Practice Essentials
The pituitary gland is the master gland of the body because it controls most of the body's endocrine functions by means of the hypothalamic-pituitary axis (see the images below). The anterior lobe of the pituitary gland secretes 6 hormones: thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), leuteinizing hormone (LH), growth hormone (GH), and prolactin (PRL). The posterior pituitary gland secretes vasopressin and oxytocin. For pituitary adenoma imaging, CT and MRI have largely replaced plain radiography because conventional radiography is poor for delineating soft tissues. [1, 2, 3, 4, 5, 6, 7, 8, 9]
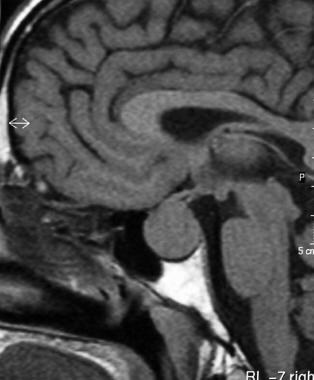
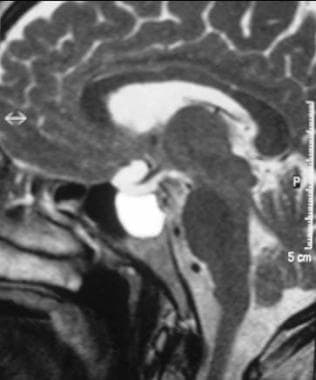
 T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
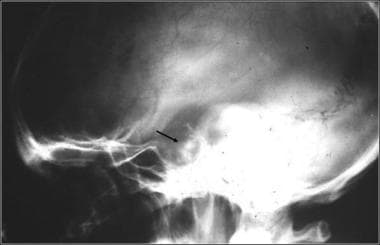 Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow)
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow)
Pituitary adenomas occur primarily in the adenohypophysis and represent 10-25% of all intracranial neoplasms; estimated prevalence in the general population is around 17%. [10, 11, 12] The World Health Organization (WHO) classifies pituitary adenomas as typical adenomas, atypical adenomas, and pituitary carcinomas and encourages taking into account tumor invasion (evaluated by MRI and/or histology) and histopathologic parameters. [13]
Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors. Small null-cell tumors measuring a few millimeters are common and found in up to 25% of autopsied pituitary glands. These may grow slowly, destroying normal pituitary function (hypopituitarism), or they may compress nearby structures and cause neurologic problems.
Functioning pituitary adenomas can be clinically classified by means of the hormone they elaborate. These tumors become symptomatic because they secrete hormones, and they are less likely than like null-cell tumors to become large enough to compress adjacent structures. As pituitary tumors grow, destruction of normal pituitary tissue results in various hormonal deficiencies. In rare cases, these tumors may spontaneously hemorrhage or become infarcted. The pressure they exert on nearby structures can produce double vision and facial numbness. The optic chiasm is directly above the pituitary gland, and upward growth of pituitary tumors frequently causes progressive visual loss. This visual loss typically begins from each side of the field of vision and leads to tunnel vision and then blindness. [14, 15]
Chen et al found that in children and adolescents with pituitary adenomas, recurrence rates after resection were higher in patients with an elevated proliferation index ≥3% (20.8%), imaging evidence of local invasion (18.2%), or both (25%). [16]
Sanmillan et al retrospectively analyzed 294 surgically treated pituitary adenoma (148 by microsurgical transsphenoidal approach and 146 by endoscopic endonasal approach). They found that pituitary adenoma volume and cavernous sinus invasion (graded with the Knosp scale), when used in combination, could predict the complexity of the surgery and the difficulty of achieving gross total resection. [4]
Clinical features
Features based on secretory ability
The clinical features of pituitary adenoma vary depending on the location and size of the tumor and its secretory capability. Pituitary adenomas typically appear during early adulthood, and no sex predilection is known. Secretory pituitary adenomas are usually small and generally do not cause neurologic symptoms or hypopituitarism, although they can. The symptoms of functioning tumors are related to the specific hormone the tumor produces. [17, 18]
Neurologic symptoms of pituitary adenomas include headaches; double vision; and loss of peripheral vision leading to blindness, facial pain, or numbness. Hypopituitarism manifests itself by lack of energy, weight loss, nausea, vomiting, constipation, amenorrhea and infertility, dry skin, increase pigmentation of the skin, cold intolerance, and mental status changes (eg, sleepiness, psychosis, collapse).
A prolactinoma is the most common pituitary tumor and may cause amenorrhea, irregular periods, galactorrhea, infertility in women, and osteoporosis. It may cause hypogonadism, loss of libido, and impotence in men.
Tumors that secrete excess GH cause gigantism in children and acromegaly in adults. Acromegaly is associated with enlargement of facial features, hands and feet, heart disease, hypertension, arthritis, carpel tunnel syndrome, amenorrhea, and impotence.
ACTH-secreting adenomas produce Cushing disease, which itself results in a widened face with acne and flushing, fatty deposits over the back of the neck, stretch marks, easy bruising, hair growth, diabetes mellitus, muscle loss, fatigue, depression, and psychosis.
Tumors that elaborate TSH produce signs and symptoms of thyrotoxicosis, such as heat intolerance, sweating, tachycardia, fine tremor, and weight loss. Some tumors may secrete more than one hormone, such as GH and PRL.
