Overview
One of the inevitable complications of spinal cord injury (SCI) is the associated osteoporosis that occurs predominantly in the pelvis and the lower extremities (see the image below). [1, 2] The acute treatment of patients with spinal cord injury has always focused on the injury itself and on the immediate complications that subsequently arise. Bone loss as a consequence of spinal cord injury has been of secondary concern historically.
Osteoporosis in persons with spinal cord injury was first studied in relation to calcium metabolism and the associated hypercalcemia and renal calculi that followed. The differences between osteoporosis induced by spinal cord injury and other causes of bone loss (disuse), such as prolonged bed rest, space travel, and lower motor neuron disorders, have since become clearer. Newer technologies allow monitoring of osteoblastic and osteoclastic activity at the microscopic level, whereas modern radiographic techniques have allowed more refined studies to be undertaken at the macroscopic level. [3, 4]
A longitudinal cohort study by Rodriguez et al found the 4-year incidence of musculoskeletal morbidities (such as osteoporosis, sarcopenia, osteoarthritis, and fractures) to be 82.4% in adults with traumatic spinal cord injury, compared with 47.5% in adults without such injury. The 4-year incidence of osteoporosis in the two groups was 24.0% and 6.2%, respectively. The report included over 9000 patients with traumatic spinal cord injury and nearly 1.5 million without. [5]
See also Spinal Cord Injuries, Autonomic Dysreflexia in Spinal Cord Injury, Functional Outcomes per level of Spinal Cord Injury, Heterotopic Ossification in Spinal Cord Injury, Hypercalcemia in Spinal Cord Injury, Prevention of Thromboembolism in Spinal Cord Injury, Rehabilitation of Persons with Spinal Cord Injuries, and Spinal Cord Injury and Aging.
Pathophysiology
The mechanism behind spinal cord injury (SCI)–induced osteoporosis is accepted as being multifactorial in the acute and chronic stages. [6, 7] These mechanisms differ from those observed in subjects without spinal cord injury after prolonged bed rest and in subjects with other neurologic deficits.
Disuse structural change and hypercalciuria
Spinal cord injury causes immediate and, in some regions, permanent gravitational unloading. The result is a disuse structural change with associated metabolic consequences. [8] Hypercalciuria is seen by 10 days following the spinal cord injury and reaches a peak 1-6 months postinjury. This level of hypercalciuria is 2-4 times that of persons without spinal cord injury who undergo prolonged bed rest. This marked increase in urine calcium is the direct result of an imbalance between bone formation and bone resorption. [9, 10]
Osteoblast and osteoclast activity
The activity of osteoblasts and osteoclasts is triggered by the spinal cord injury; however, markers of osteoblastic activity rise only slightly, whereas osteoclasts have a significant increase in their activity, peaking at 10 weeks following the injury with values 10 times the upper limits of normal (ULN). In addition, the increased bone resorption precedes the increase in osteoblastic activity. This model at the skeletal level following spinal cord injury resembles the high bone turnover rate seen in postmenopausal osteoporosis.
Bone muscle traction loss or neuronal factors
The loss of bone also may be enhanced by lack of muscle traction on bone or by other neural factors associated with spinal cord injury. [11] These other factors further separate spinal cord injury–induced osteoporosis from other causes of disuse demineralization. Absorption of calcium from the gastrointestinal (GI) tract has been found to decrease in the acute period following injury. Even so, in the past, dietary calcium reduction was commonly recommended as a way to decrease calcium excretion and prevent the complications of hypercalciuria.
