Practice Essentials
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system, destroying the myelin and the axon in variable degrees and producing significant physical disability within 20–25 years in more than 30% of patients. The hallmark of MS is symptomatic episodes that occur months or years apart and affect different anatomic locations. See the image below.
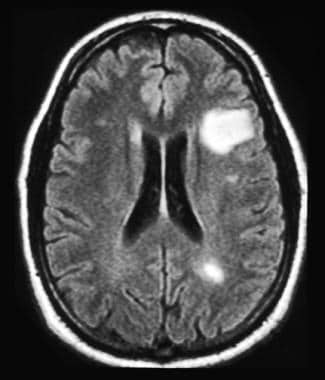 MRI of the head of a 35-year-old man with relapsing-remitting multiple sclerosis. MRI reveals multiple lesions with high T2 signal intensity and one large white matter lesion. These demyelinating lesions may sometimes mimic brain tumors because of the associated edema and inflammation.
MRI of the head of a 35-year-old man with relapsing-remitting multiple sclerosis. MRI reveals multiple lesions with high T2 signal intensity and one large white matter lesion. These demyelinating lesions may sometimes mimic brain tumors because of the associated edema and inflammation.
See Multiple Sclerosis, a Critical Images slideshow, for more information on incidence, presentation, and intervention, as well as additional resources.
Also, see the Autoimmune Disorders: Making Sense of Nonspecific Symptoms slideshow to help identify several diseases that can cause a variety of nonspecific symptoms.
Signs and symptoms
Classic MS signs and symptoms are as follows:
-
Sensory loss (ie, paresthesias): Usually an early complaint
-
Spinal cord symptoms (motor): Muscle cramping secondary to spasticity
-
Spinal cord symptoms (autonomic): Bladder, bowel, and sexual dysfunction
-
Cerebellar symptoms: Charcot triad of dysarthria (scanning speech), nystagmus, and intention tremor
-
Optic neuritis
-
Trigeminal neuralgia: Bilateral facial weakness or trigeminal neuralgia
-
Facial myokymia (irregular twitching of the facial muscles): May also be a presenting symptom
-
Eye symptoms: Including diplopia on lateral gaze (33% of patients)
-
Heat intolerance
-
Constitutional symptoms: Especially fatigue (70% of cases) and dizziness
-
Pain: Occurs in 30–50% of patients at some point in their illness
-
Subjective cognitive difficulties: With regard to attention span, concentration, memory, and judgment
-
Depression: A common symptom
-
Euphoria: Less common than depression
-
Bipolar disorder or frank dementia: May be a late finding but is sometimes found at initial diagnosis
-
Symptoms associated with partial acute transverse myelitis
See Clinical Presentation for more detail.
Diagnosis
MS is diagnosed on the basis of clinical findings and supporting evidence from ancillary tests. Tests include the following:
-
Magnetic resonance imaging: The imaging procedure of choice for confirming MS and monitoring disease progression in the CNS
-
Evoked potentials: Used to identify subclinical lesions; results are not specific for MS
-
Lumbar puncture: May be useful if MRI is unavailable or MRI findings are nondiagnostic; CSF is evaluated for oligoclonal bands and intrathecal immunoglobulin G (IgG) production
Classification
MS is divided into the following categories, principally on the basis of clinical criteria, including the frequency of clinical relapses, time to disease progression, and lesion development on MRI: [1, 2, 3, 4]
-
Relapsing-remitting MS (RRMS): Approximately 85% of cases
-
Secondary progressive MS (SPMS)
-
Primary progressive MS (PPMS)
-
Progressive-relapsing MS (PRMS)
The following 2 subgroups are sometimes included in RRMS:
-
Clinically isolated syndrome (CIS): A single episode of neurologic symptoms
-
Benign MS: MS with almost complete remission between relapses and little if any accumulation of physical disability over time
See Workup for more detail.
Management
Treatment of MS has 2 aspects: immunomodulatory therapy (IMT) for the underlying immune disorder and therapies to relieve or modify symptoms.