Rare tumors secrete LH or FSH (gonadotrophins). When pituitary tumors compromise the secretory cells, the first evidence of cellular failure usually affects the gonadotrophins. Therefore, the disappearance of menstrual periods may be the first sign of a pituitary tumor in female patients. In male patients, the most common symptom of deficiency is impotence. Isolated deficiencies of both LH and FSH do occur, but only rarely. In a male individual, LH deficiency alone leads to the appearance of a fertile eunuch. In this condition, sufficient FSH is present to permit the maturation of spermatozoa; however, because of the LH deficiency, the patient has many of the characteristics of a castrated individual. Tumors also can produce an excess of LH or FSH, and pituitary tumors that secrete only the nonspecific, hormonally inactive alpha unit of glycoprotein hormones are not rare.
Other features
Visual symptoms are generally related to compression of visual pathways and include bitemporal visual-field loss, which is denser from the superior to inferior than in other orientations, color desaturation, diplopia, and ophthalmoplegia. The funduscopic sign of long-standing chiasmal compression is primary optic atrophy. Severe optic atrophy indicates a poor prognosis for visual recovery after surgical decompression. In pregnant women, bitemporal visual-field loss and headache may indicate pituitary apoplexy.
Pituitary apoplexy is a potentially life-threatening condition. Women with pituitary adenomas and MRI evidence of subarachnoid bleeding should deliver by cesarean section to prevent apoplexy during delivery. Postpartum hemorrhage can cause infarction of the pituitary gland, leading to hypopituitarism (Sheehan syndrome).
Other problems to consider
Clinical masquerades
Clinical masquerades of pituitary adenoma include chronic retrobulbar optic neuritis, nutritional amblyopia, uncorrected refractive error, normal-tension glaucoma, and age-related maculopathy. Bilateral tilted-disc syndrome can result in a superior bitemporal field defect similar to that observed in pituitary adenoma. However, the field defect in tilted-disc syndrome is unchanging and does not respect the vertical midline, whereas the field defects in chiasm-compressive lesions are progressive and do respect the vertical midline.
Craniopharyngioma
Craniopharyngiomas are benign, slow-growing tumors that originate from epithelial remnants of the Rathke pouch at the junction of the infundibulum and the pituitary gland. These lesions are composed of both solid epithelial tissue and cystic components. The cystic components contain variable amounts of cholesterol, keratin, necrotic debris, proteinaceous fluid, and hemorrhage. Calcification is present in 75-85%.
MRI appearances vary depending on the amount of solid and cystic components and on the nature of cystic contents. Heterogeneous signal intensity on images obtained with all sequences is the most typical finding. Solid components are hypointense on T1-weighted images and hyperintense on T2-weighted images. The cysts also have a long T2; however, if they have a high cholesterol content or methemoglobin, shortening of T1 results in high signal intensity on T1-weighted images. Calcification in the tumor is better detected with CT than with MRI because MRI may not reliably depict calcification. Craniopharyngioma may also cause truncation of the dorsum sellae and upward growth into the third ventricle, which is readily identified on MRI.
Aneurysm
Aneurysms in the parasellar region may originate from the circle of Willis or intracavernous carotid arteries. MRI features include a mass of heterogeneous signal intensity due to flow effects and thrombus formation. Low signal intensity is caused by high flow and chronic thrombus; high signal intensity may represent slow flow or subacute thrombus. Flow in the patent lumen may also cause a band of artifact in the phase-encoding direction on spin-echo images. Magnetic resonance angiography (MRA) is useful in confirming an aneurysm. A potential pitfall in diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm.
Empty Sella
An empty sella occurs as a result of herniation of the arachnoid through an incompetent diaphragma sellae. Over time, cerebrospinal fluid (CSF) pulsations may enlarge the sella and compress the gland against the floor of the sella. An empty sella is usually asymptomatic and an incidental finding, but can be a manifestation of increased intracranial pressure. However, they are occasionally severe. Compression of the pituitary gland may affect function, or traction on the optic chiasm and nerves may cause visual symptoms.
Chiasmatic and hypothalamic gliomas
Gliomas of the optic chiasm and the hypothalamic pathways are primarily tumors of children and young adults. The tumors tend to be low grade, but they infiltrate along the visual pathways. Neurofibromatosis is strongly associated with optic and chiasmatic gliomas. Hypothalamic gliomas are generally aggressive and produce symptoms early, resulting in 1 of many hypothalamic syndromes: diabetes insipidus; inappropriate secretion of antidiuretic hormone (ADH); Fröhlich syndrome; or disturbances of temperature, appetite, or metabolism.
Chiasmatic gliomas are usually isointense or slightly hypointense on T1-weighted images and hyperintense on T2-weighted images. MRI is useful in determining degree of infiltration of the optic chiasm or optic nerves and for assessing posterior extension into the lateral geniculate body and the occasional exophytic growth into the suprasellar and interpeduncular cisterns. Both hypothalamic and chiasmatic gliomas are enhancing after the intravenous administration of gadolinium-based contrast agent. The multiplanar capability of MRI enables it to depict extension into surrounding structures well.
