Cancer Rehabilitation
Physical medicine and rehabilitation (PM&R) is the medical specialty principally concerned with impairments, disabilities, and handicaps that arise after acute or chronic illness. According to the 1980 classification of the World Health Organization (WHO), impairment is physiologic dysfunction or loss of anatomic integrity. Disability refers to functional consequences in relation to self-care and mobility imposed by underlying impairments. Handicap may be defined as a physical condition that interferes with a patient's ability to engage in social, educational, recreational, and vocational pursuits. In essence, handicap compromises a patient's full integration into personal relationships and family and societal roles.
Neoplastic disease can develop in virtually any organ system. This unregulated growth injures and compromises organ systems that are functioning normally. Cancer-related diseases are often treated with therapeutic modalities that, in themselves, compromise normally functioning organ systems. As a consequence, PM&R practitioners must dynamically respond both to disease progression and to the effects of various treatments that may contribute to impairment, disability, and handicap.
The rehabilitation approach to the treatment of cancer originated with the National Cancer Act of 1971. This legislation declared cancer rehabilitation as an objective and directed funds to the development of training programs and research projects. In 1972, the National Cancer Institute (NCI) sponsored the National Cancer Rehabilitation Planning Conference. This conference identified four objectives in rehabilitation of patients with cancer:
-
Psychosocial support
-
Optimization of physical functioning
-
Vocational counseling
-
Optimization of social functioning
In the 1970s, a number of models for cancer rehabilitation were initiated and supported through the NCI cancer-control program.
Cancer rehabilitation can be defined as a process that assists the cancer patient to obtain maximal physical, social, psychological, and vocational functioning within the limits created by the disease and its resulting treatment.
For excellent patient education resources, visit eMedicineHealth's Cancer Center and Women's Health Center. Also, see eMedicineHealth's patient education articles, Bladder Cancer, Brain Cancer, Breast Cancer, Mastectomy, and Ovarian Cancer.
Multidisciplinary Approach to Rehabilitation
Rehabilitation specialists have proposed several general principles regarding rehabilitation interventions for patients with cancer. Rehabilitation requires an interdisciplinary team approach because of the variety of potential problems patients may face during the course of illness. The availability of professionals from major disciplines is essential to offering comprehensive care. The patient's needs determine the team members involved. Over the years, collaboration between PM&R and the specialty of cancer medicine (ie, oncology) has been growing.
The healthcare team must develop rehabilitation goals within the limitations of the patient's illness, environment, and social support. Goals must be objective, realistic, and attainable in a reasonable time to demonstrate gains from active participation in therapy and thereby maintain the patient's motivation.
Patients, family members, and significant others must be active participants in the rehabilitation process. Patient and family involvement assists in goal setting. Interdisciplinary rehabilitation is the collaborative effort of professional members of the team working with the patient and of an accompanying support network. The rehabilitation team must provide services to patients throughout the course of illness, during all stages. Treatment plans must be individualized to meet each patient's unique and specific needs.
Physicians
Professional clinicians composing the interdisciplinary team include physicians from several specialties. Primary care physicians, surgeons, radiation oncologists, and medical oncologists make active and concurrent contributions to rehabilitation efforts to manage the disease process.
The physiatrist, a specialist in PM&R, treats neuromuscular disease, musculoskeletal disease, and functional deficits, in addition to performing electrodiagnostic procedures (eg, nerve conduction studies [NCS], electromyography [EMG]). The physiatrist also prescribes treatments performed by professionals from other disciplines, such as physical, occupational, and speech therapists. The physiatrist serves as liaison among team members, providing a considerable degree of coordination, especially when rehabilitation and clinical management of the disease are simultaneous.
Cancer rehabilitation is an emerging field within PM&R and it has many unique characteristics that may require specialized fellowship training. Fellowship training programs teach physicians to evaluate the functional needs of cancer patients during treatment and follow-up. Cancer rehabilitation is a challenging and rewarding field since it combines many aspects of medicine, such as inpatient and outpatient care, patient continuity of care, clinical assessment, diagnostic evaluation, and interventional skills. [1]
Care coordinator, or case manager
The clinical-care coordinator assists in organizing and managing the team. An important aspect of this role is initially evaluating patients referred to the rehabilitation team for consultation. Care coordinators may be nurses, social workers, or professionals in other rehabilitation-related fields. They must be familiar with the functions of team members from other disciplines to assess the patient's needs effectively.
Oncology and/or rehabilitation nurse
The role of the oncology and/or rehabilitation nurse is pivotal in cancer rehabilitation. The oncology or rehabilitation nurse typically functions as an extension of other members of the team because he or she frequently assists with treatment interventions that the physical, occupational, or speech therapists begins. Such interventions include assisting patients with exercises, mobility on the unit, self-care activities, and speech and swallowing techniques. Because nurses typically have extensive contact with patients and families, they may be most aware of the family's emotional stress and adjustment issues. Nurses sometimes function as counselors, providing substantial emotional support to patients and their families.
In addition to active involvement with representatives of most other disciplines participating in the treatment interventions, nurses are responsible for skin care, bowel and bladder management, and patient and family education. Cancer rehabilitation nurses are crucial in promoting the goal of maintaining optimal independent functioning.
Social worker
The role of the social worker can vary substantially, depending on the medical institution. Social workers often provide counseling to patients and families regarding emotional support, community resources, finances, lifestyle changes, and their participation in treatment. In some settings, social workers lead support groups and actively assist in discharge-planning activities, such as for arranging home-care services and for transfer to other healthcare settings.
Psychologist
Patients and their families often have a number of psychological and adjustment issues related to the illness, its treatment, and its resulting disabilities. The psychologist assesses and treats patients to help them manage their cancer-related psychological distress. As a member of the rehabilitation team, the psychologist assists other team members when psychological issues, either in patients or their family members, complicate efforts to provide effective therapy. The goal of consulting the psychologist is to maximize the benefit the patient derives from rehabilitation.
A Danish study determined that compared with the general population, a greater percentage of individuals who have been diagnosed with cancer are hospitalized for depression. [2] According to the report, which investigated depression-related hospitalizations occurring between 1973 and 2003, the relative risk for depression in the first year after an individual had been diagnosed with cancer ranged from 1.16 (in women with colorectal cancer) to 3.08 (in men who had been diagnosed with brain cancer). The authors concluded that depression must be recognized early and treated effectively in persons who have been diagnosed with cancer in order to avoid the need to hospitalize these individuals for depression.
Physical therapist
The role of the physical therapist includes evaluation of the patient's muscle strength, mobility, and joint range of motion (ROM). Treatment interventions the physical therapist provides may include therapeutic exercises to maintain or increase ROM, endurance activities, and mobility training (eg, transfers, gait, stair climbing). Physical therapists can also administer various therapeutic modalities depending on the needs of the individual patient. Examples of modalities that may be beneficial include the application of heat and/or cold, electrical stimulation, hydrotherapy, traction, and massage.
Occupational therapist
Occupational therapists evaluate patients' ability to carry out tasks related to self-care, including activities of daily living (ADLs), such as dressing, bathing, meal preparation, and homemaking. These professionals also assist patients to increase ability to perform ADLs, including the use of compensatory techniques and adaptive equipment. In addition, occupational therapists evaluate home environments for potential modification, and they provide instruction in driving with adaptive devices. Furthermore, they implement interventions to promote upper-extremity ROM, strength, endurance, and coordination.
Dietitian
Diet and nutrition are important factors in cancer rehabilitation. A healthy diet and adequate nutrition substantially influence the patient's ability to actively participate in an applied therapy program and are essential for radiation therapy and chemotherapy. The role of the dietitian is to evaluate the patient's current nutritional status and to provide recommendations regarding his or her specific dietary needs. Patients with cancer often require dietary supplements and alternative foods. Dietitians also assist in teaching patients and family members about the importance of appropriate diet in successful rehabilitation.
Speech therapist
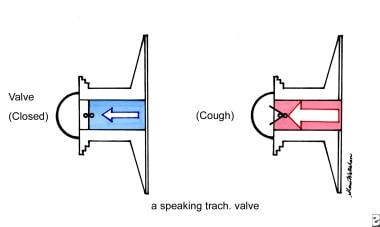
The speech therapist evaluates and treats communication deficits, dysphagia, and cognitive dysfunction in patients with cancer. Speech therapists also train patients in alternative means of speech and communication, including the use of a prosthetic larynx, adaptive communication devices, laryngeal speech, and esophageal speech. The treatment of patients with oral defects or aphasia also falls within the purview of the speech therapist. This therapist also treats swallowing deficits that result from illness or treatment.
Vocational counselor
Vocational counselors assist patients in adapting to the effect of cancer and its treatment on their employment. Vocational counselors evaluate the patient's suitability for employment and for training, if needed, and they serve as liaison between patients and their employers. Healthcare professionals often overlook the effect of cancer on the patient's vocation as an area requiring possible intervention.
Others
Although the professionals mentioned above are the most common members of the cancer rehabilitation team, practitioners from many other fields also provide important and valuable advice. These include a chaplain, a dentist, an orthotist, and a prosthetist. In addition, rehabilitation programs benefit from consultative relationships with other care-providing organizations (eg, home healthcare agencies, community hospices).
After initial screening, representatives from other disciplines conduct clinical assessments based on the patient's present needs and/or those the care coordinator identifies.
Paradigms of Cancer Rehabilitation
Dietz identified 4 categories of cancer rehabilitation that address the scope and course of the illness. [3] A variety of approaches to rehabilitation of the patient with cancer are described below.
Preventive interventions
Preventive (or "preventative") interventions lessen the effect of expected disabilities and emphasize patient education. Preventive measures also include approaches to improving the patient's physical functioning and general health status. In addition, psychological counseling before treatment can assist with the early identification of adjustment issues to allow for prompt intervention.
Restorative interventions
Restorative interventions are procedures that attempt to return patients to previous levels of physical, psychological, social, and vocational functioning. Postoperative ROM exercises for patients undergoing mastectomy and reconstructive surgery for head and neck cancer represent this category of interventions.
Supportive interventions
Supportive rehabilitation is designed to teach patients to accommodate their disabilities and to minimize debilitating changes from ongoing disease. Supportive efforts include teaching patients how to use prosthetic devices after amputation, as well as instructing the patient on use of other devices and procedures that assist in self-management, self-care abilities, and independent functioning. Other supportive efforts include provision of emotional support associated with adjustment issues while the patient is learning to cope with physical lifestyle changes.
Palliative interventions
During the palliative phase, when increasing disability and advanced disease process may be present, interventions and goals focus on minimizing or eliminating complications and providing comfort and support. Palliative goals include pain control, prevention of contractures and pressure sores, prevention of unnecessary deterioration from inactivity, and psychological support for the patient and family members.
Studies in Cancer Rehabilitation
Characteristics of patients needing rehabilitation
Lehman et al in 1978 were among the first authors to investigate the frequency of problems that cancer patients encounter in rehabilitation programs. [4] They screened 805 patients with cancer, as well as psychological and physical problems. A variety of cancers, including leukemia and cancers of the head and neck, breast, respiratory, nervous system, bladder, and bone, had been diagnosed. More than 50% of patients had problems associated with physical medicine, with a substantial portion having problems similar to those of other patients undergoing rehabilitation.
Much of the population had evidence of psychological problems. Psychological problems were more prevalent in patients with physical problems than in those without physical involvement. More than 50% of patients with physical involvement had psychological problems, and approximately 29% of patients without physical involvement had psychological difficulties. In patients with cancer of the nervous system, the incidence of psychological problems was higher than that in individuals with cancer at other sites.
The investigators concluded that many patients with cancer have coexisting physical-medicine and psychological problems and that many of these patients may benefit from rehabilitation interventions because their problems are similar to those identified in many other patient populations undergoing rehabilitation.
Ganz surveyed 500 patients with colorectal, lung, and/or prostate cancer and found that the typical patient had been living with the disease for more than 3 years. More than 80% of the sample reported problems with ambulation and, for more than 50%, the problems were severe. In addition, patients with cancer (41% with colorectal cancer, 69% with lung cancer, and 40% with prostate cancer) reported difficulty with performing ADLs. Physical problems occurred in a relatively functional sample of patients with average Karnofsky performance status (KPS) scores of 80%. More than 40% of each group had no evidence of active disease. Psychosocial problems varied widely among patients who survive longer than 1 year after their cancer was diagnosed.
