Practice Essentials
Infantile cortical hyperostosis, or Caffey disease, is a benign, rare, proliferating bone disease affecting infants. Caffey and Silverman first reported this disease as a distinct entity in 1945. Classically, infantile cortical hyperostosis disease occurs in the early part of the first year of life (< 5 mo). Infantile cortical hyperostosis is characterized by a clinical triad (fever, soft-tissue swelling, hyperirritability) and a clinching radiographic picture of underlying cortical hyperostosis (thickening or bony expansion). In addition to the skeleton, the adjacent fascia, muscles, and connective tissues are involved. [1, 2, 3, 4, 5, 6] Some researchers have suggested that infantile cortical hyperostosis has a predilection for patients with immunodeficiency disorders. [7, 8, 9]
Radiography is the most valuable diagnostic study in infantile cortical hyperostosis. Radiographs show layers of periosteal new bone formation, with cortical thickening in variable combinations of the long bones, mandible, and clavicle. [7, 4]
The bones most commonly affected in infantile cortical hyperostosis are the flat bones: mandible (75% involvement), clavicle, rib (especially the lateral arches), scapula, skull, and ilium. The tubular bones most commonly affected are the ulna bones, which usually show asymmetrical involvement. Bones rarely affected are the vertebrae, carpus, tarsus, and phalanges. Symmetrical or asymmetrical distributions may be observed, and involvement can be monostotic and polyostotic. Tubular bone involvement affects the diaphysis and spares the metaphysis and epiphysis. [7, 8, 4, 5]
The scapula is altered in 10% of cases, and exuberant hyperostosis may resemble neoplasm. Scapular involvement is also associated with neurologic deficit and diaphragmatic elevation.
When the ribs are affected, costal hyperostosis can be associated with an ipsilateral exudative pleural effusion. Bony rib fusion may occur and lead to scoliosis. In the forearm, when both the radius and ulna are affected, bony fusion is a particular risk, and the resulting synostosis may persist after the disease resolves.
(The skeletal effects of infantile cortical hyperostosis are demonstrated in the images below.)
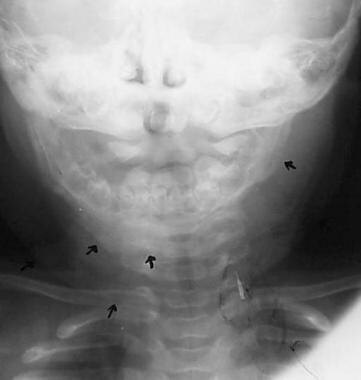 Frontal view of the mandible shows diffuse soft-tissue swelling (lower arrow on the right), right mandibular cortical thickening due to periosteal new bone formation (middle 2 arrows on the right), and mild bony proliferation of the left mandible (arrow on the left). Courtesy of Clifton Leftridge, Jr, MD.
Frontal view of the mandible shows diffuse soft-tissue swelling (lower arrow on the right), right mandibular cortical thickening due to periosteal new bone formation (middle 2 arrows on the right), and mild bony proliferation of the left mandible (arrow on the left). Courtesy of Clifton Leftridge, Jr, MD.
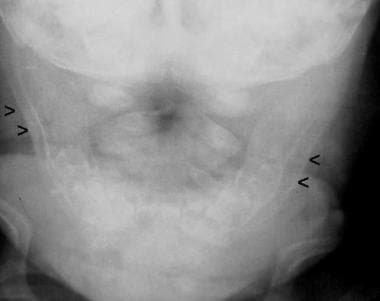 Frontal view shows cortical thickening of the mandible, which is depicted as a double contour of the cortex due to subperiosteal new bone formation.
Frontal view shows cortical thickening of the mandible, which is depicted as a double contour of the cortex due to subperiosteal new bone formation.
Two forms of infantile cortical hyperostosis have been described: prenatal and infantile. The prenatal form is rare and has a poor prognosis.
The prenatal form has been described as a more severe, congenital form of infantile cortical hyperostosis that is probably inherited as an autosomal recessive trait. Patients present with major angulation of the long bones, generalized symmetrical involvement of the skeleton, and polyhydramnios. A prenatal diagnosis can be made by detecting cortical hyperostosis of the diaphysis of long bones and ribs. Pericardial and abdominal fluid accumulations provide further evidence of fetal compromise. Polyhydramnios is almost always present, and placental enlargement has also been reported. Fetal anemia has been attributed to bone marrow encroachment by hyperostosis. [10, 11, 12]
Hyperostosis has also been reported in patients receiving therapeutic doses of prostaglandin E. Prostaglandins E1 and E2 maintain patency of the ductus arteriosus in infants born with ductus-dependent cyanotic congenital heart disease. This treatment helps provide adequate time for the infant to mature in preparation for surgical intervention. However, cortical hyperostosis can occur as a complication of long-term treatment (4-6 wk). The bony changes appear to be dose and duration dependent. Regression of the bony changes occurs on the discontinuation of treatment. [13, 14]
Overall, the age of onset, clinical signs, laboratory results, and typical radiographic features are the clues leading to the correct diagnosis of infantile cortical hyperostosis. [1, 15]
Three pathologic phases of the skeletal and soft-tissue manifestations of Infantile cortical hyperostosis have been described: early, subacute, and late.