Pituicytomas
Pituicytomas are also called choristomas, granular cell tumors, or myoblastomas and are rare largely noninfiltrating sellar or parasellar tumors in adults. They arise along the distribution of the neurohypophysis, including both the stalk and the posterior lobe. They occur in the suprasellar space, in the sella, or both. Most symptomatic pituicytomas appear as suprasellar masses. They may originate from the posterior pituitary and remain confined within the sella turcica. Pituicytoma is generally a surprise finding in that it is seldom considered in the preoperative differential diagnosis of a suprasellar lesion. The MRI signal-intensity characteristics vary depending on the cystic components. The solid parts of the tumor are enhancing.
Ectopic pituitary
An ectopic pituitary gland results in high signal intensity adjacent to the median eminence of the hypothalamus with an absence of the normal posterior pituitary bright spot on T1-weighted MRIs. An ectopic pituitary may be associated with perinatal asphyxia and disruption of the normal hypothalamic-pituitary axis. Traumatic transection of the stalk is exceptionally rare, but it can result in abnormal accumulation of posterior lobe hormones proximal to the disruption.
Metastasis
Most metastases to the pituitary gland are small, clinically silent, and rare in the clinical setting, though they are frequently reported in autopsy series. Large metastasis may cause diabetes insipidus. Leukemia, lymphoma, and cancers of the lung or breast are the most common primary origins. The demonstration of rapid growth distinguishes metastases from slow-growing pituitary adenomas.
Rathke cleft cyst
Rathke cleft cysts arise from remnants of the Rathke cleft, a fetal link between the hypothalamus and nasopharynx that obliterates in normal individuals. Rathke cleft cysts are benign cysts lined by a single layer of ciliated columnar or cuboidal epithelium, and they often contain goblet cells. When small, Rathke cleft cysts are intrasellar. However, as they grow, they extend into the suprasellar region. Rathke cleft cysts are smoothly marginated and well-defined lesions. Their MRI signal-intensity characteristics vary depending on the contents of the cyst. After gadolinium enhancement, only the capsule is enhancing; the nodular component is not enhancing. This feature helps distinguish these lesions from craniopharyngiomas. [1, 2]
Hamartoma of the tuber cinereum
Hamartomas of the tuber cinereum of the hypothalamus are benign, slow-growing tumors that consist of hyperplastic hypothalamic glial and neural tissue. The usual presentation is precocious puberty. The tumors may be sessile or pedunculated, occurring between the infundibulum and mamillary bodies. They are usually isointense to the brain on T1-weighted images and mildly hyperintense on T2-weighted images. They do not show contrast enhancement, a feature that distinguishes them from hypothalamic gliomas and germinomas.
Lymphoma
Favored sites for primary malignant non-Hodgkin lymphomas are the hypothalamus, the cavernous sinuses, and the perisellar regions. These rapidly growing tumors mostly affect patients who are immunocompromised because of chemotherapy, HIV infection, or organ transplantation. Lymphomas typically appear as homogeneous, slightly hyperintense masses on T2-weighted images. In general, lymphomas are uniformly and intensely enhancing. Cystic, hemorrhagic, and necrotic areas in these tumors are unusual.
Germinoma
Germinomas occur in the pineal and suprasellar region and affect children and young adults. Because of their propensity to invade the hypothalamus and to grow into the third ventricle, they may cause endocrine dysfunction. They are known to disseminate through CSF pathways. The tumors are usually well defined and isointense with the brain on T1- and T2-weighted images. They generally do not show necrosis, cystic change, or hemorrhage. Contrast enhancement is moderate and essential in the assessment of CSF spread of the tumor. Calcification in germinomas may make them difficult to visualize on MRIs.
Arachnoid cyst
Arachnoid cysts are CSF-containing spaces that are generally not confused with pituitary or parasellar tumors. These cysts have CSF signal intensity, they are well defined, they do not calcify, and they are not enhancing after the administration of contrast material.
Epidermoid cyst
Epidermoid cysts are benign, slow-growing tumors that arise from epithelial cell rests in the basal cisterns. They have a propensity to grow along the subarachnoid spaces and into the various crevices at the base of the brain. Intradural epidermoids are usually large, with lobulated outer margins and an insinuating pattern of growth. Epidermoid cysts are classically confused with arachnoid cysts on CT and MRI because they are similar to CSF in attenuation and intensity, respectively, and because they have similar T1 and T2 signal intensities. Diffusion-weighted images, proton density–weighted images, and fluid-attenuated inversion recovery (FLAIR) images are useful in making the distinction. Epidermoid cysts do not show contrast enhancement.
Dermoid cyst
Dermoid cysts occur in the pineal and suprasellar regions, as well as other midline locations. On histology, they have both mesodermal and ectodermal derivatives, which account for their varied appearance on MRI. The MRI appearances are those of a heterogeneous tumor. Fatty tissues in the tumor produce high signal intensity on T1-weighted images, and a fat-fluid level may be seen. These cysts may rupture, causing the cystic contents to leak into a ventricle or subarachnoid space and then produce an ependymitis or meningitis, respectively.
Meningioma
Parasellar meningiomas commonly involve the cavernous sinus and produce ophthalmoplegia. Meningiomas are hypervascular tumors that derive their blood supply from the dural vessels. They also induce an osteoblastic reaction in the adjacent bone, resulting in a characteristic focal hyperostosis, which is well depicted on plain radiographs and CT. MRI signal is isointense relative to the brain on T1- and T2-weighted images. MRI is particularly good for assessing encasement of the cavernous sinus and carotid artery. The tumor is intensely enhancing on contrast-enhanced images.