Needs of patients requiring rehabilitation
Of importance, all practitioners must keep in mind that, after the patient's condition is stabilized and after he or she is discharged from the hospital, PM&R services must be considered on an outpatient or home-based basis to maintain gains and to prevent further deconditioning. At present, the vast majority of patients are never referred for rehabilitation follow-up after discharge. The number of cancer survivors continues to grow as new therapies and interventions are developed. Cancer survivors often have early- and late-onset effects from the cancer or its treatment. These effects may be the cause of cardiovascular disease, pulmonary disease, obesity, diabetes, pain, osteoporosis, cognitive defects, and inactivity. All of these conditions must be accounted for by the healthcare team when developing a rehabilitation strategy. [5]
Quality of life
VanHarten et al devised a questionnaire to address patients' need to receive professional care related to health problems. [6] Although 258 patients with cancer were invited to participate, only 147 completed the study. The sample consisted of patients with nonmetastatic breast and colon cancer who were living in the community. For all quality-of-life (QOL) factors, patients could indicate whether they felt need for professional care to contend with cancer-related health problems; 26.5% of patients indicated a need for such healthcare.
Overall, QOL scores were relatively high. Performance of expected roles and mobility were notable problems in 26% of patients. Other patients reported that fatigue and deconditioning interfered with their functional performance and mobility. Psychological integration of the new situation into personal relations and coping with daily life were also problematic.
As a result of their survey, VanHarten et al proposed a community pilot program for patients with cancer.
Components of the program included the following:
-
Fitness and sports activities
-
Relaxation exercises
-
Patient education, especially on disease-related matters
-
Instruction and counseling of patients and relatives on coping strategies, especially dealing with crisis and fear
-
Social and cultural therapy designed to help formulate new and realistic goals in life
-
Dietary advice
In a prospective observational study, Van Weert et al examined 34 patients with cancer-related physical and psychosocial problems. [7] Their 6-week, intensive, multifocal rehabilitation program consisted of 4 components: individual exercise, sports, psychoeducation, and information. Measurements were performed before and after 6 weeks of rehabilitation to assess symptom-limited bicycle ergometry performance, muscle force, and QOL (on the RAND-36 instrument, Rotterdam Symptom Checklist [RSCL], and Multidimensional Fatigue Inventory [MFI]). Statistically significant improvements were found in symptom-limited bicycle ergometry performance, muscle force, and several domains of the QOL instruments (RAND-36, RSCL, and MFI). The rehabilitation program had immediate benefits on physiological variables, QOL, and fatigue.
A study by Foley et al indicated that a community-based, multimodal exercise program can produce clinically meaningful improvements in physical function and QOL in cancer survivors. The study involved 59 cancer survivors (91.5% female; mean age 59 years) who underwent a 12-week program of supervised, twice-weekly exercise sessions, each consisting of a total of 90 minutes of aerobic conditioning, resistance training, and balance and flexibility training. Improvements in well-being before and after the program were reported to be as follows [8] :
-
Physical well-being (13.9%)
-
Emotional well-being (6.7%)
-
Functional well-being (13.0%)
-
Total well-being (9.6%)
A reliable and valid assessment tool is vital for the rehabilitation team to gauge the patient's status before, during, and after the program, as well as on follow-up evaluation. Such an instrument allows each clinician to determine reasonable short- and long-term goals for the patient.
Oncologists were the first practitioners to assess and survey QOL in patients with cancer after the advent of chemotherapy. In the late 1940s, Karnofsky and Buchrenal developed a clinical scale to quantify functional performance in patients with cancer. Since then, a number of programs intended to ensure QOL have been developed, modified, and used.
Key elements in any QOL intervention or in ascertaining the patient's overall status in a given clinical situation include the following:
-
Physical concerns (eg, symptoms described by the patient)
-
Functional ability
-
Family well-being
-
Emotional well-being
-
Spiritual well-being
-
Satisfaction with treatment, including financial concerns
-
Sexuality and intimacy, including issues of body image
-
Social functioning
-
Occupational functioning
QOL instruments clinicians currently in cancer treatment in rehabilitation include the following:
-
Functional Living Index for Cancer (FLIC)
-
Eastern Cooperative Oncology Group (ECOG) scale
-
European Organization for Research and Treatment of Cancer (EORTC) QOL questionnaire
-
QOL index
-
Cancer rehabilitation evaluation system
-
Functional assessment of cancer therapy
-
Global adjustment-to-illness scale
Purpose and emphasis of rehabilitation
The purpose of rehabilitation for patients with cancer is similar to that for patients with other diseases. However, the pathology of the tumor, the anticipated progression of disease, and any associated treatments must be considered carefully when goals are formed. When tumor progression and treatment causes a functional decline or when the disease causes a fluctuation in abilities, rehabilitation assumes a supportive role, and its goals are adjusted to accommodate the patient's persistent anatomic and physiologic limitation.
Thorough assessment of cognitive dysfunction, physical impairments, disabilities, and handicaps is paramount before the team proceeds with rehabilitation. Emphasis is initially placed on restoring or maximizing independence with ADLs, mobility, cognition, and communication. Issues of survivorship and community reintegration, including return to work, follow.
Utilization of rehabilitation
Cancer-related disability and decrease in QOL usually occur in the later stages of the disease when the cancer has metastasized and affects multiple systems in the body. These types of disability are similar to other conditions routinely treated with rehabilitation, such as spinal cord injury, multiple trauma, and traumatic brain injury. Unfortunately, rehabilitation for cancer-related disability is an underutilized resource. [9, 10, 11] Reasons for underutilization are multidimensional. Cancer-related symptoms often have gradual onset, and patients may be hesitant to tell their oncologist about issues such as deconditioning, poor balance, impaired mobility, pain, poor nutrition, decreased cognitive function, and altered self-image. [12, 13] Furthermore, oncologists may not be in the habit of specifically asking about these impairments.
Movsas et al confirmed the findings described above. [11] They examined the rehabilitation needs of patients in a different manner in an acute medical setting. Many patients with cancer had easily remediable but unrecognized rehabilitation problems. which indicated the importance of interdisciplinary efforts to preserve patient function. An important finding was that rehabilitation was underused in the population studied.
Reasons for underuse may include the failure of the acute care staff to identify functional impairments, lack of appropriate referral for rehabilitation, lack of awareness of rehabilitation services, and lack of knowledge among family members. These barriers can be overcome by providing education and by enlisting the cooperation of the clinical oncology staff, whose background in rehabilitation and functional issues may be limited or underemphasized. The hope is that as patients’ and physicians’ awareness of cancer rehabilitation grows, referrals will increase and the specialty will continue to expand and evolve.
Breast Cancer and Rehabilitation
Introduction
Breast cancer can occur in any adult. Incidences have been increasing over the last decades for both premenopausal and postmenopausal women. Although the incidence of breast cancer increases during postmenopausal years, it is the leading cause of cancer death in women younger than 50 years. Age is not a predictor of complications, but it may affect the patient's outcome, ability to cope, and extent of psychological distress. Breast cancer is the most frequent cancer in women, and more than 85% of patients are alive 5 years after diagnosis. For these reasons, more than 700,000 survivors of breast cancer in the United States are alive within 5 years of diagnosis; their total prevalence is over 2 million. [14, 15, 16, 17]
Burstein and Winer wrote an excellent review of survivorship issues for women with breast cancer. [18]
Treatment Options
Overview
On initial presentation, clinical and pathologic staging is performed to identify prognostic factors and to determine treatment options.
Surgery and/or radiation therapy is used for local control and often successful in early-stage breast cancer. If they are smaller than 5 cm and limited to the breast and axillary nodes, most such cancers may be treated surgically with modified radical mastectomy or breast-conserving surgery. In both cases, the axilla is usually dissected. Disease-free survival rates are equal in patients undergoing mastectomy and breast-conservation surgery. Locally advanced breast cancers are treated with modified radical mastectomy, preceded or followed by chemotherapy. Irradiation of the chest wall is often considered when the risk of chest-wall or nodal recurrence is high, when primary tumors are large or multicentric, or when 4 or more axillary nodes contain metastatic cancer.
Systemic therapy (ie, chemotherapy and/or hormonal therapy) is recommended for patients who present with metastatic disease or who have risk factors for metastases. Risk factors for metastatic cancer include age younger than 35 years, positive involvement of the lymph nodes, high-grade histologies, negative estrogen receptors, large tumor, high growth fraction, aneuploid DNA content, and other biologic markers. Chemotherapy may be administered before, during, or after irradiation with parameters of timing and duration depending on the type of chemotherapy.
Estrogen and progesterone receptors can be assessed to predict the patient's response to hormonal manipulation. Tamoxifen had been the first-line adjunct hormonal therapy and was started during or after radiation therapy. Hormonal manipulation for the treatment of metastatic breast cancer may include the administration of tamoxifen. However, results of the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial suggested that an aromatase inhibitor is therapeutically superior and better tolerated than tamoxifen in postmenopausal women with primary breast cancer. Aromatase is expressed in nonovarian tissues, such as muscle and fat in both premenopausal and postmenopausal women. These nonovarian tissues become the dominant sources of estrogen in postmenopausal women.
At present, the available aromatase inhibitors belong to 1 of 2 classes. Class I inhibitors irreversibly bind aromatase and have a steroidal structure (eg, exemestane). Class II agents reversibly bind aromatase and are nonsteroidal (eg, anastrozole and letrozole). Because of the specificity of its mode of action, this class of compound is well tolerated and thus lends itself to the management of both early- and advanced-stage disease.
In metastatic breast cancer, radiation therapy is often successful in palliating symptoms from painful bony sites, brain metastases, or other metastatic sites causing symptoms or obstruction. Metastatic breast cancer rarely is curable; however, studies are underway investigating efficacy of high-dose chemotherapy followed by peripheral stem-cell rescue of bone marrow to eradicate metastatic cancer.
Current issues in breast-cancer management
Current issues in breast-cancer management include the following:
-
Necessity for axillary-node dissection and/or breast irradiation after wide excision of breast cancer in patients with a good prognosis (eg, those with small tubular, colloid, or mucinous tumors)
-
Necessity for whole-breast treatment for intraductal carcinoma
-
Timing and type of chemotherapy with surgery and radiation
-
Utility of high-dose chemotherapy with stem-cell rescue in poor-prognosis breast cancer
-
Treatment of young and old women with breast cancer
-
Role of estrogen replacement in breast cancer
Surgery and Its Acute and Chronic Morbidity
Breast-conserving surgery is increasingly used for many breast cancers because disease-free survival rates are equal for women undergoing either this procedure or non–breast-conserving surgery. Breast-conserving surgery is associated with improved body image and, perhaps, hastened psychological recovery.
Breast-conserving surgery refers to removal of the cancer along with a margin of normal breast tissue and axillary dissection. In breast-preservation surgery, wide excision implies the removal of a 1- 2-cm margin of normal tissue, whereas in segmental mastectomy, even more normal breast tissue than this is removed.
A relatively uncommon surgical procedure is quadrantectomy. This is a procedure to remove the quadrant of the breast that contains the tumor plus the underlying pectoral fascia. Any increase in the extent of surgery is associated with increased risk of both early and late complications. Most reported surgical complications are associated with axillary dissection. Debate still surrounds issues of whether axillary dissection is necessary and, if so, which parameters should be used to determine its extent.
Principles of wound healing directly affect the initiation and appropriate intensity of any rehabilitation program. Wound healing is a dynamic process that lasts months to years. Wounds initially produce inflammation that lasts a few days unless necrosis, infection, or foreign bodies are present. At the edge of an epithelial wound, basal epithelial cells migrate across the defect on fibrin strands. Epithelial cells cover the wound within 48 hours and thereafter begin to differentiate and keratinize.
Fibroblasts, from the adventitia of blood vessels, migrate into the wound on fibrin strands on day 3 and begin to synthesize collagen fibers, which begin to appear on day 4. Wound strength is related to the rate of collagen formation. By 3 weeks, most wounds achieve 15% of their ultimate strength. Strength increases at a constant rate for 4 months and then at a lower rate thereafter for more than a year. Pain at the wound site generally limits the amount of stress an individual can place on the wound.
