Practice Essentials
Endometriosis is defined as the presence of endometrial glandular tissue outside of the uterus. In contrast, adenomyosis is endometrial tissue within the myometrium. Adenomyosis once was termed endometriosis interna but currently is recognized as a distinct clinical entity. Deep infiltrating endometriosis (DIE) represents 15 to 30% of endometriosis cases and is defined as endometriosis infiltrating the peritoneum by more than 5 mm. DIE is characterized by nodules infiltrating the rectosigmoid, uterosacral ligaments (USL), vaginal fornix, rectovaginal septum (RVS) and/or bladder. [1]
Preferred examination
The initial workup begins with a history and physical examination. History and findings can be nonspecific, but occasionally, tender, nodular masses can be palpated on pelvic examination, representing fibrotic implants in the cul-de-sac. Elevations of CA-125 may be caused by endometriosis. [2] [3]
Laparoscopy is the standard modality for the diagnosis of endometriosis. [4] It is the most sensitive means of examination because only laparoscopy can identify superficial peritoneal implants; however, laparoscopy is an invasive surgical procedure. Laparoscopy can be incorporated into treatments in which endometriomas are cauterized or removed and adhesions are lysed. In addition, laparoscopy can be limited by the presence of dense pelvic adhesions, resulting in limited access to the cul-de-sac and adnexa.
For DIE, the modalities most commonly used are transvaginal ultrasound (TVUS) and MRI, which have comparable diagnostic accuracy. [1]
Plain film radiography, computed tomography (CT) scanning, and barium studies are not sensitive for the diagnosis of endometriosis. Moreover, the appearance of implants and endometriomas is nonspecific. US scanning and MRI are not sensitive for superficial lesions. US scanning is not sensitive for the detection of large implants.
The results of a Cochrane Systematic Review of imaging modalities for the noninvasive diagnosis of endometriosis concluded that none of the imaging modalities were able to detect overall pelvic endometriosis with enough accuracy to replace surgery. Specifically for endometrioma, TVUS qualified as a triage test. TVUS could also be used clinically to identify additional anatomic sites of DIE, as compared to MRI, thus facilitating preoperative planning. [5]
(Endovaginal sonogram of endometrioma is shown below.)
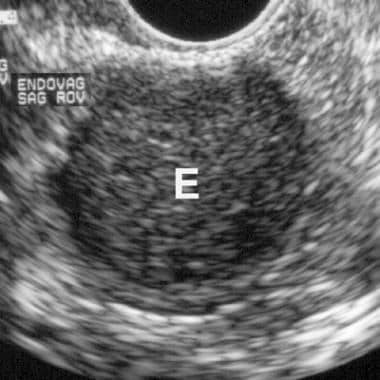 Endovaginal ultrasound scan of an endometrioma. Note the characteristic diffuse, low-level echoes of the endometrioma (E) giving a solid appearance.
Endovaginal ultrasound scan of an endometrioma. Note the characteristic diffuse, low-level echoes of the endometrioma (E) giving a solid appearance.
MRI of endometrioma is shown in the image below.
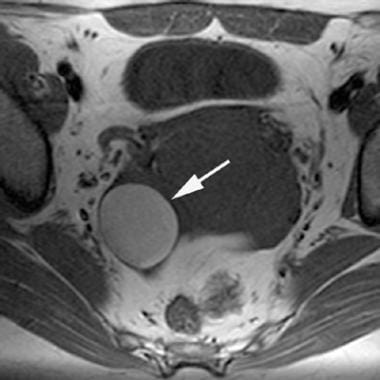 T1-weighted magnetic resonance image of an endometrioma. Note the characteristic high signal intensity (similar to that of fat) of this right-sided adnexal endometrioma (arrow).
T1-weighted magnetic resonance image of an endometrioma. Note the characteristic high signal intensity (similar to that of fat) of this right-sided adnexal endometrioma (arrow).
Pathogenesis
Two main theories exist for the pathogenesis of endometriosis. One theory is that endometrial tissue is spread by retrograde menstruation or by vascular and/or lymphatic spread. The second theory holds that the serosal epithelium of the peritoneum undergoes metaplastic differentiation into endometrium-like tissue.