Treatment of acute relapses is as follows:
-
Methylprednisolone (Solu-Medrol) can hasten recovery from an acute exacerbation of MS
-
Plasma exchange (plasmapheresis) can be used short term for severe attacks if steroids are contraindicated or ineffective [5]
-
Dexamethasone is commonly used for acute transverse myelitis and acute disseminated encephalitis
Most of the disease-modifying agents for MS (DMAMS) have been approved for use only in relapsing forms of MS. However, siponimod, ocrelizumab, ozanimod, and cladribine are also approved for active secondary progressive disease. The DMAMS currently approved for use by the US Food and Drug Administration (FDA) include the following:
A single-use autoinjector is also available for self-injection of interferon beta-1a (Rebif) in patients with relapsing forms of MS. [30]
The following agents are used for treatment of aggressive MS:
-
High-dose cyclophosphamide (Cytoxan) has been used for induction therapy
-
Mitoxantrone is approved for reducing neurologic disability and/or the frequency of clinical relapses in patients with SPMS, PRMS, or worsening RRMS
Treatment of the symptoms of MS involves both pharmacologic and nonpharmacologic measures. The following symptoms may be amenable to pharmacologic therapy:
-
Fatigue: Off-label treatments include amantadine, methylphenidate, and fluoxetine
-
Depression: Selective serotonin reuptake inhibitors are preferred
-
Spasticity: Baclofen is effective in most cases
-
Pain: Tricyclic antidepressants are first-line drugs for primary pain
-
Sexual dysfunction: Oral phosphodiesterase type 5 inhibitors (eg, sildenafil, tadalafil, vardenafil)
-
Optic neuritis: Intravenous methylprednisolone may speed recovery
See Treatment and Medication for more detail.
Background
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system (CNS), destroying the myelin and the axon in variable degrees. In most cases, the disease follows a relapsing-remitting pattern, with short-term episodes of neurologic deficits that resolve completely or almost completely. A minority of patients experience steadily progressive neurologic deterioration.
The cause of MS is not known, but it likely involves a combination of genetic susceptibility and a presumed nongenetic trigger (eg, viral infection, low vitamin D levels) that together result in a self-sustaining autoimmune disorder that leads to recurrent immune attacks on the CNS (see Etiology). Geographic variation in the incidence of MS (see Epidemiology) supports the probability that environmental factors are involved in the etiology.
MS is diagnosed on the basis of clinical findings and supporting evidence from ancillary tests, such as magnetic resonance imaging (MRI) of the brain and cerebrospinal fluid examination. (See Workup.) Traditionally, MS could not be diagnosed after only a single symptomatic episode, as diagnosis required the occurrence of repeat clinical attacks suggesting the appearance of lesions separated in time and space; however, recent guidelines allow diagnosis of MS even with a first clinical episode as long as ancillary tests support separation of lesions in time or space.
A common misconception is that any attack of CNS demyelination means a diagnosis of acute MS. When a patient has a first attack of demyelination, the physician should not rush to diagnose MS, because the differential diagnosis includes a number of other diseases. For example, MS must be distinguished from other neuroinflammatory disorders (see DDx.)
Treatment consists of immunomodulatory therapy for the underlying immune disorder and management of symptoms, as well as nonpharmacologic treatments, such as physical and occupational therapy (see Treatment). In the United States, various disease-modifying agents for MS are currently approved for use in relapsing MS.
Pathophysiology
Multiple sclerosis is an inflammatory, demyelinating disease of the CNS. In pathologic specimens, the demyelinating lesions of MS, called plaques (see the image below), appear as indurated areas—hence the term sclerosis.
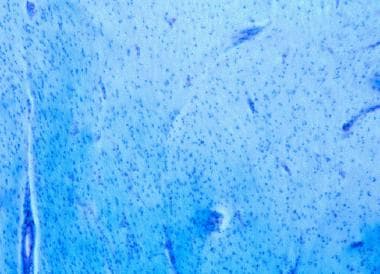 Demyelination in multiple sclerosis. Luxol fast blue (LFB)/periodic acid-Schiff (PAS) stain confers an intense blue to myelin. Loss of myelin is demonstrated in this chronic plaque. Note that absence of inflammation may be demonstrated at the edge of chronic lesions.
Demyelination in multiple sclerosis. Luxol fast blue (LFB)/periodic acid-Schiff (PAS) stain confers an intense blue to myelin. Loss of myelin is demonstrated in this chronic plaque. Note that absence of inflammation may be demonstrated at the edge of chronic lesions.