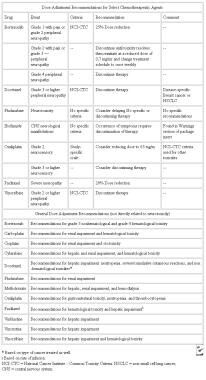
Changes in sensation are common; therefore, wounds should be treated gingerly. Because external skin sutures may provide a nidus for infection and cause extra scarring, remove them early. Factors that may impede healing include malnutrition (more common in elderly individuals than in younger patients); deficiencies of vitamin A, vitamin C, and zinc; cigarette smoking; and any conditions that decrease tissue oxygenation. Steroid use, radiation therapy, and some chemotherapy agents impede healing. The administration of doxorubicin (Adriamycin), which commonly used in adjunct chemotherapy programs, should be delayed until 4 weeks after surgery.
Early complications after mastectomy include seroma formation (10%), wound infection (7%), and skin-flap necrosis (5%). The fewest wound infections are seen when diagnoses are made by means of fine-needle aspiration. Immediate reconstruction is not associated with an increased rate of complications. Most surgeons agree that a drain must be placed after axillary dissection. The duration of drainage is not standard, but most surgeons agree that the drain can be removed when the volume of fluid draining from the wound decreases to less than 20 mL/day. The presence of a drain or a seroma can lead to infection. If seroma develops after the drain is removed, most surgeons aspirate the seroma only if the patient is uncomfortable. Do not place a drain in a lumpectomy site because cosmesis diminishes.
Complications associated with axillary dissection are secondary to nerve, vascular, and lymphatic injury. The most common complaints after axillary dissection are reduced sensation under the right arm and decreased ROM of the shoulder. Sensory deficit improves with time but may never return to normal. No known treatment exists for this adverse effect. Lymphedema can be seen immediately after surgery and results in a small increase in diameter in the upper arm only. Collateral circulation should resolve the edema within several weeks.
Chronic lymphedema and its treatment are discussed elsewhere (see the section Management of Lymphedema, below). Injury to the long thoracic nerve results in winging of the scapula. About 30% of patients develop serratus anterior muscle palsy secondary to injury to the long thoracic nerve but appear to recover by 6 months. Injury to the thoracodorsal nerve causes slight weakness in internal rotation and abduction of the shoulder from weakness of the latissimus dorsi muscle. Injury of the medial pectoral nerve results in atrophy of the lateral portion of the pectoralis major muscle. Injury to the intercostobrachial nerve results in reduced sensation along the medial aspect of the arm, and, in some patients, subsequent disabling neuralgia develops.
Breast Reconstruction
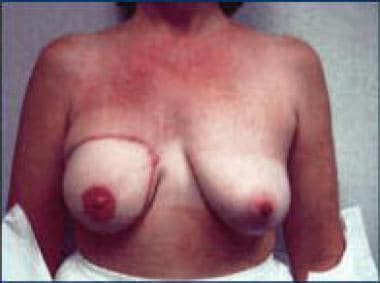
Intuition suggests that breast reconstruction offers a woman the opportunity to retain a positive self image, mitigating concern about breast cancer treatment significantly and perhaps even encouraging women to seek earlier diagnosis of breast cancer. However, the psychosocial benefit of reconstruction is only slight when patients who have undergone surgical reconstruction are compared with patients treated with mastectomy alone. Breast-preserving surgery affects body image less than mastectomy and breast reconstructive procedures do. Studies show lower scores for body image in women who have undergone breast reconstruction than in patients who have undergone breast-preserving surgery. This phenomenon may be related to the complicated nature of reconstructive surgery.
A cohort analysis of 13,388 women confirmed findings from numerous studies in that breast-augmentation surgery does not increase the risk of breast cancer and does not delay diagnosis.
Although breast-reduction surgery is never performed as cancer prophylaxis, it appears to reduce the risk of breast cancer proportionate to the amount of tissue removed. Prophylactic mastectomy has a proven role in reducing the incidence of breast cancer, both among women with a moderate or high-risk family history and among those with proven mutations of BRCA1 or BRCA2.
Methods of reconstruction
Reconstruction of the breast can be accomplished in several ways at any time after surgery. The type and timing of reconstruction do not affect biologic processes or the detection of breast cancer. For advanced cancers for which irradiation of the chest wall and regional nodes is planned, breast reconstruction should be delayed, but the intention to perform reconstructive surgery does not prevent radiation therapy if unexpected pathologic findings are discovered.
The simplest reconstruction consists of placing an expandable saline implant under the pectoralis muscle in the musculofascial layer and stretching the tissues of the chest wall to reduce tightness and firmness of the chest wall. The implant is then replaced with a permanent implant. Saline is instilled into a fill valve at regular intervals over several weeks until the expander is overfilled to 200 mL beyond the volume of the contralateral breast. After the chest wall is stretched to allow for a normal breast contour, a second operation is performed to replace the implant with a shaped prosthesis or to remove the excess fluid and fill valve. Complications include extrusion of the expander, infection, and deflation. Patients complain of chest-wall tightness and asymmetry.
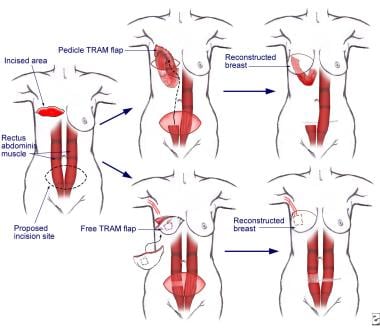
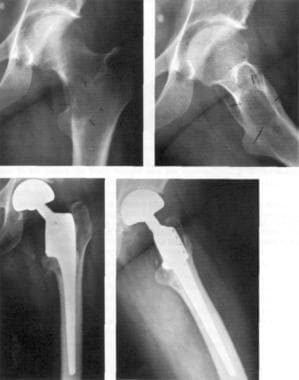
The 3 most common of the autologous procedures are the latissimus dorsi muscle flap procedure (performed by using muscles taken from the back), the procedure involving a pedicular transverse rectus abdominis muscle (TRAM flap, sometimes called conventional flap), and the free TRAM flap procedure (sometimes called the microsurgical flap). Both TRAM procedures are performed by using muscle taken from the abdomen. The deep inferior epigastric perforator (DIEP) procedure and the superior gluteal artery perforator (SGAP) flap procedure are relatively new techniques in which fat and skin without muscle are used for reconstruction. See the images below.
Flap procedures are used to transfer distant tissue with its own blood supply. Muscle and skin can be transplanted from the back (latissimus dorsi flap), abdomen (transabdominal rectus or TRAM flap), or buttocks (gluteus flap), and a microvasculature anastomosis is performed. The TRAM flap has become the flap of choice because of the volume of tissue that can be moved. However, cigarette smoking, diabetes mellitus, and obesity are relative contraindications because of decreased microcirculation. When the irradiated chest wall is reconstructed, the TRAM flap is preferred because of its vascularization.
The pedicle TRAM flap procedure requires the entire rectus abdominis muscle for construction of a new breast. The surgeon rotates the muscle, pulls it up through a previously constructed tunnel in the chest, pockets it out, and molds it into a breast. Blood supply from the superior epigastric artery and vein remain intact at their source, and they are pulled up with the muscle.
The free TRAM flap procedure requires only a portion of the rectus abdominis muscle. The surgeon fully removes a portion of the muscle from the donor site, with blood supply intact from the deep inferior epigastric vein and artery, and reattaches it to the chest wall to reconstruct the breast. The surgeon then connects the tiny vessels to recipient vessels, most often the thoracodorsal artery and vein in the axilla near the new breast, in a separate microvascular procedure.
The free TRAM flap surgery is not performed as often as other procedures in women who choose breast reconstruction after mastectomy (only 5% of reconstructions involve this procedure). However, it is a highly satisfactory option for the right candidates, and, in some cases, it may be the most logical choice.
Postprocedural care
The patient or her caregiver at home must be able to empty any remaining surgical drains and record amounts of drainage. The surgeon usually orders removal of a drain when it has less than 25 mL of output in 24 hours. Drainage from the incisions should be absent or minimal. However, for the first 2-3 days after drain removal, a small amount of serosanguineous drainage from the exit sites is normal. Abnormal drainage is foul smelling and saturates a 4 X 4-in gauze. After the drain is removed, a small piece of gauze may be placed over the drain exit, but the supportive bra should hold it in place. Tape should not be used on the reconstructed breast. For the first few weeks, showering should replace bathing in a tub.
Binding: Instruct patients in the use of a supportive bra without underwires in the hospital, usually a day after surgery. Some patients may desire an abdominal binder in addition to the supportive bra.
Smoking: Avoidance of smoking is especially important during the first few weeks of vascular and tissue healing. Also, avoidance of smoking at least 4 weeks before surgery reduces complications, such as flap necrosis and hernia after surgery.
Exercise: Encourage women after mastectomy to perform arm abduction and reaching exercises; however, advise patients to avoid these exercises after free TRAM flap surgery. The patient may be limited to lifting no more than 10-lbs. for 4-6 weeks, and the patient should keep her affected arm below the height of her shoulder for 2 weeks. However, encourage use of the arm in front of the body (as in washing the face or eating) to prevent stiffening of the joints. Some patients benefit from physical therapy (PT) to strengthen the abdominal muscle after TRAM flap surgery.
If a TRAM flap reconstruction is planned, address rehabilitation issues, and preoperatively counsel the patient about the need for a program to address back and shoulder strengthening. Decreased trunk flexion and extension strength also result from the surgery. PT focuses on strengthening exercises and compensatory movements for most patients, particularly for individuals with chronic spinal pain.
Other types of reconstruction are associated with discomfort related both to loss of tissue from their respective areas and to the actual surgical procedure. The latissimus dorsi flap procedure is less complicated than other reconstructive procedures, but an implant is required for adequate cosmesis. The most common complication is seroma formation. No functional loss of shoulder strength is observed. A gluteus maximus flap is both less painful and less morbid than a TRAM flap, but it is more technically demanding. A nipple can be constructed in all types of reconstruction by puckering skin and tattooing an areola, or by grafting skin into a nipple site and tattooing. Avoid grafts on irradiated skin.
Shoulder and Arm Rehabilitation
The goal of arm and shoulder exercises is to enable the patient to return to normal activity after axillary dissection. At 3 or 15 months after surgery, approximately 80% of patients continue to report at least 1 problem. Problems may include swelling (25%), weakness (25%), limited ROM (30%), stiffness (40%), pain (50%), and/or numbness (55%). Increasing numbers of complaints are associated with high levels of psychological distress. In the optimal situation, preoperatively evaluate the patient for strength, ROM, sensation, posture, endurance, and general functional ability. Instruct the patient regarding ROM exercises, postoperative breathing, and initial mobility after surgery. Start shoulder and arm rehabilitation as soon as the surgical incision appears healed and recurrent seroma or infection is absent; remember the principles of wound healing.
Early PT to the shoulder after axillary dissection does not increase the incidence of lymphedema. The development of seromas is most prevalent with extensive surgeries. Encourage the patient to begin gradual stretching exercises for all degrees of motion within a few days of surgery. The optimal program starts postoperatively with gentle ROM exercises of the shoulder from 45-90° in patients without reconstruction. PROM should start to 90° of flexion and abduction with external and internal rotation as tolerated. Early mobilization of the glenohumeral joint improves shoulder ROM. Recovery was faster in patients who began shoulder flexion to 40° on day 1 and 90° on day 4 than in those who had a delayed start of ROM exercises. Methods to compensate for nerve injury improve muscle strength and prevent shoulder tightness and discomfort.
Patients should begin full shoulder and arm ROM exercises as soon as the surgeon deems them safe, often after the drains are removed. Active and active-assistive exercises can be increased at this stage. Exercises, such as wall climbing, and use of pulley or wand, should be added. After all sutures are removed, exercises more aggressive than these can be incorporated.
Physical modalities may be helpful. Use ultrasound with caution, given its potential risks of promoting residual tumor cell growth or metastasis. Include stretching exercises and electrical stimulation as part of the rehabilitation program. Patients treated with mastectomy are more likely than patients receiving breast-conserving surgery to have impaired mobility. Prospective studies demonstrate that patients who receive structured PT achieve arm and shoulder function better than the function of those who do not receive such PT.
A home exercise program should be implemented, and follow-up PT assessment should be included. Massaging of scars is usually incorporated into this program around 1 month after surgery. With radiation treatment, ongoing ROM exercises are particularly important to prevent contracture formation.