Schwannomas
Schwannomas are nerve-sheath tumors that may involve cranial nerves III-XII or peripheral nerves. Schwannomas in the parasellar region arise from the trigeminal nerve and, in rare cases, from the third, fourth, or sixth cranial nerves. These tumors are benign, well encapsulated, and globular; all of these features distinguish them from broad-based meningiomas. The tumors are isointense on T1-weighted MRIs and mildly hyperintense on T2-weighted MRIs. Cystic degeneration is frequent; hemorrhage and calcification are rare. The solid portions of these tumors are strongly enhancing with the use of intravenous contrast material.
Primary and secondary tumors of the skull base
A wide variety of primary and metastatic tumors of the skull base can involve the parasellar region. Nasopharyngeal carcinomas and malignant tumors of the paranasal sinuses may invade the parasellar region, and metastases from distant primary tumors may also involve the sphenoid bone and the parasellar region. The primary tumors include chordoma, chondroma, chondrosarcoma, and plasmacytoma.
Chordomas have uniform high signal intensity on T2-weighted MRIs. Chondrosarcomas are heterogeneous on images obtained with all sequences because of their variable calcification and chondral elements, but they usually have areas of high T2 signal intensity. Chordomas characteristically arise in the midline, whereas chondrosarcomas arise off midline at the synchondroses. Alteration of the normal hyperintense clival fat is a sensitive indicator of these tumors; therefore, nonenhanced non–fat-saturated T1-weighted imaging is particularly useful. Contrast enhancement is helpful in assessing the intracranial components of these tumors.
Carotid-cavernous fistula
The fistulous communication between the carotid artery and cavernous sinus may result from a dural arteriovenous malformation, trauma, or transsphenoidal surgery. Arterial pressures cause expansion of the cavernous sinus and dilation of parasellar and orbital veins. Proptosis, chemosis, and visual loss may complicate such fistulas. Flow effects and flow artifacts on MRI may confirm the diagnosis.
Granulomatous hypophysitis
Granulomatous hypophysitis is a rare entity that mimics a pituitary adenoma both clinically and radiologically. The reported causes of granulomatous hypophysitis include tuberculosis, syphilis, sarcoidosis, mycotic granuloma, and foreign body granuloma due to a ruptured Rathke cleft cyst. Lymphocytic hypophysitis represent inflammation of the pituitary gland and may complicate pregnancy or the postpartum period. MRI findings include diffuse enlargement of the anterior lobe and pituitary stalk. The gland is strongly enhanced with gadolinium-based contrast agents, and the enhancement commonly extends along the diaphragma sellae.
McCune-Albright syndrome
McCune-Albright syndrome (MAS) may clinically mimic acromegaly or gigantism. The syndrome is characterized by polyostotic fibrous dysplasia, café au lait pigmentation of the skin, and autonomous endocrine hyperfunction. The most common form of autonomous endocrine hyperfunction in this syndrome is gonadotropin-independent precocious puberty; however, affected individuals also may have hyperthyroidism, hypercortisolism, pituitary gigantism, or acromegaly. Nonendocrine abnormalities in this disorder include hypophosphatemia, chronic liver disease, tachycardia, and sudden death (which is rare and possibly due to cardiac arrhythmias).
Imaging modalities
The clinical diagnosis of pituitary adenomas depends on the combination of symptoms and signs resulting from the size of the tumor and/or the type of hormone produced. CT and MRI have largely replaced plain radiography because conventional radiography is poor for delineating soft tissues. [5, 6, 19, 20, 21, 22, 7, 9]
Angiography is seldom performed; if indicated, CT angiography (CTA) and MRA have largely replaced conventional angiography. Angiography has a role when intervention is indicated in the cavernous sinus or in the cavernous part of the carotid artery.
Conventional single-section CT has a limited role in pituitary imaging, with a sensitivity of 17-22% in detecting microadenomas. Multidetector-row CT with 64 channels may have a role, especially in patients unable to undergo MRI. CT is best for visualizing bony detail and calcification in tumors such as germinomas, craniopharyngiomas, and meningiomas. CTA is excellent for showing the morphology of parasellar aneurysms and for presurgical planning. CT scans are valuable when MRI is contraindicated, as in patients with pacemakers or metallic implants in the brain or eyes.
MRI is generally preferred over CT for the diagnosis of pituitary adenomas because of its superior definition of small lesions in the pituitary sella and its improved anatomic definition before surgery. MRI is also preferred for postsurgical surveillance.
Somatostatin receptor scintigraphy may be used to distinguish recurrent or residual tumor from scar or necrotic tissue after surgery.
Micko et al found that optical coherence tomography in association with convolutional neural network (CNN) computing can identify pituitary adenoma tissue in biopsy samples with high sensitivity (84-100%). [23]
Limitations of techniques
Conventional radiographs are poor for delineating soft tissues. MRI is more expensive than CT, but it is the preferred imaging study for the pituitary because it improves visualization of the soft tissues and vascular structures. Other limitations of CT include suboptimal imaging of the soft tissues compared with MRI, the need for intravenous contrast medium to enhance images, and the exposure to radiation.
A potential pitfall of MRI diagnosis is a pneumatized anterior clinoid or calcification, which can simulate the flow void of an aneurysm. In addition, MRI is contraindicated in patients with pacemakers or ferromagnetic implants in the brain or eyes. With CT or MRI, pituitary adenoma remnants can be hard to differentiate from radiotherapy-induced fibrosis, especially in patients with clinically nonfunctioning pituitary adenomas, who lack circulating markers that aid in monitoring the progression or cure of the disease.