Parathyroid hormone
The body that has sustained spinal cord injury has been considered the model of premature aging, and the role of parathyroid hormone (PTH) in osteoporosis following spinal cord injury illustrates this point. Acutely, the parathyroid gland is relatively inactive, with low PTH levels observed up to the 1-year point following injury. Hypercalcemia seen immediately postinjury leads to this low level. A reversal in activity during years 1-9 is noted. [12, 13, 14]
The parathyroid gland is stimulated to the point that PTH levels are above the reference range. The result is an increase in bone reabsorption or osteoporosis related to parathyroid dysfunction in the chronic stages of spinal cord injury. This chronic-stage mechanism of osteoporosis is balanced by an increase in bone mineral in regions of the body in which weight bearing is resumed (eg, in the upper extremities, spine) and adds to the demineralization observed in regions that are chronically non–weight-bearing (eg, the pelvis, lower extremities). In addition, the prevalence of vitamin D deficiency in SCI is increased, and this may exacerbate bone loss. [15]
Etiology
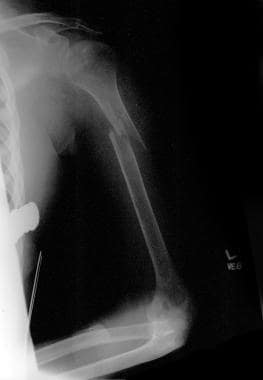
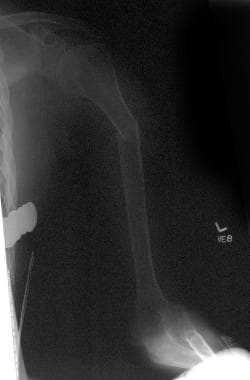
Bone loss following spinal cord injury (SCI) occurs throughout the skeletal system, with the exception of the skull. These losses are regional; areas rich in trabecular bone are demineralized to the greatest degree. The distal femur and proximal tibia are the bones most affected, followed by the pelvis and arms (see the following images). [16, 17, 18] The amount of demineralization in the skull, pelvis, and lower limbs is independent of the neurologic level.
A study by Gifre et al reported that in persons with spinal cord injury, baseline values of total femur bone mineral density (BMD) of less than 1 g/cm2 and lumbar BMD of less than 1.2 g/cm2 indicate an increased risk for osteoporosis. The study included 35 patients who had suffered spinal cord injury less than 6 months earlier, 52% of whom developed osteoporosis over a 12-month follow-up period. In addition to the BMD results, the investigators found that increased baseline values of the bone turnover markers bone-specific alkaline phosphatase (bone ALP) and serum type-1 procollagen N-terminal peptide (P1NP) also indicate a greater risk for osteoporosis. [19]
Postinjury period and degree of bone loss
A positive correlation exists between the time following the injury and the degree of bone loss. A study by Choi et al, however, found that while the duration of a spinal cord injury is negatively correlated with BMD of the neck and total proximal region of the femur, duration was not seen to be correlated with spinal BMD. [20]
Rapid loss of bone mineral occurs during the first 4 months following spinal cord injury. In patients with spinal cord injury, less than 1 year following the injury, reduction in BMDs has been noted in the femoral neck (27%), midshaft (25%), and distal femur (43%), as compared with controls. [21, 22]
Bone mineral loss continues, but to a lesser degree, in the pelvis and lower extremities over the next 10 years. [23] By 10 years postinjury, over 50% of bone content in these regions has been demineralized. The arms and trunk demonstrate an increase in bone content after the 4-month point. This gain in mineral content over the next 10-year period helps to offset some of the initial losses in the arms. The net effect is an approximate 10-21% loss of bone at the 10-year point. Interestingly, the trunk has a net gain in mineral content by 12 years postinjury.
A study by Coupaud et al indicated that in persons with spinal cord injury, peripheral quantitative computed tomography (CT) scanning reveals that the amount of time postinjury has a significant influence on cortical and trabecular measurements in the tibia and femur. This includes the bone mineral content (BMC), total BMD, and trabecular BMD of the epiphyses and the BMC, cortical BMD, and cortical cross-sectional area of the diaphyses. The study involved 26 patients, including 12 with paraplegia and 14 with tetraplegia. [24]
BMD differences in SCI population
BMD is affected not only by time postinjury but also by the type of paralysis and the type of spinal cord injury. Spastic and flaccid reflex activity and its effects on BMD are controversial
Paraplegia vs tetraplegia
Significant differences in upper extremity bone density are observed between paraplegic patients and tetraplegic patients. [25] The BMD of the arms of paraplegic patients returns to near normal by the 10-year postinjury point, which is approximately 16% more bone mineral than is found in the arms of tetraplegic patients.
Complete vs incomplete SCI
Individuals with complete injuries tend to have less BMD than those with incomplete lesions. With complete lesions, significantly lower lumbar spine BMDs have been noted (z value -1.47) in patients 1-26 years postinjury. In addition, individuals with incomplete motor spinal cord injury demonstrate greater BMD at the areas of greater lower extremity muscle strength.