Discuss lymphedema precautions with the patient before surgery, and review her condition within several days of surgery. When resting, the patient should elevate her arm higher than her heart but not over her head. Exercises using the forearm and hand should be performed immediately to help muscular propulsion of blood and lymph fluid from the lower arm. Encourage the patient to squeeze a tennis ball or other soft ball when resting. Advise the patient not to lie on her arm in the ipsilateral decubitus position and to avoid a prone position.
Discuss the effects of skin or soft tissue infections on the development of arm edema, the effect of gravity on lymph drainage, the importance of avoiding procedures on the arm that may break the skin, and the type of exercises that can improve muscle tone in the arm. Encourage the patient to be aware of the importance of weight management because edema of the arm is associated with weight gain. Advise the patient to seek medical help immediately if signs of erythema or swelling occur. Many physicians prescribe antibiotics for acute edema.
Radiation Therapy and Its Consequences
Use of radiation therapy after breast-preserving surgery is common to reduce the probability of recurrence in the breast and after mastectomy, when the risk of recurrence in the chest wall is high. The breast is treated with tangential techniques that also include irradiation of the underlying muscle, rib, and anterior surface of the lung. After mastectomy, the chest wall is treated with similar techniques, but radiation is delivered after subcutaneous tissue is damaged by production of skin flaps. The supraclavicular, axillary, and sometimes internal mammary nodes are irradiated when the risk of nodal recurrence is high. Direct anterior fields are used to treat increased volumes of rib and lung tissue. The brachial plexus is often in the node fields, but damage is uncommon with standard doses. Irradiation of the axillary nodes is associated with an increased risk of lymphedema; avoid it unless the risk of recurrence in the axillary nodes is clinically significant.
Irradiation exaggerates the effects of surgery. Fibrosis secondary to radiation in the treatment field may cause the following effects:
-
Increased obstruction of arm lymphatics (if in the radiation field)
-
Increased tightness of the chest wall and pectoralis decreasing shoulder mobility (most prevalent in patients undergoing mastectomy)
-
Pain in subcutaneous tissues, intercostal muscles, or ribs
-
Decreased pulmonary reserve (rare unless more than 10% of the lung volume is treated)
-
Rib fractures (1% risk)
Soft tissue infections, cigarette smoking, and diseases that may impair microcirculation (eg, diabetes, arteriosclerotic vessel disease) increase the probability of fibrosis. Exercise and manual massage may decrease pain and discomfort associated with fibrosis. Ointments to treat dry skin may relieve dryness and itching. Breast edema is an adverse effect unique to breast preservation and related to the extent of axillary dissection, the location and extent of breast surgery, and the size of the breast. Weight gain may aggravate breast edema. Breast edema resolves with time, but weight loss, proper breast support, and avoidance of prone sleeping position may help. Development of late breast edema is uncommon and may represent infection or recurrent cancer.
If volumes of lung tissue greater than 10% are included in the radiation fields, the patient may develop cough, shortness of breath, and low-grade fever 4-12 weeks after radiation. The physician must rule out an infectious source. Chemotherapy increases the risk of pneumonitis. Temporary, low-dose steroids may relieve symptoms of radiation pneumonitis, and antibiotics are often added empirically. Acute radiation pneumonitis resolves in 2-3 months and is not predictive of long-term pulmonary insufficiency. About 10% of lung volume must be treated to observe pneumonitis. Always compare chest radiographs with radiation portal images to confirm the etiology of the disease process.
Most patients have subclinical effects of the lung. In most patients, the diffusing capacity of carbon monoxide decreases but returns to normal levels by 24 months. However, patients who smoke cigarettes have greater deficit and less recovery than those who do not smoke. Cigarette smoking affects the tolerance of the lung to radiation; therefore, encourage patients to stop smoking. Permanent injury to the lung because of interstitial fibrosis is localized to only the radiation field and can be identified on lung radiographs. Long-term effects of lung fibrosis are related to the volume of irradiated lung and to the patient's pulmonary status before irradiation.
Radiation-induced brachial plexopathy is characterized by shoulder discomfort and progressive paresthesias and weakness in the arm and hand. About 1% of patients who receive nodal irradiation with doses greater than 50 Gy and who are usually treated with chemotherapy develop problems. If doses are limited to 50 Gy, symptoms are generally transient. Symptoms develop 3-14 months after irradiation and commonly affect the distribution of the lower plexus. Progressive neurologic dysfunction of the brachial plexus is associated with radiation fibrosis because of large fractions. The prevalence of pain, in addition to paresthesias of the hand and proximal arm weakness, may be increased. Weakness in the distribution of the upper plexus is most common. Associated arm edema secondary to irradiation is often noted. No treatment, other than symptomatic management, is known. However, cancerous infiltration of the brachial plexus can mimic these symptoms and must be ruled out.
Women treated with direct fields to the left side of the chest may have increased incidence of arteriosclerotic heart disease and, consequently, of myocardial infarctions. Women often become menopausal as a result of estrogen deprivation; this development may add to incidence of cardiovascular disease. Discuss the benefits of diet, exercise, hypertension treatment, and treatment of cholesterolemia with any patient with breast cancer, but the importance of this step is most obvious in patients treated with irradiation and chemotherapy.
Hormonal Treatment
Tamoxifen or aromatase inhibitors are commonly prescribed for women with hormone receptors positive for estrogen whose cancers are larger than 1 cm. Many premenopausal women receive tamoxifen after chemotherapy, whereas many postmenopausal women with large tumors or positive nodes receive it as single-agent adjunct therapy. Tamoxifen may be prescribed for a minimum of 5 years. In addition to the antitumoral effect, other benefits of tamoxifen may include reduced bone loss and an improved lipid profile. Tamoxifen often exaggerates symptoms of estrogen deprivation, with hot flashes (50-60%), depression (10%), weight gain, and vaginal dryness as common complaints. Examine patients annually because of a possible risk of endometrial carcinoma secondary to tamoxifen. The aromatase inhibitors have equal efficacy and a slightly improved adverse-effect profile.
Chemotherapy and Its Consequences
In the adjunct setting, chemotherapy is usually administered in 4-6 cycles of 3-4 weeks. Preconceived notions, often incorrect, can affect a woman's attitude toward chemotherapy. The clinician must anticipate these concerns, particularly nausea, hair loss, and lifestyle changes, when introducing the topic of chemotherapy. Immediate effects of chemotherapy include general fatigue, as well as nausea and vomiting, which are effectively countered with medication, including prochlorperazine, lorazepam, ondansetron, and granisetron. Patients often gain weight because food may relieve nausea, and their basic metabolic rate may decrease. Fatigue can be overwhelming and affect exercise and activity levels. Work and family issues may be important during chemotherapy because treatment can last for many months.
During therapy, many women have a diminished immune status, which puts them at risk for infection. These periods are short, but some women require increased intervals between chemotherapy cycles or use of growth factors, which are associated with their own adverse effects. Prolongation of chemotherapy may be devastating for many women who have planned for periods of disability for a certain length, who are limited in their sick absences from work, or who must rely on childcare. In general, these women should avoid being around children with the usual childhood diseases (eg, chickenpox).
Chemotherapy may render women, generally those in their late 30s or 40s, menopausal. The incidence of premature ovarian failure is about 70%, but it is lower than this in women younger than 30 years. The most common severe late effect of doxorubicin (Adriamycin) chemotherapy is cardiomyopathy, occurring in less than 1% of women with a total cumulative dose of 300 mg/m2. A previously active young woman may become dyspneic on exertion. Appropriate consultations with a cardiologist and staff from cardiac rehabilitation programs may improve the performance status of women made symptomatic by therapy. Another serious adverse effect of chemotherapy is an increased risk of leukemia, which is related to dose and type of alkylating agent (incidence of 0.7% at 10 y); this risk may increase with adjunct radiation. Current data suggest that the risk of leukemia is minimal with regimens containing cyclophosphamide that are used today.
Nonetheless, the use of adjuvant chemotherapy clearly benefits women with early breast cancer. A meta-analysis of randomized trials of adjuvant prolonged polychemotherapy in women with early breast cancer demonstrated that, in terms of survival advantage, relatively short regimens of approximately 3-6 months were as effective as the longer chemotherapy regimens. Polychemotherapy provided an absolute improvement of 7-11% in 10-year survival among women younger than 50 years at presentation; for women 50-69 years of age, the absolute improvement in 10-year survival was 2-3%.
Anthracycline-containing regimens were slightly more active than the previous standard combination chemotherapy of cyclophosphamide, methotrexate and 5-fluorouracil (CMF), with the former producing a moderate improvement over the latter with respect to the percentage of patients surviving and being disease-free after 5 years. The benefit of anthracycline-containing regimens is particularly evident in premenopausal patients, and increasing evidence suggests that 6 or more cycles of the 3 drug regimens are more effective than the 4 cycles of doxorubicin and cyclophosphamide (ie, Adriamycin and cyclophosphamide [AC]) that has become popular.
Taxanes, such as paclitaxel, have promising activity in patients with node-positive primary breast cancer. Preliminary results from a large, multicenter study showed that patients treated with AC followed by paclitaxel had a significantly better disease-free survival and overall survival than patients treated with only AC. Moreover, the addition of paclitaxel to AC was well tolerated. Further results from this trial are awaited with interest, particularly because preliminary results from other studies have not yet confirmed these findings.
Encourage women to be active and to seek support. Evidence suggests that participating in support groups or having a confidant increase probability of survival. Continuation of regular activities during chemotherapy is beneficial. In 1 study, 41% of women found that treatment was easier than expected. By focusing on delayed benefits of chemotherapy (ie, survival issues), women can cope with short-term adverse psychological effects. In some professions, women are not allowed to continue working during therapy (eg, firefighter, airline pilot), and they are placed on medical disability. The Americans with Disabilities Act (ADA) protects women with breast cancer from workplace discrimination in most settings. The Family Medical Leave Act (FMLA) also requires flexibility in scheduling for patients and family members to accommodate treatments.
Exercise
While there is not a criterion standard when prescribing exercise, an experienced rehabilitation team can prescribe an exercise regimen to optimize each patient’s health. For the general population, the benefits of exercise on weight and on the cardiovascular system are undisputed. Women with breast cancer who participated in aerobic exercise have improved QOL. Obesity is a minor risk factor for breast cancer; it is associated with additional complications of breast-cancer treatment (eg, lymphedema) and is associated with an increased risk of breast-cancer recurrences.
Exercise improves the functional capacity of patients with breast cancer who are receiving adjunct chemotherapy. Weight gain is common during chemotherapy and apparently connected with loss in muscle tissue, which may contribute to reduced functional capacity and a lowered metabolic rate during adjunct chemotherapy. Increased lean body weight is observed in patients who exercise while receiving chemotherapy.
A study by Scott et al indicated that in patients with breast cancer, a continuous exercise program, that is, one administered both during and after adjuvant chemotherapy, has greater benefits with regard to cardiorespiratory fitness than does exercise engaged in only during or after (ie, concurrently or sequentially with) the chemotherapy. Patients in the study received either usual care or had three sessions per week walking on a treadmill, with the treadmill sessions lasting 20-50 minutes at 55-100% peak oxygen consumption. Those engaging in the treadmill program either underwent about 16 weeks of concurrent or sequential exercise or approximately 32 weeks of continuous exercise. The investigators found that only the continuous program was associated with a significant improvement in peak oxygen consumption. [19]
In animal models, exercise did not induce metastases and was associated with a decreased number of metastases. Exercise also attenuates cachexia in animals.
Management of Lymphedema
Any dissection of axillary lymphatics and nodes places a woman at risk for edema of the arm. Axillary surgery and irradiation can lead to lymphedema, which may be caused by direct damage to axillary lymphatics. Fibrosis of the axilla secondary to surgery and/or radiation causes venous and lymphatic obstruction by compressing major vascular trunks and blocking regeneration of lymphatic and venous collaterals. Additional radiation therapy, trauma, and infection are other causative factors. Increase in arm circumference immediately after surgery is common and should resolve within weeks. No standardization exists in the literature as to the type and location of measurement and the implications of such measurement. Most clinicians agree that a difference in circumference of more than 2 cm between the arms has clinical significance.
Nonetheless, lymphedema may be classified as 1 of 3 stages. The first stage is where pitting is associated with edema and temporarily reduced with elevation of the arm. In the second stage, the edema does not reverse spontaneously. Protein-rich edema persists and can lead to proliferation of connective tissue. With such changes, fibrosis occurs and brawny edema is seen on clinical evolution. In the last stage, lymphostatic elephantiasis, the patient has enormous volume with cartilage-like hardening of dermal tissue along with papillomatous outgrowths.