Computed Tomography
In the ideal situation, CT of the pituitary gland is performed with multidetector-row CT. [24] Volumetric (64-section) CT scanners produce coronal reformations with a resolution near that of direct coronal acquisitions. Single-channel axial and helical CT of the pituitary gland is best performed in the direct coronal plane by maximally extending the patient's neck while the patient is either supine or prone. This method allows for the demonstration of pituitary abnormalities with radiation dose to the lens that is lower than that possible with axial imaging.
As an alternative, pituitary CT can be performed in the axial plane with thin (1-mm) contiguous sections after the intravenous administration of 100 mL of contrast medium. Exposure parameters are approximately 120 kV, 200 mA, 2-second scanning time, and a soft tissue algorithm. Images are reformatted in the coronal and sagittal planes.
Microadenomas are small, round tumors embedded in the parenchyma of the pituitary gland. Microadenomas uncomplicated by hemorrhage or cyst formation are typically isoattenuating or hypoattenuating relative to the adjacent normal pituitary gland. They may not be visible on nonenhanced CT scans. Enhancement after the administration of contrast material occurs but is delayed compared with the immediate, intense enhancement of the normal pituitary gland. This effect is due to the absence of a blood-brain barrier while the adenoma remains hypoattenuating. Therefore, about two thirds of microadenomas typically appear hypoattenuating on dynamic, rapid-sequence, contrast-enhanced CT scans, and one third show early enhancement.
Macroadenomas have variable appearances. Most are isoattenuating relative to the cortex on nonenhanced CT scans and show moderate enhancement on enhanced scans. Calcification is rare (1-8%). Necrosis, cyst formation, and hemorrhage may result in lesions of mixed attenuation. CT also shows bony changes and the mass lesion, with expansion of the sella turcica with or without erosion or depression of the sellar floor toward the sphenoid sinus. Secreting adenomas protrude or expand into the cavernous sinus more often than do nonsecreting macroadenomas. [25] In summary, microadenomas are hypoattenuating relative to the normal pituitary on dynamic enhanced CT scans, and macroadenomas are isoattenuating on nonenhanced CT scans and intensely enhancing on enhanced scans.
CTA is extremely useful in preoperative planning for macroadenomas. The relationship of the tumor to the anterior cerebral arteries and optic nerve are critical to the surgeon. Image guidance protocols for thin-section CT are also useful during surgery itself.
Although MRI is the imaging modality of choice to study pituitary adenomas, CT still has a role because many patients cannot undergo MRI. CT is also useful to depict calcification, which may influence the differential diagnosis. CT may contribute to preoperative planning, particularly in regard to pneumatization and the anatomy of the sphenoid sinus. The drawback of CT is that its ability to show the soft tissue is suboptimal compared with that of MRI. In addition, contrast medium is used to enhance images, and patients are exposed to radiation.
A study of 28 patients with surgically confirmed functional pituitary microadenomas showed that dynamic contrast-enhanced multisection CT combined with image reconstruction holds promise in detecting MRI-occult pituitary microadenomas. In this study, 15 cases were correctly diagnosed by MRI, while dynamic contrast-enhanced multisection CT correctly diagnosed 26 cases. The accuracy of localization was markedly better for adrenocorticotropic hormone-secreting microadenomas, increasing from 32% on MRI to 85% by dynamic contrast-enhanced multisection CT. [26]
The sensitivity of conventional CT in detecting pituitary microadenomas is 17-22%. The accuracy of modern scanners has not yet been evaluated.
Magnetic Resonance Imaging
In children, the height of normal pituitary gland can be evaluated as a function of age. Pituitary gland heights (PGHs) have been measured on strictly sagittal T1-weighted images obtained with 3- to 7-mm-thick sections. The measurement is taken at the greatest height, which is usually the midpoint. PGH physiologically enlarges at birth, at puberty (6-7 mm), during pregnancy (< 10 mm), and after birth (< 12 mm).
Sex-related differences in the PGH are observed. In the age group of 10-69 years, pituitary height is greater in female individuals then in male individuals. In pediatric patients aged 0-9 years, both sexes have minimal pituitary height. Maximal height is observed in the 10- to 19-year age group. The height gradually decreases with age after 20 years. In a study by Suzuki et al, no females had a PGH of 9 mm or more, and no males had a PGH of 8 mm of more. [27]
On MRI, the normal anterior pituitary gland and its stalk return uniform isointensity relative to gray matter. These structures also show intense enhancement after the administration of contrast agent. The gland may be hyperintense in neonates and in pregnant women. The normal posterior pituitary appears bright on T1-weighted MRIs. In about 10% of individuals, MRIs show focal pituitary abnormalities, which are thought to represent protein molecules in the gland.
On T2- and nonenhanced T1-weighted MRIs, the normal anterior lobe of the pituitary gland is isointense relative to the gray matter of the brain. The posterior lobe is hyperintense on T1-weighted images. The high proteinaceous content of the neurosecretory vesicles in the posterior pituitary lobe partly accounts for this appearance.
Nonneoplastic cysts are seen in 20% of autopsies and may represent a normal variant or glandular degeneration. In women of childbearing age, the pituitary gland varies substantially in size over the course of the menstrual cycle. In these women, the normal gland may have a convex superior surface, and it may appear to bulge out of the sella turcica.