Early phase
The early phase of infantile cortical hyperostosisis is characterized by an acute intraperiosteal inflammatory reaction consisting of edema and cellular infiltration, with subsequent thickening of the periosteum. The inflammatory process can extend into the neighboring soft tissues, and cortical resorption may be present.
Subacute phase
In the subacute phase of infantile cortical hyperostosis, inflammation diminishes, the periosteum thickens, and ossifying periostitis subsequently develops. Beneath the periosteum, layers of immature lamellar bone are produced; these can be exuberant in nature. Bony deposition may occur in the neighboring soft tissues.
Late phase
The late phase of infantile cortical hyperostosis involves the removal of peripheral bone, beginning along the inner surface and extending outwardly. Cortical remodeling may also be observed.
Differential Diagnosis
Some of the most important conditions in the differential diagnosis are osteomyelitis, bone tumors, scurvy, hypervitaminosis A, and battered baby syndrome. These need to be excluded when diagnosing infantile cortical hyperostosis. [16]
Ophthalmic manifestations include periorbital swelling and edema, unilateral proptosis, chemosis, and conjunctival hyperemia. Many cases are misdiagnosed and treated as orbital cellulitis, angioedema, or even dacryocystitis. [17]
Imaging Modalities
Radiography
Radiography is the most valuable diagnostic study in infantile cortical hyperostosis. Radiographs show layers of periosteal new bone formation, with cortical thickening in variable combinations of the long bones, mandible, and clavicle. Plain radiographs may show soft-tissue swelling and/or cortical hyperostosis (with doubling or tripling of the normal width of the bone). The periosteal reaction progresses to subperiosteal new bone formation.
Radiographic findings can range from a subtle indistinctness of the cortical margin (mild periosteal reaction) associated with soft-tissue swelling to a thick bony cloaking of the diaphysis of long bones. [18, 19]
Although infantile cortical hyperostosis is an abnormality of bone formation, destructive lesions of the skull or tubular bones have been identified.
(The radiographic features of infantile cortical hyperostosis are demonstrated in the images below.)
 Frontal view of the mandible shows diffuse soft-tissue swelling (lower arrow on the right), right mandibular cortical thickening due to periosteal new bone formation (middle 2 arrows on the right), and mild bony proliferation of the left mandible (arrow on the left). Courtesy of Clifton Leftridge, Jr, MD.
Frontal view of the mandible shows diffuse soft-tissue swelling (lower arrow on the right), right mandibular cortical thickening due to periosteal new bone formation (middle 2 arrows on the right), and mild bony proliferation of the left mandible (arrow on the left). Courtesy of Clifton Leftridge, Jr, MD.
 Frontal view shows cortical thickening of the mandible, which is depicted as a double contour of the cortex due to subperiosteal new bone formation.
Frontal view shows cortical thickening of the mandible, which is depicted as a double contour of the cortex due to subperiosteal new bone formation.
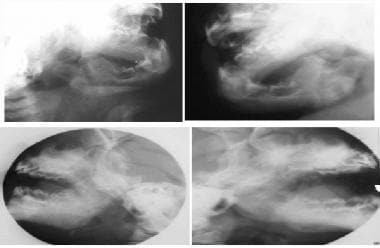 Oblique (top row) and lateral (bottom row) views of the mandible show cortical thickening and bony expansion. Courtesy of Clifton Leftridge, Jr, MD.
Oblique (top row) and lateral (bottom row) views of the mandible show cortical thickening and bony expansion. Courtesy of Clifton Leftridge, Jr, MD.
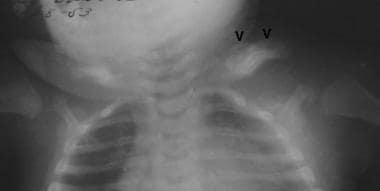 Frontal view of the chest shows diffuse cortical thickening of the upper left clavicular border with a localized fusiform bony component in the midportion of the bone. There is also new bone formation involving the left scapula with diffuse sclerosis and marginal irregularity. Courtesy of Clifton Leftridge, Jr, MD.
Frontal view of the chest shows diffuse cortical thickening of the upper left clavicular border with a localized fusiform bony component in the midportion of the bone. There is also new bone formation involving the left scapula with diffuse sclerosis and marginal irregularity. Courtesy of Clifton Leftridge, Jr, MD.
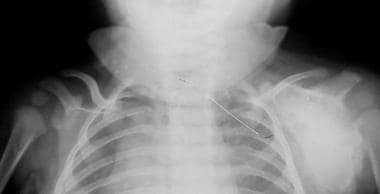 Frontal view shows deformity of the left scapula, which is enlarged and densely sclerotic. Courtesy of Clifton Leftridge, Jr, MD.
Frontal view shows deformity of the left scapula, which is enlarged and densely sclerotic. Courtesy of Clifton Leftridge, Jr, MD.