Late arm edema is associated with the patient's age, the extent of cancer in the axilla, the extent of axillary dissection, and the dose and techniques for irradiation. Nearly 33% of patients older than 55 years and 25% of patients in whom more than 15 nodes are dissected develop a difference of 2 cm or greater in the circumference of their arms at 3 years. By comparison, late breast edema is less common after axillary dissection is performed in conjunction with breast-preservation surgery. Therefore, always consider the presence of an infection or recurrent cancer as a possible cause of late edema.
Perform medical assessment to determine the cause of swelling. Rule out or treat infection, venous thrombosis, or cancer recurrence. Prescribe antibiotics if the development of edema is acute. Make serial measurements of both arms with the olecranon as the reference point. Assess shoulder, arm, and hand strength; sensory changes; color; turgor; pulses; and mobility. In rare cases, long-standing lymphedema can lead to lymphangiosarcoma, a highly aggressive tumor with poor survival despite forequarter amputation.
Conservative management of lymphedema should include preventive and mechanical modalities as needed. Pharmacologic means include antibiotic prophylaxis to prevent and treat cellulitis and lymphangitis. Drugs such as anticoagulants, hyaluronidase, pyridoxine, benzopyrenes, and others have been used but have no proven therapeutic value. Preventive care should emphasize identification of patients at highest risk of lymphedema. Comorbid illnesses such as hypertension, heart disease, diabetes and kidney disease can contribute to edema also. Patients should understand lymphatic drainage, the pathology leading to lymphedema, as well as the signs, symptoms, and complications of lymphedema.
Self-care instructions include the following:
-
Proper nutrition with balanced nutrition and increased protein and lowered salt intake
-
Weight management
-
When possible, the arm should be elevated above the level of heart.
Home exercise program includes the following:
-
ROM exercises
-
Exercises and techniques to improve venous drainage
-
The importance of gravitational drainage
Static resistance exercises and positional changes need to be incorporated into daily activities, including positioning for sleep.
Traditionally, no heavy lifting with the involved arm, typically less than 15 lb - However, although weight lifting has generally has been proscribed for women with breast cancer–related lymphedema, in a randomized, controlled trial of twice-weekly progressive weight lifting in 141 breast cancer survivors with stable lymphedema of the arm, Schmitz et al found that, compared with the control group, the weight-lifting group had greater reductions in the self-reported severity of their lymphedema symptoms (P=0.03) and experienced more improvement in upper- and lower-body strength (P< 0.001 for both). [20]
In addition, the incidence of lymphedema exacerbations was lower in the weight-lifting group than in the control patients (14% vs 29%, P=0.04).
Injury and infection should be avoided, as follows:
-
No venipuncture or finger sticks on the involved side
-
Skin breaks should be cleaned with mild soap and water, followed by antibacterial ointment use.
-
Recommend long-sleeved shirts and bug-repellents for prevention of bug bites.
-
Use of gloves during gardening
-
Use of an electric razor for shaving
-
Good nail care, including not cutting the cuticles
-
Gauze wrapping instead of tape use
Physician should be notified about rashes, erythema, swelling, pain, increased warmth or localized infection. Daily cleaning and lubrication of skin is indicated.
Avoid constrictive pressure on the arm (eg, no blood pressure cuff, no constrictive bands).
Recommend follow-up with the physician on a regular basis and with any sudden change in arm circumference or evidence of infection.
Complex lymphedema therapy is used to treat peripheral lymphedema and typically has 2 phases, acute and maintenance. The acute phase of therapy consists of manual compression, external compressive bandaging, and specific therapy exercises, including manual and massage techniques. Patients and family members should be taught these techniques. The goals for the patient during the maintenance phase are to be able to wear specially fitted pressure gradient garments during the day, with compression bandaging or a compression device at night. Intermittent pneumatic pressure devices are used in the management of lymphedema. However, such devices may be most effective in low-protein venous edema in which fluid is directly forced back into the blood vessels. With lymphedema, such tissue fluid may simply be displaced into an adjacent region.
External compression can place increased proximal demands on the existing intact lymphatic system. Pressures over 45 mm Hg may further damage lymphatic structures. With increased pressures, pain and hematomas are common in the involved site. Patients with severe edema required prolonged compressive bandaging and close follow-up with therapists (typically several times a week for at least 3-4 wk). Afterward, results can be maintained with continued bandaging and use of manual techniques at home.
A nonelastic bandage may have to be left on in excess of 12 h/d. After the volume of the limb is stabilized, the use of manual techniques and compression garment (often customized) may be sufficient. With exacerbations of lymphedema, use of a nonelastic bandage may be necessary, along with outpatient PT for close supervision. Compression garments are ideally replaced every 3-4 months because they tend to lose their elasticity.
Counsel the patient regarding the permanent nature of the condition and how to prevent its progression. Remember that, with increased interstitial protein level, progressive fibrosis and chronic inflammation can ensue. Although treatment is time-consuming, particularly in its initial phases, it is associated with improved body image and function, which increase QOL. Arm swelling has been associated with increased psychiatric morbidity, as reflected by anxiety, depression, and poor adjustment to breast cancer. Consider psychological intervention when lymphedema is obvious to the casual observer.
Investigators in the Netherlands reported long-term impairments, disabilities, and QOL-related issues. Pain (60%) and reduction of grip strength (40%) were the most frequent impairments. The prevalence of impaired ROM and edema was 9-16% and 15%, respectively. Mean group scores for QOL differed significantly for physical functioning, vitality, and health perception compared with those for a healthy female group. Radiotherapy and chemotherapy were significant factors in the prediction of impaired ROM.
Another group of clinical investigators reported their findings in 105 survivors of breast cancer. The patients were interviewed to obtain data about their health and economic changes in the 5 years after diagnosis and initial treatment. An age- and work-matched group of 105 women without cancer were also interviewed. Key changes in functional status and economic outcomes (eg, changes in market earnings, household income, insurance coverage) were measured. Severity of impairment was compared between the study and control groups. Also tested was the adversity of economic outcomes in relation to the women's impairment, regardless of their breast cancer status.
The analysis revealed statistically significant evidence with regard to each of the relationship tested. Survivors of breast cancer were more likely than control subjects to be functionally impaired at 5 years, and women with impairment were most likely to have reduced work effort and to experience downturns in market earnings, among other outcomes.
Korpan et al reviewed the effects of exercise on breast cancer treatment–related lymphedema. Weight-lifting exercise did not worsen lymphedema when individuals wore a compression garment on the affected limb. Hydrotherapy pool exercise decreased mild-to-moderate lymphedema 29% after 3 months of weekly sessions. [21]
Exercise facilitates lymph drainage via 2 mechanisms. First, exercise compresses lymph vessels with muscle contraction. Second, exercise alternates intrathoracic pressure with respiration. These 2 mechanisms assist lymph drainage from the extremities, into the thoracic duct, and back into circulation.
Systemic Effects of Cancer-related Deconditioning
Injury to Organ Systems
Cancer syndromes, either as a consequence of tumor-induced organ-system injury or of toxic therapeutic interventions, can produce inactivity in the patient. Fatigue and, in advanced conditions, asthenia, cachexia, and anorexia, compound underlying injuries to organ systems. Effects of inactivity contribute to morbidity and mortality by predisposing organ systems to further pathophysiologic risks. Various deleterious effects of inactivity have been documented in both healthy individuals and patients with cancer. [22, 23]
Musculoskeletal effects
In healthy individuals on complete bed rest, strength declines at a rate of 1-1.5% per day, or about 10% per week. Muscle torque may decline as much as 24% in lower-extremity muscles after 5 weeks of bed rest. Loss of strength is often greater in the proximal lower extremities than in the upper extremities; this outcome leads to impairments when the patient walks or assumes a sitting or standing posture.
Muscle shortening occurs in addition to loss of muscle force. Muscle shortening, in conjunction with changes in periarticular and intra-articular tissues, contributes to joint contractures. If local edema and hemorrhage are present, collagen formation escalates, producing tightness of the soft tissue. In the presence of underlying muscle weakness, as might be seen with a lesion of the lower or upper motor neuron, decreased levels of activity add to weakness already present. In these settings, dynamic muscle imbalance further increases the risk of joint contracture.
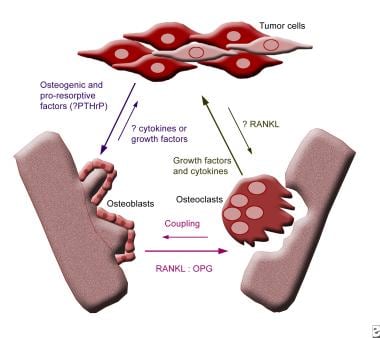
Urinary calcium excretion increases within 2-3 days of bed rest and continues to increase over 4-7 weeks. This hypercalciuria may result from a loss of muscle pull on bony surfaces and eventually leads to disuse osteoporosis. In young individuals, shift of calcium from bone to the circulatory system is heightened and exceeds maximal urinary excretion, sometimes resulting in hypercalcemia. Underlying skeletal metastatic disease or paraneoplastic production of compounds similar to parathyroid hormone (PTH) may place patients at risk for hypercalcemia. In 1 study of subjects on bed rest, 8 hours of sitting and 4 hours of supine exercise per day were insufficient to diminish hypercalciuria, whereas standing 3 hours per day was helpful.
Respiratory effects
When a person assumes a recumbent position, the diaphragm moves cephalad because pressure from intra-abdominal contents effectively decreases intrathoracic size. Lying down initially increases pulmonary blood flow as blood redistributes from the lower extremities; however, within 60-90 minutes, pulmonary blood flow returns to baseline or below the level observed when the patient is sitting. Abdominal-muscle activity predominates over rib-cage motion when the patient is lying down, producing a shallow breathing pattern and increasing the respiratory rate. Reduced activity in diaphragmatic and intercostal muscle contributes to weakness of the respiratory musculature, just as inactivity causes weakness in the musculature of the extremities.
Reduced rib-cage motion can lead to tightness of the costovertebral and costochondral joints. As a result of these anatomic changes, functional residual capacity declines, and closing volume (point during expiration where the alveoli close), which changes in position do not affect, may increase beyond functional residual capacity, producing atelectasis. Coughing to clear secretions is more difficult in the supine position than in other positions; therefore, pooling of secretions occurs in the dependent portions of the lungs. In the converse, blood flow is greatest to these same lung portions, leading to a ventilation-perfusion (V/O) mismatch and producing arterial hypoxemia.
Several factors increase the risk of respiratory complications in patients with cancer compared with the general population. Coughing or taking deep breaths may be painful for the patient with rib metastases or for the patient who has undergone surgical procedures of the chest and abdomen. Lung involvement because of primary tumor, metastatic disease, malignant pleural effusion, or complications of chemotherapy or radiation further contributes to reduced oxygenation, retained secretions, and the risk of pneumonia. Frequent changes in position may improve secretion clearance and V/O mismatch in patients on bed rest. Encourage patients to take deep breaths at regular intervals and to use incentive spirometers and pulmonary resistive exercises. Stretching and strengthening of the trunk and abdominal muscles can help prevent or treat rib cage tightness and weakness.
Urinary effects
Voiding in a supine position inhibits effective bladder evacuation. Stasis of urine occurs within the renal pelvis, and this urinary stasis, in conjunction with the hypercalciuria associated with immobilization, predisposes a patient to development of stones in the urinary tract. Retention of urine or use of indwelling catheter increases risk of urinary tract infections. Patients with cancer involving bladder-outlet obstruction (as in prostate cancer), or with impaired bladder emptying caused by involvement of the sacral nerves or spinal cord are at added risk when they are required to void on a bedpan.
Prevention of urinary complications involves limiting the use of indwelling catheters as much as possible. If long-term catheter use is required, consider a condom catheter in the male patient or intermittent catheterization in the female patient. Provide a bedside commode for patients with intact spontaneous voiding to allow them to void in a relatively upright position when they can be transferred. Allow patients bathroom privileges as soon as they can move about.