(The pituitary, cysts, adenomas, and metastases on MRI are displayed in the images below.)
 Sagittal T2-weighted MRI shows a hyperintense cystic lesion above the pituitary gland due to a Rathke pouch cyst in the region of the pars intermedia.
Sagittal T2-weighted MRI shows a hyperintense cystic lesion above the pituitary gland due to a Rathke pouch cyst in the region of the pars intermedia.
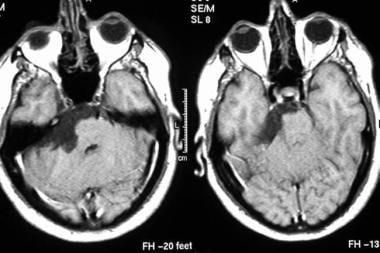 Axial contrast-enhanced T1-weighted MRIs show an epidermoid cyst. Note the low signal intensity in the tumor along crevices of the subarachnoid space.
Axial contrast-enhanced T1-weighted MRIs show an epidermoid cyst. Note the low signal intensity in the tumor along crevices of the subarachnoid space.
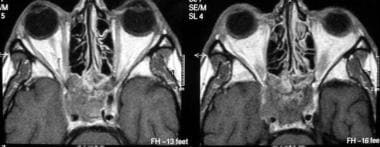 Axial contrast-enhanced T1-weighted MRIs show a metastasis to the sphenoid bone and the cavernous sinus from a primary site in the colon.
Axial contrast-enhanced T1-weighted MRIs show a metastasis to the sphenoid bone and the cavernous sinus from a primary site in the colon.
Microadenomas
In the evaluation of pituitary microadenomas, the key issues are the presence or absence of an adenoma, the location of the adenoma, and its invasive effects on the adjacent structures. High-resolution, thin-section MRI of the pituitary gland in the coronal and sagittal planes is the imaging modality of choice.
Pituitary microadenomas are usually slightly hypointense to isointense to the gray matter on T1-weighted images and range from isointense to hyperintense on T2-weighted images unless they contain intratumoral hemorrhage or cystic changes. Microadenomas are often difficult to detect unless rapid-sequence contrast-enhanced T1-weighted images are obtained. These tumors appear as foci of relatively low enhancement.
Macroadenomas
Macroadenomas show a heterogeneous enhancement pattern that reflects varying degrees of necrosis, cyst formation, and hemorrhage.
In macroadenomas, the aims of imaging are to precisely demarcate the boundary of normal tissue against the tumoral tissue, to assess for invasion of the cavernous sinus, and to demonstrate any mass effect on neighboring structures (eg, optic chiasm). Also critical is the relationship of the lesion to the nearby vasculature. These factors are important from the surgeon's perspective. Invasion of the cavernous sinus is related to biologically aggressive neoplasms and increases the risk of morbidity and mortality with surgical procedures even though the tumor remains histologically benign in most cases.
In a study by Connor et al, in 49 patients who had undergone transsphenoidal surgery for macroadenomas with potential unilateral parasellar involvement, the preoperative MR imaging features that were useful in predicting complete removal of the parasellar component of a pituitary adenoma (as assessed by postoperative MRI) were depiction of the lateral and inferolateral compartment of the cavernous sinus and decreasing encasement of the intracavernous carotid artery. [28]
According to Patel et al, postoperative early high-resolution volumetric MRI is valuable in determining the presence or absence of residual tumor after transsphenoidal pituitary surgery for macroadenomas. Early scans were 100% sensitive and 91% specific for predicting greater than 98% resection. The authors noted that cavity volume almost always decreases after surgery, and a lack of such a decrease should alert the surgeon to the possibility of persistent compression of the optic apparatus that may warrant reoperation. [29]
In one study, the use of 3T intraoperative MRI during transsphenoidal microsurgery on pituitary macroadenomas led to an increase in both the extent of tumor resection (in 8 of 73 patients, or 10.9%) and the rate of radical resections (69% instead of 60%). [30]
Enhanced imaging
High-resolution gadolinium-enhanced dynamic MRI has emerged as the most technically refined tool for evaluating pituitary microadenomas. This method makes use of the short temporal window in which contrast between the adenoma and the normal pituitary gland is high; this technique allows for optimal delineation of the tumor. This technique is superior to standard contrast-enhanced T1-weighted MRI in detecting microadenomas, particularly when no contour abnormality is present. This method also provides greater clarity than that of standard sequences after contrast agent is infused.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
On dynamic contrast-enhanced imaging, enhancement occurs in an expected sequence because of the unique and separate blood supplies to the pars nervosa, infundibulum, and pars distalis by the inferior hypophyseal artery, superior hypophyseal artery, and the portal system, respectively.
The earliest enhancement of normal structures is seen in the infundibulum and in the posterior lobe of the pituitary gland, followed by gradual enhancement of the anterior lobe from the junction of the infundibulum to the peripheral portion of the anterior lobe of the pituitary gland. Peak enhancement of pituitary adenomas usually occurs after the marked enhancement of the normal pituitary gland appears. By virtue of the differential enhancement pattern, adenomas are best seen in the early phase of gadolinium-enhanced dynamic imaging; they appear as hypointense lesions against the hyperintense background of the normally enhancing pituitary gland.