The theory that retrograde menstruation causes endometriosis is supported by the analysis of peritoneal fluid in women. [6] As many as 90% of women have blood in the peritoneal fluid during the perimenstrual period. In addition, endometrial cells have been found in the peritoneal fluid. The pattern of endometriosis is consistent with retrograde menstruation and is most common in the ovary, followed by the other dependent areas of the pelvis. Vascular and/or lymphatic spread is supported by noting the occasional distal (extraperitoneal) sites of endometriosis, including the lungs and central nervous system (CNS). [7] In addition, teenage girls with obstructive uterine or vaginal anomalies show retrograde menstrual bleeding, and endometriosis is common in these patients.
Metaplasia is the conversion of peritoneal epithelium into endometrial epithelium. The theory that metaplasia causes endometriosis is supported by the fact that endometrial cells and peritoneal cells derive from the same celomic wall epithelium. [7] This theory also is supported by development of endometriosis in women who lack normal endometrial tissue, such as those with Turner syndrome or uterine agenesis. In addition, rare cases of endometriosis have been found in the prostatic utricle of men.
Clinical manifestations
Clinical presentations of endometriosis often consist of chronic pelvic pain and infertility; however, many patients are asymptomatic. [8] The earliest visible manifestations of endometriosis are whitish peritoneal plaques. The foci of endometrial tissue are small subserosal nodules with a brown appearance on gross examination; termed powder burns, they are seen on laparoscopic examination. Over time, the repeated hemorrhaging can produce extensive fibrosis surrounding the endometrial tissue, which can result in adhesions to adnexal structures or to bowel and can obliterate the pelvic cul-de-sac.
When the ovaries are involved, they can become enlarged with cystic, blood-filled spaces that, on gross examination, are termed chocolate cysts, or endometriomas. Endometriomas can become large and multilocular. The endometrial-cell lining can become obliterated over time, making the pathologic distinction between an endometrioma and a hemorrhagic cyst difficult in some cases. [7] The wall of the endometrioma becomes thickened and fibrotic, with hemosiderin deposition. [9] The high concentration of iron in the cysts causes the distinctive appearance on MRI [10] and has been proposed to relate to the induction of carcinomas in endometriosis that form in some patients. [9, 11]
Staging
Staging of endometriosis depends on the degree and complications of endometrial implants. The American Society for Reproductive Medicine (ASRM) classification of endometriosis categorizes endometrial implants, such as location and the depth of penetration, as well as the degree of cul-de-sac obliteration and adhesions. The findings on laparoscopy can be used to classify patients into 4 classes, from mild (stage I) to severe (stage IV). [12] In 2017, the World Endometriosis Society consensus statement on the classification of endometriosis proposed a classification toolbox that includes the revised American Society for Reproductive Medicine and, where appropriate, the Enzian and Endometriosis Fertility Index staging systems. [13]
Patient education
For patient education information, see the Women's Health Center, as well as Female Sexual Problems and Endometriosis.
Radiography
Plain film radiographs are not indicated in the radiologic workup of endometriosis. No specific findings are found on plain films. [14] The findings on contrast studies (a barium enema and an intravenous [IV] urogram) are nonspecific.
Uncommonly, women with rectal pain or bleeding from endometriosis involvement of the bowel may undergo a barium enema examination. A tethered submucosal mass that is centered at the anterior midrectum—representing cul-de-sac implants involving the rectal wall—is typical. [14]
An IV urogram in patients with endometriosis may show ureteral obstruction at or below the pelvic brim. [15] This obstruction may be from an endometriosis implant invasion into the ureter or from a mass effect from endometrioma.
The appearance of endometriosis on a barium enema contrast study can be mimicked by rectal carcinoma or serosal metastases.
Computed Tomography
CT scanning typically is not performed in the radiologic evaluation of endometriosis because the appearance of endometriosis and endometriomas on CT scans is nonspecific. If CT scanning is performed, endometriomas appear as cystic masses. A slightly high attenuation crescent lying dependently within the cyst has been described as a more specific feature. [16]
Complications of endometriosis, such as bowel obstruction, are evident on CT scans. Ureteral obstruction may cause hydronephrosis.
The appearance of endometriomas and endometriosis on CT scans is easily mimicked by pelvic inflammatory disease, as well as by benign or malignant ovarian tumors. CT scanning should not be relied on for the diagnosis.