Examination of the demyelinating lesions in the spinal cord and brain of patients with MS shows myelin loss, destruction of oligodendrocytes, and reactive astrogliosis, often with relative sparing of the axon cylinder. [31] In some MS patients, however, the axon is also aggressively destroyed.
The location of lesions in the CNS usually dictates the type of clinical deficit that results. As neural inflammation resolves in MS, some remyelination occurs, but some recovery of function that takes place in a patient could be due to nervous system plasticity. MS is also characterized by perivenular infiltration of lymphocytes and macrophages, as demonstrated in the image below. Infiltration of inflammatory cells occurs in the parenchyma of the brain, brainstem, optic nerves, and spinal cord.
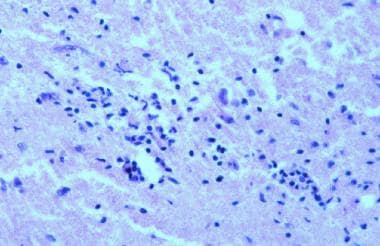 Inflammation in multiple sclerosis. Hematoxylin and eosin (H&E) stain shows perivascular infiltration of inflammatory cells. These infiltrates are composed of activated T cells, B cells, and macrophages.
Inflammation in multiple sclerosis. Hematoxylin and eosin (H&E) stain shows perivascular infiltration of inflammatory cells. These infiltrates are composed of activated T cells, B cells, and macrophages.
One of the earliest steps in lesion formation is the breakdown of the blood-brain barrier. Enhanced expression of adhesion molecules on the surface of lymphocytes and macrophages seems to underlie the ability of these inflammatory cells to penetrate the blood-brain barrier.
The elevated immunoglobulin G (IgG) level in the cerebrospinal fluid, which can be demonstrated by an oligoclonal band pattern on electrophoresis, suggests an important humoral (ie, B-cell activation) component to MS. In fact, variable degrees of antibody-producing plasma cell infiltration have been demonstrated in MS lesions. The image below provides an overview of demyelination.
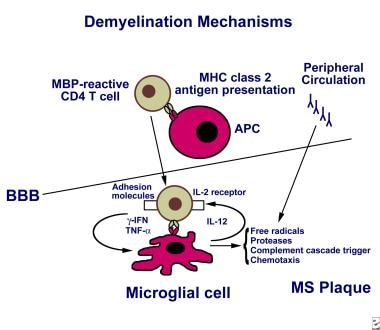 The mechanism of demyelination in multiple sclerosis may be activation of myelin-reactive T cells in the periphery, which then express adhesion molecules, allowing their entry through the blood-brain barrier (BBB). T cells are activated following antigen presentation by antigen-presenting cells such as macrophages and microglia, or B cells. Perivascular T cells can secrete proinflammatory cytokines, including interferon gamma and tumor necrosis factor alpha. Antibodies against myelin also may be generated in the periphery or intrathecally. Ongoing inflammation leads to epitope spread and recruitment of other inflammatory cells (ie, bystander activation). The T cell receptor recognizes antigen in the context of human leukocyte antigen molecule presentation and also requires a second event (ie, co-stimulatory signal via the B7-CD28 pathway, not shown) for T cell activation to occur. Activated microglia may release free radicals, nitric oxide, and proteases that may contribute to tissue damage.
The mechanism of demyelination in multiple sclerosis may be activation of myelin-reactive T cells in the periphery, which then express adhesion molecules, allowing their entry through the blood-brain barrier (BBB). T cells are activated following antigen presentation by antigen-presenting cells such as macrophages and microglia, or B cells. Perivascular T cells can secrete proinflammatory cytokines, including interferon gamma and tumor necrosis factor alpha. Antibodies against myelin also may be generated in the periphery or intrathecally. Ongoing inflammation leads to epitope spread and recruitment of other inflammatory cells (ie, bystander activation). The T cell receptor recognizes antigen in the context of human leukocyte antigen molecule presentation and also requires a second event (ie, co-stimulatory signal via the B7-CD28 pathway, not shown) for T cell activation to occur. Activated microglia may release free radicals, nitric oxide, and proteases that may contribute to tissue damage.