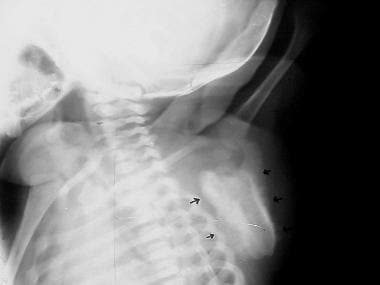 Lateral view of the left scapula in the same patient seen in the previous image. Cortical irregularity consistent with hyperostosis is evident on this view. Courtesy of Clifton Leftridge, Jr, MD.
Lateral view of the left scapula in the same patient seen in the previous image. Cortical irregularity consistent with hyperostosis is evident on this view. Courtesy of Clifton Leftridge, Jr, MD.
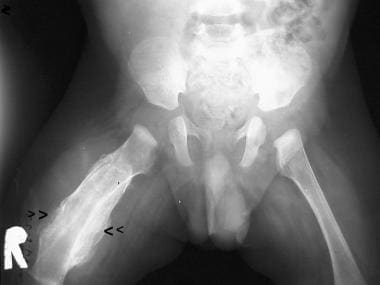 Pelvis and femora. The diaphysis of the right femur is completely encased by a thick deposition of laminated subperiosteal new bone formation. Consequently, the cortex widens, resulting in cortical hyperostosis. Note that the epiphysis and metaphysis have been spared. Courtesy of Clifton Leftridge, Jr, MD.
Pelvis and femora. The diaphysis of the right femur is completely encased by a thick deposition of laminated subperiosteal new bone formation. Consequently, the cortex widens, resulting in cortical hyperostosis. Note that the epiphysis and metaphysis have been spared. Courtesy of Clifton Leftridge, Jr, MD.
Magnetic Resonance Imaging
T1- and T2-weighted magnetic resonance imaging (MRI) scans reveal the periosteal reaction in infantile cortical hyperostosis, which appears before the characteristic radiographic findings of hyperostosis.
MRI provides excellent differentiation between bone and soft tissues. The modality also allows an evaluation of the extent of soft-tissue involvement, which includes edema. Soft-tissue edema has decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. Marrow edema has increased signal intensity on T2-weighted MRI.
Compared with plain radiography overall, MRI adds little important additional information for the clinical evaluation of infantile cortical hyperostosis, but it is useful when infection and neoplasia are considered more likely diagnoses.
MRI may be used to exclude subperiosteal hemorrhage; however, it is rarely used in this way. MRIs depict hemorrhage with subsequent new bone formation.
Computed Tomography
CT scan findings in infantile cortical hyperostosis include soft-tissue swelling; periosteal reaction (ossifying periostitis), which can progress to abundant subperiosteal new bone formation; and cortical thickening (cortical sclerosis due to the deposition of new bone). CT scanning is rarely necessary and is generally avoided because of its high radiation burden. [20]
Ultrasonography
Soft tissue may be easily identified with ultrasonography, which is easy to perform on infants. Early periosteal new bone formation is also easily visualized with high frequency (10-14 MHz) transducers.
Ultrasonography may also help diagnose the prenatal cases, showing similar appearance to osteogenesis imperfecta. [17]
A soft-tissue mass would have nonspecific appearances and could not reliably exclude infection or neoplasia.
Nuclear Imaging
The distribution of radiotracer accumulation is similar with bone and gallium scans. Accumulation of the radiopharmaceutical in the involved bones is markedly increased during the active phase of the disease.
The characteristic "bearded-child" appearance is due to the intense and diffuse abnormal accumulation of radiotracer in the mandible.
Nuclear medicine scans are positive before radiographic signs develop. In addition, nuclear medicine studies may be useful for documenting the extent of skeletal involvement.
-
Frontal view of the mandible shows diffuse soft-tissue swelling (lower arrow on the right), right mandibular cortical thickening due to periosteal new bone formation (middle 2 arrows on the right), and mild bony proliferation of the left mandible (arrow on the left). Courtesy of Clifton Leftridge, Jr, MD.
-
Frontal view shows cortical thickening of the mandible, which is depicted as a double contour of the cortex due to subperiosteal new bone formation.
-
Oblique (top row) and lateral (bottom row) views of the mandible show cortical thickening and bony expansion. Courtesy of Clifton Leftridge, Jr, MD.
-
Frontal view of the chest shows diffuse cortical thickening of the upper left clavicular border with a localized fusiform bony component in the midportion of the bone. There is also new bone formation involving the left scapula with diffuse sclerosis and marginal irregularity. Courtesy of Clifton Leftridge, Jr, MD.
-
Frontal view shows deformity of the left scapula, which is enlarged and densely sclerotic. Courtesy of Clifton Leftridge, Jr, MD.
-
Lateral view of the left scapula in the same patient seen in the previous image. Cortical irregularity consistent with hyperostosis is evident on this view. Courtesy of Clifton Leftridge, Jr, MD.
-
Pelvis and femora. The diaphysis of the right femur is completely encased by a thick deposition of laminated subperiosteal new bone formation. Consequently, the cortex widens, resulting in cortical hyperostosis. Note that the epiphysis and metaphysis have been spared. Courtesy of Clifton Leftridge, Jr, MD.
-
The periosteum as it relates to long-bone anatomy.