Spasticity vs flaccidity
Some controversy exists surrounding the protective effect of spasticity on bone mineral content. Studies have found a decrease in losses of bone density in patients exhibiting spasticity, compared with the flaccid group.
Age
In the last few decades, only one study has included age as a risk factor for osteoporosis. For every 1-year increase in age, the rate at which osteoporosis of the knees developed was shown to increase by 3.54%. In another study, rates for femoral (including hip) fractures in patients following spinal cord injury were found to be greater than those in the general population by factors of 104 and 24 at age 50 years and 70 years, respectively. [26] (See also Spinal Cord Injury and Aging.)
Clinical Evaluation
Osteoporosis is a subclinical condition in and of itself. Thus, no associated clinical signs or symptoms exist for this entity. The most common way osteoporosis is discovered in patients with spinal cord injury is when radiographs are taken following fractures; the radiographs reveal the fracture and significant bone loss. See Radiologic Studies.
Similarly, no overt physical examination findings exist that lead to the diagnosis of osteoporosis. However, patients with spinal cord injury may be predisposed to knee effusions due to osteoporosis, heterotopic ossification, trauma, and benign hydrarthrosis.
Biomechanical Markers
The biomechanical markers that have been measured in studies of spinal cord injury (SCI)–induced osteoporosis include serum calcium, phosphorous, alkaline phosphatase, 1,25–dihydroxyvitamin D and calcitonin, and urinary calcium and hydroxyproline.
These markers may not be followed routinely in the ongoing care of the person with spinal cord injury. However, the sensitivity and early response of these markers indicate that they would be useful in the early identification of patients with spinal cord injury who are at risk of developing severe osteoporosis. [10, 27, 28]
Radiologic Studies
Advances in technology have resulted in the ability to precisely quantify bone density. However, osteoporosis is still most commonly diagnosed in patients with spinal cord injury (SCI) after they have sustained a fracture and radiographs have been obtained.
A survey study by Dionyssiotis et al of how professionals working in spinal cord injury medicine assess and manage osteoporosis in patients with SCI reported that the likelihood of testing for bone loss during the post-acute phase (4-18 months after injury) was 41.5%, and following low-impact fracture, 42.7%. The likelihood of testing during the chronic phase was higher, at 51.2%. [29]
Radiography
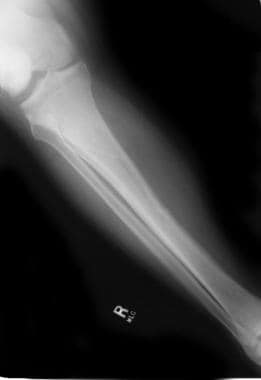
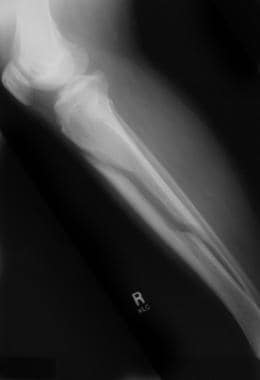
Significant bone loss is demonstrated along with the fracture. See the images below.
QCT scanning
Quantitative computed tomography (QCT) scans can isolate densitometric and geometric changes in cortical and trabecular components of bone. [30] This kind of testing allows for volumetric measurements, grams per cubic centimeter (g/cm3), which is the most precise measurement of bone density.
DXA scanning
The most commonly used method for clinical studies, dual-energy radiographic absorptiometry scan, (DXA) records absolute bone mineral densities (BMDs) in various regions of the body. [28, 31, 32, 33] This allows for comparison of BMDs in patients with spinal cord injury with measurements from uninjured individuals of similar age, race, and sex.
These imaging studies are not used commonly in the standard of care of patients with spinal cord injury. However, Moreno [34] advocates periodic measurements of lumbar spine, hip, and knee (distal femur and proximal tibia) BMD for persons with chronic SCI.
The aforementioned survey study by Dionyssiotis and colleagues of professionals working in spinal cord injury medicine reported that about 70% of respondents used hip DXA to assess bone density in patients with spinal cord injury. [29]
Management Approaches
Changes occur rapidly in the skeleton of a patient with spinal cord injury (SCI), and interventions must be undertaken quickly. The fact that there are no effective treatments to restore bone mineral once it has been lost makes early treatment even more imperative. Thus, early prevention is the main focus in treating spinal cord injury–induced osteoporosis. [14, 35, 36, 37, 38, 39, 40]
Typically, conservative treatment is pursued, with healing reported in 3-4 weeks. Soft splints may be required. Hard splints and materials should not be used.