Magnetic Resonance Imaging
The appearance of endometriomas on MRIs is variable and depends on the concentration of iron and protein in the fluid, which are products of blood degradation. [15, 17, 18] Most endometriomas have the gross appearance of chocolate cysts, representing highly concentrated blood products. MRI demonstrates these endometriomas as cystic masses with very high signal intensity on T1-weighted images and very low signal intensity on T2-weighted images.
MRI is an accurate technique in distinguishing endometriomas from other masses. MRI is more accurate in distinguishing benign from malignant ovarian masses than ultrasonography. [19, 20, 21] In one study, MRI showed a sensitivity of 90-92% and a specificity of 91-98% for the diagnosis of endometrioma in patients with adnexal masses. [17] MRI is not sensitive for superficial implants; therefore, the modality should not be relied on to rule out endometriosis. False-negative findings are usually seen in patients with only peritoneal implants. These are generally too small to resolve with MRI or any noninvasive imaging technique. False-positive results can occur because cystic neoplasms or functional cysts can mimic endometriomas. Most hemorrhagic cysts and neoplasms do not show the degree of T1 and T2 shortening shown by endometriomas, because the concentration of iron even in whole blood is much less than that in endometriomas. [10, 22, 23]
(See the images below.)
 T1-weighted magnetic resonance image of an endometrioma. Note the characteristic high signal intensity (similar to that of fat) of this right-sided adnexal endometrioma (arrow).
T1-weighted magnetic resonance image of an endometrioma. Note the characteristic high signal intensity (similar to that of fat) of this right-sided adnexal endometrioma (arrow).
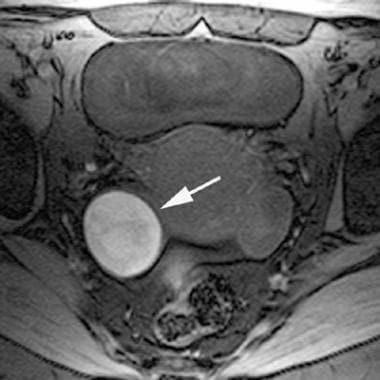 Fat-saturated T1-weighted magnetic resonance image of an endometrioma. In this right adnexal endometrioma (same lesion as in the previous image), fat saturation has been applied. Note that the endometrioma's (arrow) signal intensity does not decrease. This signal characteristic differentiates endometriomas from fatty adnexal masses, such as dermoids.
Fat-saturated T1-weighted magnetic resonance image of an endometrioma. In this right adnexal endometrioma (same lesion as in the previous image), fat saturation has been applied. Note that the endometrioma's (arrow) signal intensity does not decrease. This signal characteristic differentiates endometriomas from fatty adnexal masses, such as dermoids.
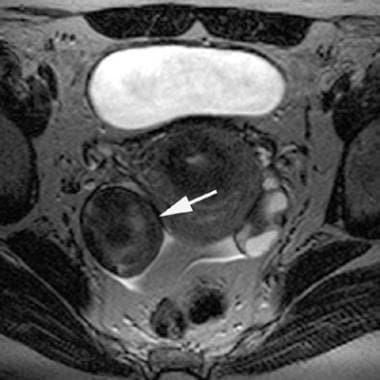 T2-weighted magnetic resonance image of an adnexal endometrioma (arrow; same lesion as in the previous image). Note the characteristic low T2-weighted signal. This low T2 signal is a result of the high iron concentration in the endometrioma.
T2-weighted magnetic resonance image of an adnexal endometrioma (arrow; same lesion as in the previous image). Note the characteristic low T2-weighted signal. This low T2 signal is a result of the high iron concentration in the endometrioma.
This low signal intensity on the T2-weighted images is termed shading and occasionally occurs in a gradient from higher to lower signal intensity. This pattern of signal intensities results from the high iron concentration in the endometrioma and is rarely seen in other masses of any type. [10, 22]
In a study to determine the sensitivity and specificity of the T2 dark spot sign in helping distinguish endometriomas from other hemorrhagic adnexal lesions, 16 of 45 endometriomas, zero of 25 hemorrhagic cysts, and 2 of 4 serous cystadenomas demonstrated dark spots. Sensitivity, specificity, positive predictive value, and negative predictive value of T2 dark spots for differentiating endometriomas from other hemorrhagic cystic ovarian masses were 36%, 93%, 89%, and 48%, respectively. The T2 shading sign was found to be sensitive but not specific for endometriomas. [24]
Multiple high signal lesions, usually in the ovaries, on T1-weighted images are also highly suggestive of endometriosis.