Immune cells in MS
Molecular studies of white matter plaque tissue have shown that interleukin (IL)-12, a potent promoter of inflammation, is expressed at high levels in lesions that form early in MS. B7-1, a molecule required to stimulate lymphocytes to release proinflammatory cytokines, is also expressed at high levels in early MS plaques. [31] Evidence exists of elevated frequencies of activated myelin-reactive T-cell clones in the circulation of patients with relapsing-remitting MS and higher IL-12 production in immune cells of patients with progressive MS.
Decreased function of T-lymphocytes with a regulatory role (Tregs) has been implicated in MS. [32] These Tregs are CD4+ CD25+ T cells that can be identified by their expression of a transcription factor known as Foxp3.
Conversely, the cytokine IL-23 has been shown to drive cells to commit to a pathogenic phenotype in autoimmune diseases, including MS. These pathogenic CD4+ T cells act reciprocally to counteract Treg function and can be identified by their high expression of the proinflammatory cytokine IL-17; they are therefore referred to as TH 17 cells. [33]
Tregs and TH 17 cells are not the only critical immune cells in the pathogenesis of MS. Immune cells such as microglia (resident macrophages of the CNS), dendritic cells, natural killer (NK) cells, and B cells are gaining increased attention by MS researchers. In addition, nonimmune cells (ie, endothelial cells) have also been implicated in mechanisms that lead to CNS inflammation. [34]
Spinal MS
Approximately 55–75% of patients with MS have spinal cord lesions at some point during the course of the disease. Spinal MS is often associated with concomitant brain lesions; however, as many as 20% of patients with spinal lesions do not have intracranial plaques. No strong correlation has been established between the extent of the plaques and the degree of clinical disability.
Spinal MS has a predilection for the cervical spinal cord (67% of cases), with preferential, eccentric involvement of the dorsal and lateral areas of the spinal cord abutting the subarachnoid space around the cord. The gray matter may be involved.
Myelocortical MS
Myelocortical MS (MCMS) is a new subtype of MS identified in 2018. It is marked by demyelination of the spinal cord and cerebral cortex but not of cerebral white matter. Researchers studied the brain and spinal cords from 100 patients with MS who had died between May 1998 and November 2012. Twelve of these individuals (12%) had demyelinated lesions in the spinal cord and cerebral cortex, but not in cerebral white matter. Researchers then compared the demyelinated lesion area in tissue sections of cerebral white matter, spinal cord, and cerebral cortex of individuals with MCMS with those collected from individuals with traditional MS and found that only the typical MS patients had lesions in the cerebral white matter. This suggests that neurodegeneration can be independent of demyelination in MCMS patients. [35]
Optic neuritis in MS
Approximately 20% of patients with MS present with optic neuritis (ON) as a first demyelinating event, and 40% of patients may experience ON during the course of their disease. Sequential episodes of optic nerve involvement and a longitudinally extensive myelopathy suggest a separate disorder, known as neuromyelitis optica [NMO], or Devic disease (see the images below). [36] Although Devic disease is sometimes categorized as an MS variant, typical MS therapies are ineffective in Devic disease, and most experts consider Devic disease to be separate from MS.
Etiology
The cause of MS is unknown, but it is likely that multiple factors act in concert to trigger or perpetuate the disease. It has been hypothesized that MS results when an environmental agent or event (eg, viral or bacterial infection, exposure to chemicals, lack of sun exposure) acts in concert with a genetic predisposition to immune dysfunction.
Genetic and molecular factors
The concordance rate for MS among monozygotic twins is only 20–35%, suggesting that genetic factors have only a modest effect. The presence of predisposing non-Mendelian factors (ie, epigenetic modification in 1 twin), along with environmental effects, plays an important role. For first-degree family members (children or siblings) of people affected with MS, the risk of developing the disorder is sevenfold higher than in the general population, but familial excess lifetime risk is only 2.5–5%. [37]
Different variants of genes normally found in the general population, commonly referred to as polymorphisms, may lead to different gradations of cellular expression of those genes and therefore of the proteins that they encode. With MS susceptibility, it may be that a polymorphism within the promoter region of a gene involved in immune reactivity generates an exaggerated response (eg, elevated expression of a proinflammatory gene) to a given antigen, leading to uncontrolled immune cell proliferation and autoimmunity.