Other secondary morphologic features, such as infundibular displacement and focal glandular convexity, are readily demonstrable. Underlying bone destruction and tumoral calcification can be seen, but they are better demonstrated with CT than with MRI.
Degree of confidence
MRI is the examination of choice in pituitary and parasellar tumors because it depicts the complex anatomy around the sella well. Almost 30 various pathologic entities occur in this region, and most can be differentiated with MRI. These entities can be broadly divided on the basis of their involvement, as follows: intrasellar, infundibular, suprasellar, anterior part of the third ventricle and/or optic chiasm, and sphenoid and/or cavernous sinus. MRI is a good modality of choice to evaluate the organ of origin. [31, 32, 33, 34, 35, 36, 37, 38, 39]
Yin and associates studied the MRI data of 142 patients retrospectively with proven sellar lesions to evaluate the performance of MRI in the diagnosis of sellar lesions. Thirty patients were found to have pituitary adenoma cystic lesions, and sella were diagnosed in 24. Further classification resulted in 66 cases of parenchymatous lesions and 22 cases of cystic and parenchymatous lesions. The authors found MRI-based classification of sellar lesions useful for the diagnosis and differential diagnosis. [40]
According to Yun et al, based on a retrospective review of MRI scans and tumor histology of 50 patients who underwent endoscopic endonasal resection for nonfunctional adenomas, preoperative MRI analyses can be helpful, although not definitive, in predicting adenoma consistency. The investigators found that fibrous adenomas are associated with higher collagen content and are more difficult to resect, with higher rates of subtotal resection. [3]
False positives/negatives
In about 10% of the healthy adult population, MRIs show pituitary abnormalities compatible with the diagnosis of asymptomatic pituitary adenomas. Most pituitary adenomas remain asymptomatic and do not require treatment.
False-positive results are possible with asymptomatic focal pituitary lesions, such as cysts of the pars intermedia, metastases, infarctions, epidermoid cysts, and abscesses. These lesions may appear as areas of low signal intensity after the administration of gadolinium-based contrast agent. False-positive pituitary MRIs have also been reported in patients with ectopic ACTH secretion and the Cushing syndrome. In patients with Cushing syndrome, the incidence of pituitary adenomas at surgery is high; therefore, a focal hypointense area after gadolinium enhancement is accepted as being diagnostic of a pituitary microadenoma in a patient with pituitary endocrinopathy. [41]
Nuclear Imaging
Somatostatin receptors
Somatostatin receptors have been demonstrated in vitro in most GH-secreting pituitary adenomas and in some TSH- and PRL-secreting pituitary tumors. These receptors have not been consistently demonstrated in nonfunctioning adenomas. Recurrent or residual pituitary tumors are hard to distinguish from postoperative fibrosis with MRI and CT.
Scintigraphic visualization of residual tumor is possible with labeled somatostatin analogs, such as indium-111 diethylenetriaminepentaacetic acid (DTPA)-octreotide. This method allows scar tissue to be differentiated from recurrent tumor and helps in identifying patients who may benefit from somatostatin analogs.
Scintigraphy with technetium-99m pentavalent dimercaptosuccinic acid, or 99mTc (V)DMSA, also helps in detecting most pituitary GH- and PRL-secreting adenomas and nonfunctioning adenomas, with tumor-to-background ratios as high as 25. Functional imaging of pituitary remnant adenomas (>10 mm) with 99mTc (V)DMSA depicts viable remnants of pituitary adenomas. This approach may be of value in patients with clinically nonfunctioning adenomas to monitor the effects of radiation therapy. [42]
Indium-111-DTPA-octreotide scintigraphy is a technique for imaging somatostatin receptors in many neuroendocrine tumors (eg, pituitary adenomas). This agent is highly sensitive and a suitable tracer to evaluate the state of somatostatin receptor in pituitary adenomas. In one study, 111In-DTPA-octreotide single photon emission CT (SPECT) depicted adenomas in 21 (78%) of 27 patients who had 1 nonsecreting, 10 GH-secreting, and 10 PRL-secreting tumors.
The role of 111In-DTPA-octreotide scintigraphy in assessing nonfunctioning pituitary tumors has not been firmly established. Treatment with untagged octreotide may arguably interfere with tracer uptake in pituitary tumors. Therefore, patients undergoing scintigraphy should likely stop treatment for 2-3 days before the test. [43]
Studies have shown a good correlation between high 111In-DTPA-octreotide uptake and the response to octreotide therapy in patients with TSH- and GH-secreting pituitary adenomas. This method of imaging may facilitate the development of noninvasive treatments of somatostatin receptor–positive pituitary adenomas that might benefit from octreotide treatment. Such studies may be a breakthrough for poor surgical candidates, for patients who refuse surgery, or for presurgical and/or postsurgical imaging.
Naswa et al reported a case of multiple endocrine neoplasia type-1 syndrome associated with an ectopic pituitary adenoma in which somatostatin receptor–based PET/CT imaging using 68Ga-DOTANOC was instrumental in the diagnosis of ectopic pituitary adenoma. [44]
Angiography
Catheter angiography has a limited role in the management of pituitary adenomas. Conventional contrast-enhanced angiography is now virtually obsolete because MRA and CTA can depict the positions of the intracavernous and supraclinoid carotid arteries and because they are useful in differentiating pituitary mass lesions from aneurysms. In rare cases, the diagnoses of a substantially thrombosed aneurysm requires intra-arterial digital subtraction angiography (DSA).
