornuate uterus appears banana shaped without the usual rounded fundal contour and triangular appearance of the fundal cavity. Uterine zonal anatomy is normal. If present, a rudimentary horn can be observed as a soft-tissue mass with echogenicity to that of myometrium. If obstructed, a rudimentary horn with functioning endometrium may present as a complex hemorrhagic cystic structure.
In class III (didelphys uterus), 2 separate normal-sized uteri and cervices are observed. A vaginal septum may be difficult to visualize. The 2 uterine horns are usually widely splayed, and endometrial and myometrial zonal widths are preserved.
In class IV (bicornuate uterus) anomalies, US may demonstrate 2 uterine cavities with normal endometrium. The most important imaging finding is a concave fundus with a fundal cleft greater than 1 cm. This has been shown to be a reliable means of distinguishing bicornuate from septate uteri. 3D US may play a useful role in making this diagnosis but, as of yet, is not widely available. An increased intercornual distance (>4 cm) may be observed. The cleft is visualized best on oblique coronal images in the plane of the long axis of the uterus. The septum separating the 2 horns demonstrates echogenicity identical to that of myometrium. The inferior portion of the septum (extending for a variable length inferiorly) may be fibrous.
In class V (septate uterus), US may demonstrate a convex or flattened fundal contour. The intercornual distance usually is normal or decreased (< 4 cm), and each uterine cavity is usually small. The septum may be composed of muscle or fibrous tissue and is not a reliable means of distinguishing septate from bicornuate uteri. A more reliable means to differentiate the 2 involves examining the fundal contour (see class IV). Confirm ambiguous US findings using MRI.
In class VI (arcuate uterus), US may detect the abnormality, but typically, it is not clinically significant because arcuate uterus has no significant negative effects on pregnancy outcome.
In class VII (DES related), US may detect the abnormality as uterine hypoplasia. Typically, DES-related uterus is diagnosed confidently using HSG.
Degree of confidence
Some studies have found that 3D US is highly sensitive (up to 100%) and specific (up to 100%) in helping diagnose major müllerian anomalies. [40] Studies have also found 2D transvaginal sonography to be a highly effective means of diagnosis, with 75-100% sensitivity and up to 95% specificity. Positive predictive value was higher with 3D scanning than with 2D scanning (100% vs 50%, respectively).
Nicolini et al found that transabdominal 2D US failed to visualize the uterine cavity adequately in as many as 35% of patients, although it adequately imaged the uterine fundus in 90% of patients. [41]
In a study by Raga et al, 3D US detected all of 12 congenital uterine anomalies and correctly classified the anomalies according to AFS class in 11 of 12 patients. One false-negative result involved bicornuate uterus misdiagnosed as septate uterus because of a leiomyoma that caused the fundal contour to appear convex. [42]
-
Uterus, müllerian duct abnormalities. This was difficult to differentiate as septate or bicornuate uterus using hysterosalpingography. It was a surgically proven case of bicornuate uterus.
-
Uterus, müllerian duct abnormalities. Surgically proven case of bicornuate uterus. Correct diagnosis may be suggested based on hysterosalpingography findings, which are, most notably, the widened intercornual distance (>4 cm) and the widened intercornual angle (>60°).
-
Uterus, müllerian duct abnormalities. T-shaped uterus. Classic configuration of the uterine cavity in a typical diethylstilbestrol-exposed uterus (American Fertility Society class VII). Uteri are typically hypoplastic. In this patient, no maternal history of diethylstilbestrol exposure was found.
-
Uterus, müllerian duct abnormalities. T2 fast spin-echo MRI image of septate uterus acquired in the oblique plane along the long axis of the uterus. Note that the outer fundal contour (superior border) is flat or slightly concave, which is sufficient to make the diagnosis of septate uterus.
-
Uterus, müllerian duct abnormalities. Septate uterus. Note that a longer septum divides the uterine cavity. Outer fundal contour is flat.
-
Uterus, müllerian duct abnormalities. MRI image of septate uterus. The patient has a thin, fibrous septum that cannot be resolved distally at the fundus. More importantly, the outer fundal contour remains convex, thus excluding a bicornuate uterus.
-
Uterus, müllerian duct abnormalities. Bicornuate uterus. The midline uterine external fundal cleft (superior border) has a depression greater than 1 cm, excluding septate uterus from the differential diagnosis. This image is of bicornuate bicollis, since 2 cervices are present. Bicornuate uterus is distinguished from didelphys uterus because some degree of fusion has occurred between the lower uterine segments (ie, they are fused, although the cavities are not communicating).
-
Uterus, müllerian duct abnormalities. Didelphys uterus. Complete separation and full development of both müllerian ducts is noted. (a) Two vaginas and 2 cervices; (b) 2 distinct cervices; (c) 2 uterine horns are widely splayed; (d) cross section of uterine bodies and cervices.
-
Uterus, müllerian duct abnormalities. Unicornuate uterus. Patient has full development of a single uterine horn and a normal-appearing cervix. This anomaly was one of many in this patient with Goldenhar syndrome.
-
Uterus, müllerian duct abnormalities. Normal uterus. Note a single uterine cavity and flat or convex outer fundal contour.
-
Uterus, müllerian duct abnormalities. Septate uterus. Midline septum can be of variable length and can be muscular or fibrous. In the diagram, the septum is shown as an extension of the uterine myometrium.
-
Uterus, müllerian duct abnormalities. Septate uterus. The midline septum can extend for a variable length and can be muscular or fibrous. In the diagram, the septum is thin and linear as expected in the fibrous type. Since the composition of the septum varies, whether it is composed of muscle or fibrous tissue is not a means to distinguish septate from other forms of uterine anomalies.
-
Uterus, müllerian duct abnormalities. Bicornuate uterus. Note the partial fusion of the lower uterine segment and persistently separated upper uterine segments. Of key importance is the prominent fundal cleft (>1 cm), which distinguishes the anomaly from septate uterus.
-
Uterus, müllerian duct abnormalities. Unicornuate uterus. Note the failure of the development of one half of the uterus. This form may be associated with a rudimentary horn arising from the contralateral müllerian duct.
-
Uterus, müllerian duct abnormalities. Didelphys uterus. Note the complete separation but full development of each müllerian duct.
-
Uterus, müllerian duct abnormalities. Arcuate uterus. Mild thickening of the midline fundal myometrium resulting in fundal cavity indentation but normal outer fundal contour. Some authors consider it a normal variant. It is not associated with an increased risk of obstetric/gynecologic complications.
-
Uterus, müllerian duct abnormalities. Diethylstilbestrol-exposed uterus. Myometrial hypertrophy results in a T-shaped uterine cavity and cavity irregularity, which is pathognomonic for the anomaly. Typically, the uteri are hypoplastic.