With deformity of the extremity from fracture (eg, displacement of bones), surgical intervention of open reduction and internal fixation may be required. Thus, an orthopedic consultation may be warranted in such cases.
Pharmacologic Therapy
To date, the bisphosphonates are the most well-studied class of medications to prevent demineralization following spinal cord injury (SCI). These agents are potent inhibitors of osteoclastic bone resorption and have been found to have a positive effect in preventing spinal cord injury–induced osteoporosis. In a study by Gilchrist et al on patients with chronic SCI, alendronate 70 mg weekly was shown to prevent total body and hip bone loss at 1-year postinjury. [41] In addition, a 2-year course of daily alendronate (10 mg) prevented ongoing bone loss at the distal tibia following SCI. [42]
Parathyroid hormone (PTH) promotes new bone formation, leading to increased bone mineral density (BMD). Teriparatide is a biologic product containing a portion of human PTH, which primarily regulates calcium and phosphate metabolism in bones. Teriparatide is approved for patients at high risk of fracture due to primary osteoporosis, hypogonadal osteoporosis (men), or postmenopausal osteoporosis (women).
Animal research has suggested that coenzyme Q10 may be an effective treatment for SCI-induced osteoporosis, with a study by Zhang et al indicating that the agent had a variety of physiologic effects in spinal cord–injured rats that reduced the likelihood of bone loss. [43]
Physical Therapy
The effect of remobilization on spinal cord injury–induced osteoporosis has been fairly well studied. Weight-bearing exercises with standing frames and bikes, using forms of functional electrical stimulation (FES), have been shown to be effective when started within 6 weeks of injury. However, these same programs in the population with chronic spinal cord injury are ineffective in preventing osteoporosis or restoring bone mineral. [44, 45, 46, 47, 48, 49] (FES-induced lower extremity cycling has not been shown to increase bone density in the hip parameters of patients with chronic injury. [44, 45, 46, 49, 50] )
Complications
The most measurable complication of osteoporosis following spinal cord injury (SCI) is pathologic fracture. The historical incidence of fractures in the population with spinal cord injury has been 1.45-6% while the prevalence of fractures is reported to be 25-46%; however, this historically low incidence may be deceptive, because most patients with such injury who sustain subsequent traumas and fractures are not treated in spinal cord injury centers. [51] In addition, these studies on fractures have come from inpatient charts.
The Model Spinal Cord Injury System produced figures on fracture rates based on time following injury, with incidences of 14% at 5 years, 28% at 10 years, and 39% at 15 years postinjury. These incidence rates were based on outpatient studies and have been confirmed.
The sites of fractures correspond to the sites of greatest osteoporosis, with fractures most commonly occurring in the supracondylar region and the tibia. [52] A bone mineral density (BMD) fracture threshold of 50% appears to exist for the knee, and this most likely is the BMD fracture threshold for most regions in the body. [53]
Fracture rates in the lower extremities are 10 times greater in patients with complete spinal cord injury than in patients with incomplete injuries. Paraplegic patients are at higher risk than are tetraplegic patients, due to the higher level of function that paraplegic individuals have with regard to mobility and participation in physical activities.
The inciting events that lead to fractures are frequently unknown or are associated with relatively minimal traumas. This is because less torque is needed to produce failures in bone in persons with spinal cord injury than in individuals who have not sustained such injury.
Patient Education
Patients with spinal cord injury (SCI) should be educated regarding appropriate nutritional intake of calcium and vitamin D, as well as the benefits of early mobilization. Transfer techniques and wheelchair sport safety are also important educational areas that can help to limit the amount of osteoporosis and prevent the fractures that may result.
Appropriate amounts of calcium intake and early mobilization are the main means of limiting mineral loss; however, there is no known way to completely prevent osteoporosis in this population.
For patient education information, Osteoporosis Center, as well as Osteoporosis and Osteoporosis Medications.
-
Fracture of an osteoporotic bone in a patient with a spinal cord injury.
-
Fracture of an osteoporotic bone in a patient with a spinal cord injury.
-
Osteoporotic femur in a patient with a spinal cord injury.
-
Osteoporotic femur in a patient with a spinal cord injury.