Venography continues to have a role during catheter navigation for venous sampling during a workup for causes of Cushing syndrome. Bilateral simultaneous venous sampling from the inferior petrosal sinuses after a peripheral injection of corticotropin-releasing hormone (CRH) can confirm or rule out a central (pituitary) source of ACTH by showing a substantial gradient between the pituitary (central) and peripheral values of plasma ACTH and thus confirm Cushing disease.
Degree of confidence
The sensitivity and specificity of bilateral, simultaneous venous sampling from the inferior petrosal sinuses after a peripheral injection of CRH in confirming or ruling out a pituitary source of ACTH is 96-100%. The positive predictive value of this test is high because virtually no false-positive results occur. To some extent, it can even help in lateralizing the site of the pituitary tumor to guide microsurgery. False-positive results are virtually unknown with venous sampling from the inferior petrosal sinuses. False-negative findings are uncommon.
-
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
-
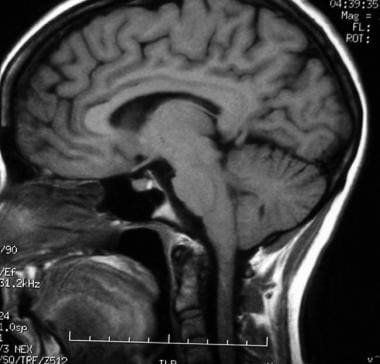
T1-weighted sagittal MRI through the pituitary fossa shows a normal pituitary gland.
-
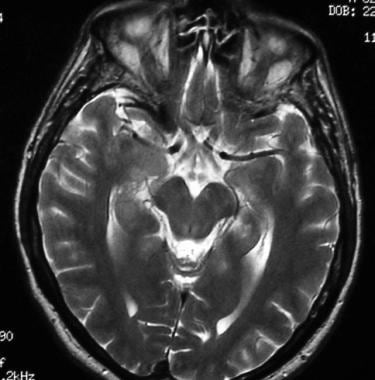
T2-weighted axial MRI shows a normal optic chiasm and optic nerves.
-
Ectopic pituitary gland. Note the high signal intensity in median eminence and the absent normal posterior pituitary hyperintensity.
-
Sagittal T1-weighted MRI shows a nonenhancing pituitary macroadenoma.
-
Sagittal contrast-enhanced T1-weighted MRI shows a nonenhancing, hypointense lesion. Pituitary adenomas are most commonly hypointense on T1-weighted MRIs and hyperintense on T2-weighted MRIs.
-
Empty sella. Note the cerebrospinal fluid signal intensity in the herniated arachnoid space through the diaphragma sellae sella (which is partially filled with cerebrospinal fluid).
-
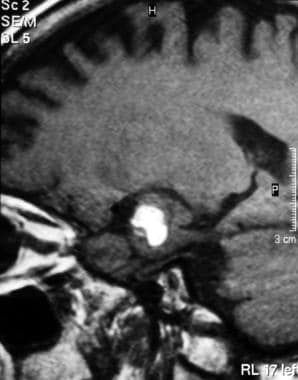
A 37-year-old woman presented with pituitary apoplexy. Note the high signal intensity in the pituitary gland secondary to hemorrhage on this T1-weighted MRI.
-
Sagittal T1-weighted MRI shows hemorrhage in a huge pituitary adenoma.
-
Sagittal contrast-enhanced T1-weighted MRI shows a huge, enhancing pituitary adenoma.
-
Coronal T1-weighted MRI shows a nonenhancing pituitary macroadenoma invading the left cavernous sinus (arrow).
-
Sagittal T2-weighted MRI shows a hyperintense cystic lesion above the pituitary gland due to a Rathke pouch cyst in the region of the pars intermedia.
-
Coronal T2-weighted MRI shows a large partially cystic craniopharyngioma that is causing obstructive ventriculomegaly.
-
Coronal fluid-attenuated inversion recovery (FLAIR) MRI of the brain shows an aneurysm of the intracranial carotid artery that is compressing the optic chiasm.
-
T1-weighted MRI shows a pearly, subarachnoid epidermoid cyst and high signal intensity.
-
Axial contrast-enhanced T1-weighted MRIs show an epidermoid cyst. Note the low signal intensity in the tumor along crevices of the subarachnoid space.
-
Sagittal T1-weighted image of the brain shows a hypothalamic hamartoma. Note the nonenhancing, small hypothalamic lesion with signal intensity similar to that of the gray matter in the region of the hypothalamus.
-
Axial contrast-enhanced T1-weighted MRIs show a metastasis to the sphenoid bone and the cavernous sinus from a primary site in the colon.
-
Coronal contrast-enhanced T1-weighted image shows meningeal nodular enhancement at the base of the brain due to sarcoidosis.
-
Axial contrast-enhanced T1-weighted MRI shows intense meningeal enhancement due to tuberculous meningitis. Note also the ring enhancement of a granuloma.
-
Coronal contrast-enhanced T1-weighted image shows intense enhancement in the region of the sphenoid and cavernous sinuses due to a fungal infection in a patient with immunocompromise.
-
Two coned lateral radiographs of the pituitary fossa show progression of disease. Left, Image shows ballooning of the pituitary fossa. Right, Image obtained 2 years latter shows bone destruction.
-
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow)
-
Radiograph shows a thickened heel pad in a patient with acromegaly.