GI effects
Inactivity results in impaired colonic function. Immobilized subjects have increased adrenergic stimulation, resulting in decreased peristalsis and increased sphincter contraction. Studies using radiopaque markers demonstrate an increase in colonic transit time and decline in mass propulsive waves of the colon in immobilized individuals. Constipation may occur when the patient is receiving opioids for pain control and may result in fecal impaction. Administration of chemotherapy may result in nausea, vomiting, and anorexia. These factors, in combination with the negative nitrogen balance associated with bed rest, may further contribute to cachexia and hypoproteinemia. Early encouragement of patients to use the bathroom or commode and practice of a consistent bowel program, including use of stool softeners and bulk-forming agents, can reduce risks of constipation.
Cardiovascular effects
Hemodynamic changes associated with compromise within the cardiovascular system begin within a few days of recumbency. Healthy young men lose 300-500 mL of plasma volume within the first week of bed rest. Plasma volume declines more than red cell mass does, increasing blood viscosity, which is thought to contribute to the risk of deep vein thrombosis (DVT). Hypotension in connection with upright positioning has been observed in patients within a week of their beginning a regimen of bed rest. When healthy individuals are elevated to an upright position, venous return declines, decreasing stroke volume and cardiac output. Adrenergic sympathetic stimulation normally occurs, producing increase in the heart rate and vasoconstriction of peripheral and splanchnic blood vessels, maintaining blood pressure.
After prolonged recumbency, the circulatory system is unable to produce adequate vasoconstrictive response to changes in posture, leading to fall in blood pressure and tachycardia when the patient rises to a standing position. Stroke volume and cardiac output decline, producing lightheadedness and syncope secondary to inadequate cerebral perfusion. Additional symptoms (eg, burning in the lower extremities, nausea, diaphoresis) have also been documented after recumbency, though clinically significant decreases in blood pressure may not be found in all patients when they assume a standing position.
Decreased cardiac efficiency is also affected in response to exercise. Increases in stroke volume in response to exercise are not maintained, and cardiac output declines. In patients with coexisting coronary artery disease, changes on standing may precipitate myocardial ischemia. Maximal oxygen consumption decreases by as much as 15% when healthy individuals exercise in an upright position after 10 days of bed rest. After this postural response is lost, 3-4 weeks may be required to establish normal postural responses.
Thrombogenic risks
Bed rest, in association with other risk factors, may result in DVT, and risk for thrombosis increases with the length of bed rest. In addition to changes in blood viscosity, mechanical compression of veins may contribute to venous stasis. Patients with cancer, because of associated hypercoagulable states, are predisposed to form venous clots. Several strategies can help prevent and mediate cardiovascular complications, though early mobilization of the patient is the most effective approach. Maintenance of adequate fluid and salt intake is another simple measure for alleviating symptoms associated with cardiovascular symptoms.
Dynamic resistance exercises in the supine position assist with maintaining plasma volume. Abdominal strengthening and lower-extremity exercises (eg, ankle pumps) improve venous stasis and can be performed in conjunction with the use of elastic stockings and abdominal binders to maintain blood pressure in orthostatic patients. Use of reclining wheelchairs or tilt tables may help the patient gradually adjust to an upright posture if orthostatic symptoms are a problem. Introduce pharmacotherapy in cases of suspected autonomic neuropathy-related orthostasis. Recommend that the patient begin sitting upright as soon as possible because lack of orthostatic stress significantly contributes to impaired exercise capacity.
Nervous effects
Balance and coordination decline in patients on bed rest. This decline may increase a patient's risk of falling. Confinement of a patient to a hospital bed can also cause sensory deprivation, which affects perception and cognition. Changes in concentration, sensory distortion, and hallucinations have been documents in both healthy subjects and hospitalized patients. Alterations in intellectual and perceptual testing have also been found in patients on bed rest. Early activity with access to sensory stimulation can help in preventing changes in intellectual or perceptual capacities.
Integumentary effects
Hospitalized patients are at high risk for pressure ulcers, which have an incidence of 7.7% incidence within 3 weeks of admission. Geriatric patients are at particular risk for pressure ulcers because of the aging-associated loss of subcutaneous tissue, decreased connective tissue elasticity, and decreased secretion levels of sebaceous and sweat glands. Sustained pressure over bony prominences results in ischemic injury, and, because muscle and subcutaneous tissues are more sensitive to injury than the epidermis, the initial appearance of a sore may not reflect the severity of the underlying injury. Several factors contribute to skin breakdown; these include pressure, shearing forces, friction, and moisture.
Patients with cancer at increased risk for pressure sores include not only the elderly but also patients with impaired alertness, altered sensation or movement, poor nutrition, and/or incontinence. Prevention involves first the identification of high-risk patients and then intervention with repositioning schedules in a bed or chair, use of support surfaces or beds to reduce pressure, reduction of shearing forces during transfers or repositioning, minimization of skin exposure to moisture, and maintenance of adequate nutrition.
Therapeutic Exercise to Mitigate Deconditioning
Norwegian clinical investigators reviewed the role of therapeutic exercise in the amelioration of cancer-related weakness and fatigue. Interventions included aerobic exercise training (10 studies) and resistance exercise (2 studies). Researchers used a wide range of instruments to assess health-related QOL and physical exercise capacity. The studies indicated promising effects on both physiologic and psychological outcomes.
Guo et al [24] conducted a retrospective review of 60 asthenic patients (30 with solid tumors and 30 with hematologic malignancy) who were admitted for inpatient rehabilitation. The study reviewed the differences in total, motor, and cognitive functional independence measure (FIM); hospital and rehabilitation length of stay; and FIM efficiency. The study concluded that both groups benefited from inpatient rehabilitation as patients with both types of malignancy made similar functional gains.
Randomized clinical studies are few, small, and mainly focused on patients with breast cancer. Complete knowledge about the type of physical exercise most beneficial for patients at different stages of the disease progression is still lacking. Future work should identify fewer and more specific endpoints.
A best-evidence statement from the Cincinnati Children’s Hospital Medical Center provides the following recommendations for the use of physical therapy during and after hematopoietic stem cell transplantation (HSCT) in pediatric patients [25] :
-
It is strongly recommended that physical therapists provide exercise therapy, including endurance and strength training, throughout the HSCT process to reduce/minimize the effects of immobility and the consequences of the HSCT treatment; this will contribute to improved quality of life (QOL)
-
It is recommended that a physical therapist do the following in the pretransplantation phase: (1) complete a physical therapy examination to establish a baseline level of function and QOL; (2) make recommendations for treatment (based on) specific to the evaluation results, as appropriate; and (3) educate families regarding the benefits of structured exercise therapy throughout the HSCT process to reduce the effects of immobility and consequences of the HSCT treatment
-
It is strongly recommended that during the HSCT inpatient stay, starting at admission or the initiation of the preparative treatment regimen and continuing until discharge, a physical therapist provide a supervised exercise program of low to moderate intensity to positively affect QOL; this would include: (1) aerobic endurance training and (2) exercises dealing with strength, balance/coordination, and flexibility
-
It is recommended that during the HSCT inpatient stay, a physical therapist provide interventions to promote functional mobility, including with regard to transfers, walking and stair climbing, and relaxation through progressive relaxation exercises
-
It is strongly recommended that following discharge from the HSCT inpatient stay, a physical therapist provide a supervised, scheduled, moderate-intensity exercise program (with the goal of returning the individual to functional baseline level or of continuing until progress is no longer demonstrated), including the following: (1) aerobic endurance training and (2) exercises dealing with strength, balance/coordination, and flexibility
-
It is recommended that during the outpatient program following HSCT, a physical therapist provide interventions to promote functional mobility, including with regard to transfers, walking, and stair climbing
Rehabilitation for Head and Neck Cancer
Head and Neck Cancer: Overview
The American Cancer Society estimated that 9880 new cases of laryngeal cancer (7920 in men and 1960 in women) and 3770 related deaths (of 2960 men and 810 women) occurred in the United States in 2005. An estimated 2500 cases of hypopharyngeal cancer are diagnosed each year. About 60% of larynx cancers start in the glottis. Another 35% develop in the supraglottic region, and the remaining 5% occur in the subglottis. [26, 27, 28, 29, 30, 31]
Swallowing and mastication
Swallowing and mastication are the most salient deficits that arise as a result of the treatment of cancer of the oropharynx.
Swallowing occurs in 3 stages. The first is voluntary, and the other 2 are reflexive. Swallowing starts with voluntary contraction of the mylohyoid muscles, which throw the bolus back onto the posterior pharyngeal wall. The rich sensory innervation, provided by glossopharyngeal nerves, then triggers complex, coordinated movements of the involuntary phases of swallowing. These movements involve the base of the tongue, the soft palate, the larynx, the posterior pillars of the fauces, and the pharynx.
Mastication is a complex process resulting from fine and coordinated movements of the mandible at the temporomandibular joint carried out by 4 main muscles originating from the base of the skull, the temporal arch, and the temporal fossa (outer pterygoid, inner pterygoid, masseter, and temporal), and 3 secondary ones on the floor of the mouth (digastric, geniohyoid, and mylohyoid). Therefore, act of chewing is permitted because of the anatomic and functional integrity of active structures (eg, muscles) and passive structures (eg, mandibular lever, teeth, mucosal lining, salivary glands).
Treatment modalities
As with other cancers, the treatment modalities for head and neck cancers depend on the site, size, and histopathology of the tumor and on evidence of metastasis. The treatment is often defined on a consensus-based system of grading and staging.
The combination of radiation therapy with concurrent chemotherapy, primarily platinum based, has curative potential in many patients with advanced squamous cell carcinoma (SCC) of the oropharynx, hypopharynx, and larynx. These treatment regimens are particularly attractive for patients in whom the alternative treatment involves surgical resection of a large portion of the tongue base, oropharynx, hypopharynx, or larynx.
The oncologic efficacy of chemoradiation therapy in this population in comparison with radiation therapy alone has been well documented. However, little detailed information is available on the long-term health-related QOL (HRQOL) outcomes after chemoradiation therapy to treat advanced head and neck cancer or on randomized trials comparing chemoradiation therapy with surgery plus radiation therapy. [32] Despite this lack, the presumption persists that posttreatment QOL is generally better with chemoradiation therapy than with surgery plus radiation therapy.
Many patient, disease, and treatment factors influence ultimate long-term QOL. Health status outcomes are often globally referred to as QOL outcomes. However, they should precisely be separated into objective and subjective QOL outcomes; that is, functional or performance status outcomes (what an individual can do) and evaluations of the way an individual perceives and reacts to what he or she is capable of doing, respectively.
Outcomes
Health status outcomes are multidimensional and include several areas or domains. A single healthcare intervention can have positive effects in 1 area and negative effects in another. Circumstances that determine how a person perceives or is affected by performance status (QOL) in a specific area of functioning vary substantially between individuals. These are subject to adaptation over time, and exert a variable influence on overall QOL for different individuals.
In addition, the general assumption that nonoperative intervention uniformly leads to superior QOL also fails to account for the potential for a nonfunctioning but anatomically preserved organ. Some health states in which an organ is preserved (eg, chondronecrosis, chronic aspiration) may be less desirable than not having the organ. Furthermore, advances in ablative surgical techniques, surgical reconstruction, and rehabilitation after surgery may help preserve and restore function.
Patients treated for head and neck cancer can present with some of the most significant posttreatment morbidity of any group of patients with cancer. Functional deficits can affect nutrition, swallowing, communication, dental health, and the musculoskeletal system. The usual treatment involves surgery and/or radiation, though chemotherapy is most frequently used as a neoadjunct agent. Underlying comorbid illnesses or problems, such as alcohol abuse, poor nutritional status, and cardiopulmonary diseases, are more common in these patients than in others.
Extensive surgical treatment can lead to visible deficits and may interfere with socialization and employment. Therefore, functional deficits associated with treatments should be considered with diagnosis of head and neck cancers. In general, treatment selection is the first step in that process because each treatment at each disease site has specific effects on function. Some treatments have been designed with the goal of preserving function.
Counseling the patient and family
As in other medical and surgical procedures that generate new impairments (eg, amputation), counseling of the patient and family members is important. If possible, facilitate discussion among multidisciplinary team members about procedures planned in the context of potential functional effects and rehabilitation needs. Professionals involved should include the physical therapist, occupational therapist, speech and/or language therapist, dentist and/or maxillofacial prosthodontist, audiologist, physician and surgeon, dietitian, and social worker. In addition, the patient and family members or significant others should be involved.