Research on single-nucleotide polymorphisms (SNPs) that confer risk of more severe disease or of developing particular forms of MS will be of great interest to the clinicians treating this complex disorder in the early stages. To date, however, HLA-DRB1 is the only chromosomal locus that has been consistently associated with MS susceptibility. Multiple other polymorphisms that may act in concert to predispose to MS have been described with genome-wide approaches, but their individual contribution to risk is not nearly as high as the risk conferred by the HLA locus. [38]
Genes that instead of conferring susceptibility to MS confer relative protection against it are also being investigated, and clues are emerging from within the major histocompatibility complex (MHC) region. For example, it has been suggested that the HLA-C*05 allele confers protection against MS. [39]
Molecular mimicry has been proposed as an etiologic process in MS. The molecular mimicry hypothesis refers to the possibility that T cells in the peripheral blood may become activated to attack a foreign antigen and then erroneously direct their attack toward brain proteins that share similar epitopes.
Viral infection
Another hypothesis is that a virus may infect the immune system, activating self-reactive T cells (myelin reactive) that would otherwise remain quiescent. A virus that infects cells of the immune and nervous systems can possibly be reactivated periodically and thus lead to acute exacerbations in MS.
Epstein-Barr virus (EBV) infection has been found to become periodically reactivated, but a possible causative role in MS has been difficult to prove. Evidence supporting EBV infection as an etiologic factor includes (1) long-term studies showing a higher association with MS in individuals with early presence of serum antibodies against specific EBV antigens and (2) high expression of EBV antigens within MS plaques. [40]
Evidence that argues against an etiologic role for EBV infection includes the fact that MS is a highly heterogeneous disease; EBV might help trigger some cases but not others, making associations in populations difficult. In addition, it is possible that EBV reactivation is an effect rather than a cause (ie, instead of viral reactivation being the trigger for MS, reactivation might be an epiphenomenon of a dysregulated immune system).
Environmental factors
Geography is clearly an important factor in the etiology of MS. The incidence of the disease is lower in the equatorial regions of the world than in the southernmost and northernmost regions. However, a systematic review by Alonso and Hernán found that this latitude gradient became attenuated after 1980, apparently due to an increased incidence of MS in lower latitudes. [41]
Apparently, whatever environmental factor is involved must exert its effect in early childhood. If an individual lives in an area with low incidence of MS until age 15 years, that person's risk remains low even if the individual subsequently moves to an area of high incidence.
On the other hand, certain ethnic groups (eg, Eskimos), despite living in areas of higher incidence, do not have a high frequency of MS. Therefore, the exact role played by geography versus genetics is not clear.
Vitamin D levels
Low levels of vitamin D have been proposed as one environmental factor contributing to the development of MS. Vitamin D has a role in regulating immune response, by decreasing production of proinflammatory cytokines and increasing production of anti-inflammatory cytokines; also, high circulating levels of vitamin D appear to be associated with a reduced risk of MS. [42]
Thus, lower vitamin D levels due to lower sunlight exposure at higher latitudes may be one reason for the geographic variations in MS incidence, and the protective effect of traditional diets high in vitamin D could help explain why certain areas (eg, Norway) have a lower incidence of MS despite having limited sunlight. [43] This hypothesis would also provide an explanation for the correlation between childhood sun exposure and MS in monozygotic twins discordant for MS. [44]
Chronic cerebrospinal venous insufficiency
A controversial hypothesis proposes a vascular rather than an immunologic cause for some cases of MS. In 2008, Paolo Zamboni described an association between MS and chronic cerebrospinal venous insufficiency (CCSVI). [45]
The CCSVI hypothesis posits that stenosis of the main extracranial venous outflow pathways results in compromised drainage and a high rate of cerebral venous reflux. The CCSVI hypothesis has been linked with the potential effects of iron deposition in the brain parenchyma, which some authors suggest is modestly to strongly predictive of disability progression, lesion volume accumulation, and atrophy in some patients with MS. [46, 47]
A small, open-label study suggested that internal jugular vein and azygous vein angioplasty had a positive effect on MS symptoms in patients with CCSVI. [48] A meta-analysis found a positive association between CCSVI and MS, but poor reporting of the success of blinding and marked heterogeneity among studies of CCSVI precluded definitive conclusions. [49]
Because of the potential danger of such experimental procedures in treating this unproven vascular condition, the US Food and Drug Administration (FDA) has issued a warning. See FDA issues alert on potential dangers of unproven treatment for multiple sclerosis.