Peritoneal implants initially are small serosal lesions and usually escape detection. Larger, fibrotic implants of endometriosis are seen on MRI as spiculated nodules of very low signal intensity on T2-weighted images. [25, 26] These commonly occur in the cul-de-sac; they less commonly appear on the bladder dome, rectum, or umbilicus or in pelvic surgical scars. [25] Fat-saturated T1-weighted images are helpful to identify the punctate high signal intensity from hemorrhage in these lesions. [27, 28]
Cul-de-sac implants result in adhesions that constrict it. [29] Dilated fallopian tubes occasionally are seen on MRI in patients with endometriosis; these demonstrate high signal intensity on T1-weighted images, which is indicative of bloody fluid. [15, 30]
MRI can also demonstrate the complications of endometriosis, such as bowel implants and ureteral obstruction. [15] Since longer imaging times are required for MRI, antiperistaltic medication can improve visualization of the bowel.
Ultrasonography
Patients with suspected endometriosis referred for US scanning evaluation should receive a transvaginal study, because this is more sensitive for smaller endometriomas. [31, 32, 33, 34] The kidneys should be examined for hydronephrosis.
The typical US scan finding in endometriosis is a cystic mass with diffuse, low-level echoes. [35, 33] Punctate echogenicities in the wall may be present and increase diagnostic confidence. [36]
(See the images below.)
 Endovaginal ultrasound scan of an endometrioma. Note the characteristic diffuse, low-level echoes of the endometrioma (E) giving a solid appearance.
Endovaginal ultrasound scan of an endometrioma. Note the characteristic diffuse, low-level echoes of the endometrioma (E) giving a solid appearance.
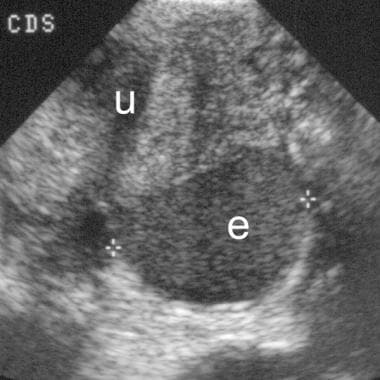 Sagittal view shows an endometrioma (e) in the cul-de-sac with diffuse, low-level echoes. The endometrioma lies directly posterior to the uterus (u).
Sagittal view shows an endometrioma (e) in the cul-de-sac with diffuse, low-level echoes. The endometrioma lies directly posterior to the uterus (u).
However, endometriomas can vary in appearance. For example, they may appear cystic (simple or complex), or they may resemble a solid mass (see the image below). [36, 30] Punctate echogenicities in the wall of endometriomas are less commonly seen but add specificity to the diagnosis. [14]
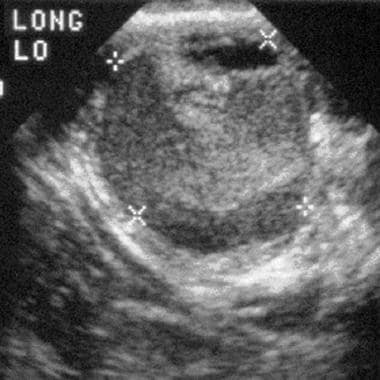 Transverse view of a left ovarian endometrioma shows a heterogeneous appearance, with diffuse, low-level echoes interspersed with echogenic and anechoic areas.
Transverse view of a left ovarian endometrioma shows a heterogeneous appearance, with diffuse, low-level echoes interspersed with echogenic and anechoic areas.
Small implants typically are not seen with US scanning. [2]
Doppler waveform analysis is not helpful in differentiating endometriomas from other masses. Low-resistance waveforms resembling malignancy are encountered in endometriomas. [19, 34] (See the image below.)