Discuss potential adverse effects or morbidity from each treatment available for the patient's disease site and stage, and identify the patient's preferences for treatment options. After the treatment of choice is determined for a particular patient, address the optimal schedule of interventions by the various rehabilitation team members. If the tumor treatment of choice is a surgical procedure, discuss whether specific parts of the procedure can be modified to facilitate the patient's postoperative function without compromising the possibility of successfully removing the tumor. For example, in patients with oral cancer who undergo surgical treatment, reconstruction may be modified to facilitate postoperative speech and swallowing.
When the decision has been made regarding optimum tumor treatment for the patient, each professional on the rehabilitation team should counsel the patient and evaluate the patient's function before treatment, formulating a plan for initiating rehabilitation after treatment. Because the patient can communicate most easily before treatment, pretreatment assessments are critical.
Some therapies may be preventive, and they may begin before and continue throughout treatment. For example, the patient who receives radiotherapy and chemotherapy needs oral ROM exercises to maintain movement of the lips, tongue, and jaw. Initiate these exercises before radiation therapy, and advise the patient to continue doing the exercises 4-6 times daily. If possible, these should be performed for 5-10 minutes each time throughout the course of radiation and for at least 3 months thereafter. The physical therapist may likewise need to give the patient shoulder exercises to maintain shoulder ROM if nerves innervating the shoulder are resected in a radical neck dissection.
Dental evaluation is important before radiation treatment because dental caries can develop or progress with postradiation xerostomia. Dental extraction, if needed, should be done before radiation treatment. Oral hygiene is essential as a preventive strategy. The dentist and/or maxillofacial prosthodontist may need to take oral impressions before surgery and to be in the operating room during an oral surgical procedure to fit and place a temporary intraoral prosthesis until a permanent prosthesis can be constructed.
Immediately after treatment, counsel patients undergoing tumor-removal surgery regarding the functional effects of surgery and the kinds of therapy they need. Patients must realize that they must be active participants in the different components of their rehabilitation program (eg, development of intraoral prosthetics, PT, speech or swallowing therapy).
If treatment, radiation, or surgical procedures affects swallowing, evaluate patients with posttreatment videofluorography as soon as they can attempt to swallow. This approach is both cost-effective and efficient. The patient may be able to begin oral intake immediately after undergoing a modified barium swallow study if swallowing is functional or if particular swallowing therapy (eg, postural change, swallowing maneuvers) prove effective. Relying solely on bedside approach to assess swallowing without radiographic study is usually slow because the clinician is tentative about the exact nature of patient's swallowing ability. By using a radiographic study of swallowing, design a therapy and/or rehabilitation program so the patient can return to oral intake as soon as possible.
If the patient's ability to communicate is compromised, as after total laryngectomy, the speech and/or language therapist should provide the patient with alternative means of communication to facilitate interactions with nursing staff, family, and others. Throughout this recovery, have the social worker visit with family members and the patient to provide psychosocial counseling, as well as to assist the family in obtaining needed resources when the patient goes home. The social worker usually remains in contact with family and patient, continuing to provide counseling and follow-up resources after the patient arrives home. The location of the patient's tumor and the nature of treatment dictate the type of rehabilitation needed.
Palatal Cancer
In general, the patient who receives surgery to remove a tumor of the hard palate is examined preoperatively by the maxillofacial prosthodontist to provide intraoral obturator prosthesis at the time of surgery. When the patient awakens after surgery, the temporary prosthesis is already in place. This prosthesis is redesigned after the patient's healing is complete after 2-4 weeks or longer. With this temporary prosthesis in place, patient's speech and swallowing often remain relatively intact.
Surgical removal of part or all of the soft palate often requires a palatal bulb that extends posteriorly into the surgical defect. If the palate is resected only partially, fitting the prosthesis may be more difficult than if the entire soft palate were removed. Success of the palatal bulb prosthesis depends on the capacity of the patient's lateral pharyngeal walls to move inward to meet the prosthesis and achieve velopharyngeal closure during speech and swallowing. Sufficient space between the prosthesis and the walls of the pharynx is important to enable comfortable nasal breathing, but enough motion of the pharyngeal wall is needed to contact the prosthesis and close off the passageway to the nose at critical times during speech production and swallowing. Design of this prosthesis can be difficult, particularly in patients who have had radiotherapy to the pharynx because radiotherapy can reduce motion of the pharyngeal wall.
Some patients who undergo removal of the soft palate can never wear prostheses successfully enough to provide obturation of the velopharyngeal space, because they have inadequate pharyngeal-wall activity. In these patients, the prosthesis may need to be large enough that it completely blocks the passage to the nose; therefore, it is uncomfortable. If the prosthesis is too small, air can pass through the nose, leaving the patient with nasality during speech and leakage of food up the nose during swallowing. Sometimes, optimal results are not achieved despite participation of the most had prosthodontist and speech or language therapist in the design of a palatal bulb prosthesis. The same difficulties occur with attempts at surgical reconstruction of the soft palate. In general, prostheses are more successful than surgical procedures in patients with soft palate tumors.
Oral Cancer
Surgical procedures involving the tongue
In general, the percentage of the oral tongue and tongue base that is resected and the nature of the surgical reconstruction govern the extent of the patient's speech and swallowing problems after surgery. This generalization is true whether patients' disease is at an anterior or posterior site. Clinically significant speech and swallowing defects result regardless of the extent of reconstruction if resection of the tongue is more than 50%.
All patients with tumors of the oral cavity should undergo dental assessment before treatment. If at all possible, save the teeth necessary to stabilize any prosthetic device the patient may need after treatment. Although the patients' viable teeth are at risk for radiation-induced necrosis, spare at least 3 teeth to permit function of the prosthetic device.
Criteria for selecting patients for implant-based oral rehabilitation after cancer treatment are the following:
-
Adequate patient motivation, expectation, and
-
resources
-
A reasonable oncologic prognosis
-
Good oral hygiene
-
Bone of adequate quality and volume and in a suitable arch relationship
-
Adequate oral function (particularly of the tongue and for swallowing)
-
No medical contraindications to further surgery
Some surgeons and radiation oncologists advise the use of hyperbaric oxygen therapy when implants or reconstruction surgery involves viable tissue material, especially if radiation therapy is used at the high dose ranges.
Nature of oral reconstruction and its effects
The nature of reconstruction in the oral cavity after resection of a tumor may substantially facilitate or impair the patient's speech and swallowing abilities. In general, the best reconstruction is primary closure, in which no foreign tissue from another part of the body is introduced into the oral cavity. Primary closure probably is best because the patient retains maximal oral sensation. However, primary closure is not appropriate in the anterior floor of the mouth, where primary closure, often done by using tongue tissue, may exacerbate functional abnormalities both in speech and swallowing by tying the tongue into the surgical defect.
A new oral-reconstruction procedure with sensate flaps has been developed to restore oral sensation. In this procedure, a flap of tissue from another part of the body is introduced into the oral cavity. This technique involves anastomosing nerves, as well as blood vessels, from the flap to oral tissues. To date, no clear data about functional effects of this procedure are available. See the images below.
Resection of the anterior oral cavity
Resection of part of the anterior floor of the mouth and tongue generally results in changes in speech articulation and swallowing associated with reduced ROM and shaping of the anterior tongue. The anterior tongue serves to produce sounds for speech, such as "t, d, s," and "z," as well as to lift and contact the food and bring it laterally to the teeth for chewing. The anterior part of the tongue also contributes to forming food into a bolus before it is swallowed. The anterior tongue initiates the oral stage of swallowing by propelling food backward. All these functions can be affected by resection of the anterior floor of the mouth and the tongue.
If surgical reconstruction after resection further inhibits tongue motion, increased functional deficit is anticipated. Resection of the anterior portion of the mandible is not performed, generally because of severity of the cosmetic defect. The patient who has undergone resection of the anterior oral cavity may have some delay in triggering the pharyngeal swallow because of postoperative changes in tongue motion. Oral tongue motion contributes to sensory input for triggering the pharyngeal stage of swallowing. Provide patients with speech and swallowing therapy as soon after healing as possible. Motor control of the pharyngeal stage of swallowing is not impaired unless the muscles of the floor of the mouth are cut in anterior resection. Muscles on the floor of the mouth contribute to lifting the larynx and opening the upper esophageal sphincter during swallowing.
Resection of the posterior oral cavity
Patients who undergo resections of the posterior oral cavity may have severe rehabilitation problems, depending on the reconstructive technique used to close the surgical wound after resection. Functional effects of mandibular reconstruction have not been defined well. A patient who has undergone this resection typically has both speech and swallowing problems stemming from the removal of tongue tissue and/or the type of reconstruction used. Resections of the posterior oral cavity usually affect the efficiency of oral aspects of swallowing, including chewing and propelling of food toward the back of the mouth and triggering of the pharyngeal stage of swallowing. They also affect the pharyngeal stage of swallowing.
Patients can return to intelligible speech, full oral intake, and a fairly normal diet after receiving speech and swallowing therapy and placement of an intraoral prosthesis (ie, a device to augment or reshape the palate). The function of this prosthesis is to sufficiently reshape the hard palate to permit interface of the palate with the remaining section of the tongue if the patient has a sufficient degree of remaining tongue mobility.
Pharyngeal and Laryngeal Cancer
Resection of the pharyngeal wall
The patient who has undergone radiotherapy or surgery in the pharyngeal wall generally has posttreatment difficulty in exerting adequate pressure on food to efficiently propel it through the pharynx for swallowing. Notable quantities of food may remain in the pharynx after the swallow, and the patient may aspirate. Postural techniques sometimes compensate for pharyngeal resections, which tend to be on 1 side, whereas radiotherapy has bilateral effects.
Dietary restrictions may be appropriate for some patients because they have difficulty propelling thick foods through the pharynx because pressures required in this situation are greater than those needed for liquids. For patients who have undergone high-dose radiotherapy and who have resultant difficulty in pharyngeal-wall function, the supraglottic swallow assists in the swallowing process by accelerating laryngeal elevation and improving airway closure. In general, these patients have little if any change in their speech patterns.
Laryngeal resection
The patient who undergoes laryngectomy generally has some change in voice quality (eg, hoarseness), as well as difficulty in protecting the airway during swallowing. A number of rehabilitation procedures involving volitional airway protection during swallowing can be taught to these patients, along with exercises to improve ROM of residual structures in the larynx. The patient who has undergone vertical partial laryngectomy or hemilaryngectomy typically returns to oral intake at approximately 10 days to 2 weeks after surgery. The patient who has undergone supraglottic laryngectomy generally takes longer to recover swallowing functions to permit oral intake, usually a month or more, even with good, aggressive swallowing therapy. These patients often have no speech or voice problems.
Total laryngectomy
The patient who has undergone a total laryngectomy obviously has no source of voice production any longer and needs to replace the function of the larynx with an artificial larynx, esophageal speech, or tracheoesophageal puncture (TEP) voice restoration (ie, placement of a surgical prosthetic device). The TEP procedure has come into widespread use because it restores voice production quickly, and the patient does not need to go through the long process of learning esophageal speech. See the images below.
To be a good candidate for TEP, the patient must be willing to maintain a small prosthesis in the puncture site and perform stomal care. TEP involves creating a hole or puncture connecting the superior aspect of the stoma to the esophagus. The patient wears a prosthesis inside the tract to prevent backflow of food into the airway. TEP is a relatively simple surgical procedure, and, after several days, the patient can use the tract for voice production by exhaling and covering the stoma, which redirects the air through the prosthesis in the puncture site and into the pharynx and esophagus. Airflow into the pharynx and esophagus vibrates flaccid tissue, creating the sound of voice. The quality of the voice is generally changed (eg, lower pitch, roughness), but the speech is intelligible.
Unlike other nonindwelling systems, the indwelling voice prosthesis can be inserted immediately, at the time of primary TEP. No temporary stenting of the fistula tract with a feeding tube is done. The advantages of this technique are numerous, provided a device of sufficient length is used. In most patients an 8- or 10-mm device is long enough to compensate for postoperative surgical edema of the tracheoesophageal wall.
The advantages are summarized below:
-
The retrograde insertion technique, performed by using a special trocar and cannula for the TEP and a disposable guidewire (Provox), diminishes the risk of separation of the tracheoesophageal wall.
-
The voice prosthesis somewhat stabilizes the tracheoesophageal wall.
-
The flanges of the voice prosthesis give optimal protection against leakage of saliva and gastric reflux.