Given the paucity of supporting evidence, most MS experts also question the CCSVI hypothesis and do not recommend this therapy. Nevertheless, CCSVI has received widespread attention in the lay press and MS support groups, so physicians should be prepared for inquiries from patients on this highly controversial subject.
Hepatitis B vaccine
Worldwide anecdotal reports suggesting a connection between hepatitis B vaccination and MS prompted the US Centers for Disease Control and Prevention (CDC) to investigate this possibility. The CDC concluded that the weight of the available scientific evidence does not support the suggestion that hepatitis B vaccine causes or worsens MS. [50]
On the basis of the CDC findings, a National Multiple Sclerosis Society expert panel concluded as follows: “People with MS should not be denied access to health-preserving and potentially-life saving vaccines because of their MS, and should follow the CDC guidelines for any given vaccine.” [51]
Epidemiology
United States statistics
Prevalence estimates for MS in the United States vary from 58 to 95 per 100,000 population. [52] According to the National Multiple Sclerosis Society, 400,000 individuals in the United States are affected by MS. [53] Misdiagnosis is common, however.
As is true of autoimmune diseases in general, MS is more common in women. The female-to-male ratio of MS incidence has increased since the mid-20th century, from an estimated 1.4 in 1955 to 2.3 in 2000. [41] MS is usually diagnosed in persons aged 15–45 years; however, it can occur in persons of any age. The average age at diagnosis is 29 years in women and 31 years in men.
International statistics
Worldwide, approximately 2.1 million people are affected by MS. The disease is seen in all parts of the world and in all races, but rates vary widely. [53] In general, the prevalence of MS tends to increase with latitude (eg, lower rates in the tropics, higher rates in northern Europe), but there are many exceptions to this gradient (eg, low rates among Chinese, Japanese, and African blacks; high rates among Sardinians, Parsis, and Palestinians).
The presence of these exceptions implies that racial and ethnic differences affect risk. In addition, a substantial increase in MS incidence has been reported from different regions, suggesting that environmental factors, as well as geographic and genetic ones, play an important role in MS. [54] (See Etiology.)
Epidemiologic studies indicate an increase in MS prevalence in Latin America. Susceptibility to MS and clinical behavior of the disease varies genetically in Latin America; for example, MS apparently does not occur in Amerindians with Mongoloid genes. [55]
Prognosis
If left untreated, more than 30% of patients with MS will develop significant physical disability within 20–25 years after onset. Several of the disease-modifying agents used in MS have slowed disability progression within the duration of research trials; whether these effects will be maintained over longer periods is not known.
Less than 5–10% of patients have a clinically milder MS phenotype, in which no significant physical disability accumulates despite the passage of several decades after onset (sometimes in spite of multiple new lesions seen on MRI). Detailed examination of these patients in many instances reveals some degree of cognitive deterioration.
Male patients with primary progressive MS have the worst prognosis, with less favorable response to treatment and rapidly accumulating disability. The higher incidence of spinal cord lesions in primary progressive MS is also a factor in the rapid development of disability.
Life expectancy is shortened only slightly in persons with MS, and the survival rate is linked to disability. Death usually results from secondary complications (50–66%), such as pulmonary or renal causes, but can also be due to primary complications, suicide, and causes unrelated to MS. The Marburg variant of MS is an acute and clinically fulminant form of the disease that can lead to coma or death within days.
Patient Education
Patients should be educated on the purposes of medications, doses, and the management of adverse effects. Patients and caregivers need education on appropriate management of problems related to pain, fatigue, and spasticity, as well as on issues related to bowel, bladder, and sexual function. For patients with advanced disease, caregivers need hands-on training in transfer techniques, as well as in management of skin integrity, bowel programs, and urinary collection devices.
Patients with MS report a high incidence of falling. Contributing factors are similar to those in other populations with neurologic diseases. Patients with MS can benefit from receiving information about preventing falls from their healthcare practitioner. [56]
To ensure a successful outcome, family members and caregivers should be included in any education provided. Community agencies, such as the state chapters of the National Multiple Sclerosis Society, can provide valuable information concerning community resources, as well as social support and education.
Patients may benefit from referral to comprehensive and professional organizations and Web sites that are dedicated to MS. Among these, the National Multiple Sclerosis Society is highly recommended for information on current hypotheses, ongoing research, general resources, and educational programs. Other highly recommended MS-related Web sites include MultipleSclerosis.com and The Consortium of Multiple Sclerosis Centers.