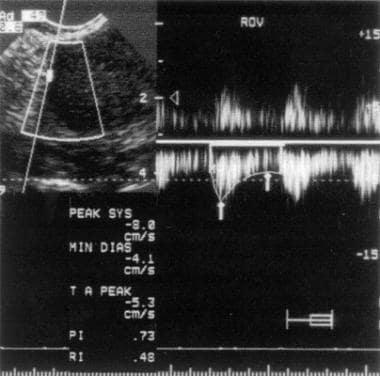 Duplex ultrasound scan of an endometrioma. Note the resistive index of 0.48, indicating a low-resistance waveform. This value can also be seen with an ovarian neoplasm, yielding false-positive results.
Duplex ultrasound scan of an endometrioma. Note the resistive index of 0.48, indicating a low-resistance waveform. This value can also be seen with an ovarian neoplasm, yielding false-positive results.
In a study of ultrasound findings in women with decidualized endometriomas surgically removed during pregnancy, most endometriomas (14/17, 82%) were described as manifesting vascularized rounded papillary projections with a smooth contour in an ovarian cyst with one or a few cyst locules and ground-glass or low-level echogenicity of the cyst fluid. [37]
Degree of confidence
US scanning is not as specific as MRI in the evaluation of endometriosis. The appearance of a cystic mass with homogeneous, diffuse, low-level echoes is highly suggestive of an endometrioma. However, other appearances are much less specific and can be mimicked by hemorrhagic cysts, tubo-ovarian abscesses, and cystadenomas. [36] US scan characteristics of endometriomas overlap with other pathologies, such as ovarian neoplasms. Endometriomas are commonly bilateral or multicystic, furthering their resemblance to malignancies. [38]
Since small endometrial implants are not seen reliably on US scans, US scanning is not a sensitive technique to diagnose endometriosis.
Guerriero and colleagues found that the sensitivity and specificity of endovaginal ultrasonography were 83% and 89%, respectively, when the technique was used to differentiate endometriomas from other ovarian cysts. [35]
Low-resistance Doppler waveforms resembling malignancy are often encountered in endometriomas. [19, 39]
False-positive findings may occur because hemorrhagic cysts, tubo-ovarian abscess, and cystadenomas may resemble endometriomas. [19, 34, 38]
False-negative findings can occur because endometrial implants are too small to visualize on US scans. In addition, endometriomas without the typical appearance described above may be misdiagnosed as hemorrhagic cysts, tubo-ovarian abscesses, and cystadenomas. [38]
Questions & Answers
Overview
How is endometrioma/endometriosis staged?
How is endometrioma/endometriosis diagnosed?
What is the role of imaging in the diagnosis and treatment of endometrioma/endometriosis?
What is the pathogenesis of endometrioma/endometriosis?
Which clinical findings are characteristic of endometriosis?
Which clinical findings are characteristic of endometrioma?
What is the role of radiography in the workup of endometrioma/endometriosis?
What is the role of CT scanning in the workup of endometrioma/endometriosis?
What is the role of MRI in the workup of endometrioma/endometriosis?
What is the role of ultrasonography in the workup of endometrioma/endometriosis?
What is the accuracy of ultrasonography for the evaluation of endometrioma/endometriosis?
-
Endovaginal ultrasound scan of an endometrioma. Note the characteristic diffuse, low-level echoes of the endometrioma (E) giving a solid appearance.
-
Duplex ultrasound scan of an endometrioma. Note the resistive index of 0.48, indicating a low-resistance waveform. This value can also be seen with an ovarian neoplasm, yielding false-positive results.
-
Sagittal view shows an endometrioma (e) in the cul-de-sac with diffuse, low-level echoes. The endometrioma lies directly posterior to the uterus (u).
-
Transverse view of a left ovarian endometrioma shows a heterogeneous appearance, with diffuse, low-level echoes interspersed with echogenic and anechoic areas.
-
T1-weighted magnetic resonance image of an endometrioma. Note the characteristic high signal intensity (similar to that of fat) of this right-sided adnexal endometrioma (arrow).
-
Fat-saturated T1-weighted magnetic resonance image of an endometrioma. In this right adnexal endometrioma (same lesion as in the previous image), fat saturation has been applied. Note that the endometrioma's (arrow) signal intensity does not decrease. This signal characteristic differentiates endometriomas from fatty adnexal masses, such as dermoids.
-
T2-weighted magnetic resonance image of an adnexal endometrioma (arrow; same lesion as in the previous image). Note the characteristic low T2-weighted signal. This low T2 signal is a result of the high iron concentration in the endometrioma.