For patient education information, see the Brain & Nervous System Center.
-
The mechanism of demyelination in multiple sclerosis may be activation of myelin-reactive T cells in the periphery, which then express adhesion molecules, allowing their entry through the blood-brain barrier (BBB). T cells are activated following antigen presentation by antigen-presenting cells such as macrophages and microglia, or B cells. Perivascular T cells can secrete proinflammatory cytokines, including interferon gamma and tumor necrosis factor alpha. Antibodies against myelin also may be generated in the periphery or intrathecally. Ongoing inflammation leads to epitope spread and recruitment of other inflammatory cells (ie, bystander activation). The T cell receptor recognizes antigen in the context of human leukocyte antigen molecule presentation and also requires a second event (ie, co-stimulatory signal via the B7-CD28 pathway, not shown) for T cell activation to occur. Activated microglia may release free radicals, nitric oxide, and proteases that may contribute to tissue damage.
-
MRI of the head of a 35-year-old man with relapsing-remitting multiple sclerosis. MRI reveals multiple lesions with high T2 signal intensity and one large white matter lesion. These demyelinating lesions may sometimes mimic brain tumors because of the associated edema and inflammation.
-
MRI of the head of a 35-year-old man with relapsing-remitting multiple sclerosis. This MRI, performed 3 months after the one in the related image, shows a dramatic decrease in the size of lesions.
-
Inflammation in multiple sclerosis. Hematoxylin and eosin (H&E) stain shows perivascular infiltration of inflammatory cells. These infiltrates are composed of activated T cells, B cells, and macrophages.
-
Demyelination in multiple sclerosis. Luxol fast blue (LFB)/periodic acid-Schiff (PAS) stain confers an intense blue to myelin. Loss of myelin is demonstrated in this chronic plaque. Note that absence of inflammation may be demonstrated at the edge of chronic lesions.
-
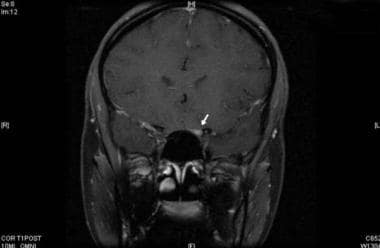
Gadolinium-enhanced, T1-weighted image showing enhancement of the left optic nerve (arrow).
-
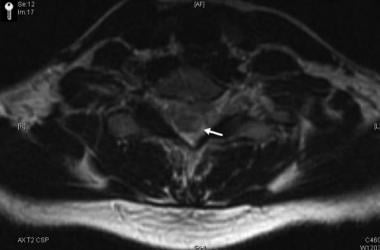
Corresponding axial images of the spinal cord showing enhancing plaque (arrow). The combination of optic neuritis and longitudinally extensive spinal cord lesions constitutes Devic neuromyelitis optica.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Emergency Department Management
- Treatment of Acute Relapses
- Immunomodulatory Therapy for Relapsing-Remitting MS
- Treatment of Aggressive MS
- Immunomodulatory Therapy for Progressive MS
- Experimental Agents
- Stem Cell Transplantation
- Treatment of MS in Pregnancy
- Symptom Management
- Rehabilitation
- Surgery for Alleviating Symptoms
- Deterrence and Prevention
- Consultations
- Long-Term Monitoring
- Show All
- Guidelines
- Medication
- Medication Summary
- Immunomodulators
- Corticosteroids
- Immunosuppressants
- Sphingosine 1-Phosphate Receptor Modulators
- Dopamine Agonists
- Skeletal Muscle Relaxant
- Neuromuscular Blockers, Botulinum Toxins
- Alpha2-Adrenergic Agonists
- Benzodiazepines
- Stimulants
- Anticonvulsants, Other
- Anticonvulsants, Hydantoin
- Selective Serotonin/Norepinephrine Reuptake Inhibitors
- Nonsteroidal Anti-Inflammatory Drugs
- Antispasmodic Agents, Urinary
- Laxatives
- Acetylcholinesterase Inhibitors, Central
- Antidiarrheals
- Potassium Channel Blockers
- Show All
- Questions & Answers
- Media Gallery
- Tables
- References