-
The prosthesis reduces irritation of the stoma and the fistula tract (possibly because of less migration) compared with a feeding tube taped to the skin around the stomal area.
-
Because the indwelling prosthesis is positioned flush against the posterior tracheal wall, it does not interfere with a cannula or a heat and moisture exchanger (HEM) after surgery.
-
Patients can become familiar with maintenance of the voice prosthesis soon after their operation, with the help of the nurses.
-
It eliminates early postoperative prosthesis fitting, when the stoma is incompletely healed, when it may still be sore, and when the patient's mental and physical status is not yet optimal.
-
At around day 10 after surgery, the patient can immediately focus on voicing itself, which can give him or her a tremendous psychological boost.
-
Postoperative radiotherapy is not contraindicated, and most patients develop a useful voice before this treatment starts.
-
The first replacement usually occurs after months, when wound healing is completed, surgical edema has subsided, and the patient is generally in improved physical and mental condition.
Although sometimes debated, this approach is obviously no challenge to the leading role of the speech therapist on the multidisciplinary rehabilitation team.
The only disadvantages are the presence of a feeding tube in the nose and throat for 10 days and temporary deterioration of the voice during postoperative radiotherapy.
To some extent, potential pulmonary problems can be prevented. This possibility has led to the application of HME during the early postoperative period. The availability of hydrocolloid adhesives allows its use on surgical wounds. The advantages of this method include eliminating the need for noisy external humidifiers (improved patient comfort and cost-effectiveness), minimizing the decrease in breathing resistance, protecting and caring for the stoma, familiarizing the patient with HMEs at an early stage, and easing occlusion of the stoma after voicing can start.
With respect to voice quality, patients using prosthetics and those using esophageal speaking reported an improvement after regular use of HMEs. With the availability of dedicated (valved) devices, compliance with HME use has clearly improved. Moreover, optimizing stomal occlusion by means of an HME has improved voice quality even further owing to a notable increase in the maximum phonation time and dynamic range. One might conclude that pulmonary protection and rehabilitation with HMEs improve the pulmonary status and QOL of patents after laryngectomy and that they have a positive effect on vocal rehabilitation. The reassuring role of the speech therapist is important for this last issue because most patients regain a useful voice (with the original quality in most). At least, these patients can communicate throughout this difficult period.
If patients elect to learn esophageal voice techniques, arrange weekly therapy sessions. The patient learns to voluntarily push or inhale air into the esophagus and release it to create vibration in the pharynx and esophagus. Learning this procedure is time-consuming, and months or years of training may be required to speak well.
Total laryngectomy also changes the swallowing mechanism, requiring the patient to increase effort and pressure to swallow after surgery. However, the patient should be able to eat a full normal diet after total laryngectomy.
Neck cancer
Structures such as the jugular vein, sternocleidomastoid muscle, submandibular gland, and spinal accessory nerve are removed during radial neck dissection. Surgical complications can include problems with the wound and injury to the cranial nerves, including VII, X, and XII. Lymphedema may also occur.
Deficits commonly noted include the following: paralysis of trapezius and lateral displacement of scapula, head-rotation dysfunction due to sternocleidomastoid paralysis, contraction of pectoralis due to radiation and lack of opposition by trapezius, impingement syndrome due to lost scapulothoracic stability and scapulohumeral rhythm, and an inability to abduct fully unless in gravity-eliminated plane.
Common interventions include stretching the pectoralis muscle, stretching the bilateral scalenes, strengthening of the serratus and teres muscles, strengthening the rotator cuff in a gravity-eliminated plane to stabilize the scapula, and avoiding upright abduction and flexion exercises.
Postoperative Effects of Radiotherapy on Patient's Function
In general, postoperative radiotherapy adds to functional complications of treatment for head and neck cancers, frequently prolonging the course of functional rehabilitation and making rehabilitation more difficult than it otherwise would be. Patients with partial laryngectomy who cannot eat when they begin radiotherapy require substantially prolonged recovery periods before returning to oral intake. Patients who have undergone total laryngectomy and begin radiotherapy and who are unable to produce regular esophageal voice of at least 3-4 syllables with a single inhalation often lose their ability to produce esophageal voice for a long time during and after radiation. In addition, if the salivary glands are in the path of the radiotherapy, xerostomia may result, making swallowing difficult.
Many patients cannot continue rehabilitation strategies during postoperative radiation therapy because their tissues become too swollen and irritated. This common adverse effect of radiation slows rehabilitation and often causes patients to lose some function they have regained. Proper support and planning can help the patient and his or her family members adjust to this temporary setback.
Rehabilitation Process
Rehabilitation for patients with head and neck cancer begins with treatment planning in which all the previously cited rehabilitation professionals are represented. At this time, integrate rehabilitation and treatment plans for the patient and provide appropriate counseling. Arrange for each of the rehabilitation professionals to meet with the patient before treatment begins to define patient's goals.
Rehabilitation is not a passive process. The patient must be an active participant. Allow the dentist and/or maxillofacial prosthodontist and the speech or language therapist time to perform a detailed pretreatment assessment. The social worker frequently conducts in-depth psychosocial interviews. Pretreatment assessments become difficult as third-party payment officials authorize shorter and shorter hospital stays for patients undergoing treatment for head and neck cancer.
Patients often enter the hospital the day of surgery. When possible, hold a pretreatment conference at least 1 week in advance of treatment to notify the rehabilitation professionals of the patient's potential needs and to allow them time to schedule appointments with the patient and relevant others. Immediately after surgical treatment, counsel the patient regarding the potential functional effect of treatment.
Continue counseling the patient throughout the course of treatment. When treatment is completed, therapy in all areas can often begin aggressively. Although the best option may be to continue rehabilitation interventions throughout the course of radiation therapy, patients may not feel well enough to participate.
After treatment is completed, rehabilitation professionals can begin a variety of assessment and treatment sessions, providing the patient with needed information to continue rehabilitation on a daily basis at home with a variety of exercises. Compacting of scheduled visits into the same afternoon or day often facilitates patient's active participation in the rehabilitation process.
Rehabilitation professionals must remain actively involved with patients who develop a recurrence of disease or a second or even third primary cancer. In these patients, functional abilities and rehabilitation needs should be reassessed, and the rehabilitation specialists can provide support throughout the patients' second or third treatment regimen. Well-coordinated rehabilitation services are vital for these patients.
Interventions for rehabilitation of speech and swallowing
Interventions aimed at rehabilitation of the speech and swallowing mechanisms typically begin with a radiographic study of the swallowing process to define the nature of the patient's swallow physiology after surgical procedures that may have necessitated anatomic revision or physiologic changes associated with various types of treatments. Often the potential effects of these intervention strategies can be assessed during radiographic study.
Some therapies (eg, postural changes, variety of ROM exercises) can immediately compensate for awareness of food, as can swallowing maneuvers designed to improve selected aspects of the various phases of swallowing. These swallowing maneuvers involve taking voluntary control of selected components of the pharyngeal stage of swallowing, such as closing of the true vocal folds and the entrance of the airway, improving laryngeal elevation and that of the upper sphincter opening into the esophagus, and improving pressure generated on the food bolus. Instruct patients to practice these maneuvers or other exercises 5-10 times per day for 5 minutes to improve muscle function. The patient must occasionally use such voluntary controls during each swallow to enable oral intake.
In the patient with oral cancer, impairments in speech and swallowing are often largely related to reduced ROM due to tumor resection combined with radiation therapy. Compensatory techniques can allow the patient to commence supervised oral intake of food and enhance speech production. Exercise programs can enable the patient to eventually eat without these compensatory techniques. Compensatory strategies in swallowing typically involve changing the position of the head to alter the direction of the flow of food through the mouth and pharynx, sensory stimulation to heighten sensation, surgical procedures, or radiotherapy. ROM exercises often improve the efficiency of both speech and swallowing.
Speech production relies on the ability of the tongue to make complete or near-complete contacts with the palate at various locations. The degree and site of contact or approximation determine the nature of the sound produced. Likewise, during swallowing, the tongue must make complete contact with the hard palate sequentially from front to back to propel the food into the pharynx. The force of gravity alone does not provide an efficient swallow. Therefore, in the patient with reduced range of lip and tongue motion, ROM exercises can improve both speech and swallowing processes.
Instruct patients in these procedures and have them practice independently at home with a clear understanding of when they are successful. The effects of exercise are measurable easily in terms of the degree of motion seen in the tongue or lips. If the surgical resection procedure involves a large amount of tissue, particularly over half the tongue, the ROM exercises alone are not enough to restore sufficient function to provide for speech articulation and the swallowing process. In this situation, request design of a prosthesis to reshape or lower the palate to meet maximal ROM of the tongue.
Throughout speech and swallowing rehabilitation, the social worker or other psychosocial counselor provides the patient with needed psychosocial support. Current data are insufficient to determine the necessary duration of speech and swallowing interventions for each patient type until maximum recovery is attained. Several studies of swallowing recovery with therapy have been completed in patients after partial laryngectomy. Otherwise, no clear data define the average length of time before achievement of maximal speech, swallowing, psychosocial, and other functions. This lack of precise temporal guidelines makes goal setting difficult.
Functional outcomes for patients with head and neck cancer reveal persistent severe pain that may be caused by tumor recurrence, treatment sequelae, or other factors. Pain is often mixed nociceptive and neuropathic. The incidence of residual dysphagia may approach 80%; however, this condition may not restrict use of oral opioids and other analgesics. Other common physical impairments are disfigurement and jaw dysfunction.
Overall (global) QOL in patients surviving head and neck cancer tends to improve over time and may be better than that of healthy controls. Speech and swallowing problems (as the patient assesses them) and pain are probably the most important factors determining the patient's general well-being after 12 and 24 months. However, the relationship is complex, as these items represent only part of 1 of the several domains contributing to the QOL construct. Therefore, their effect on social, sexual, occupational, and family functioning varies according to circumstances and the individual's coping skills.
Patients with eating and speech concerns have the highest levels of dissatisfaction with body image/appearance and greater cognitive and behavioral difficulties as opposed to those without such concerns. In a study of 280 surgically treated patients with oral cavity, midface, and cutaneous cancers of the head and neck, participants with eating and speech concerns showed higher levels of interest in psychosocial interventions to address appearance-related difficulties. This finding suggests there is need for more comprehensive psychosocial care for these patients after completion of functional rehabilitation. [33]
Although pain, dysphagia, and psychological distress are important QOL correlates, predictors of QOL may also exist to help identify patients who are likely to have difficulty late in their recovery. De Graeff et al reported that a high level of depressive symptoms, low performance status, and combined modality treatment were significant predictors of physical and psychological morbidity after treatment. Hammerlid et al found that depression and physical function at diagnosis were independent predictors of global QOL at 3 years. These factors should be actively sought. Some aspects of pain are predictable. For example, if the neck is surgically treated, shoulder pain and discomfort is worse than if no neck dissection were performed. Chaplin and Morton showed that the prevalence of pain and discomfort did not differ with the type of neck dissection performed, though Kuntz and Weymuller et al reported that the degree of discomfort and pain was increased after radical neck dissection.
Cancer of the Musculoskeletal System and Its Rehabilitation
Musculoskeletal Cancers: Overview
Musculoskeletal tumors are rare, accounting for less than 0.2% of all cancers, with approximately 2400 primary bone sarcomas reported each year in the United States. The most common primary tumor is the sarcoma. By contrast, carcinomas or hematologic malignancies metastatic to bone are more common than primary bone cancer. Approximately 350,000 people die with bone metastasis each year in the United States. [34, 35]
The behavior of sarcomas differs from that of carcinomas and hematologic malignancies. Sarcomas may occur in osseous and nonosseous musculoskeletal tissues. Approximately two thirds of extremity tumors are soft tissue sarcomas. These types of tumors are most prevalent in the second and third decades of life. They occur most commonly in the lower extremities, followed by the head, neck, and/or trunk; they least often occur in the upper extremity. The survival rate is presently 70-80%.
Osteogenic sarcoma is a cancer in which the neoplastic cells directly produce osteoid. It is the most common primary tumor of bone, and it is characterized by complex genetic changes, including loss and amplification of chromosomal regions. Mutation of p53, a gene important for cell-cycle regulation, is commonly associated with osteogenic sarcoma and correlated with high levels of genetic instability.
Treatment options
Chemotherapeutic regimens vary but usually consist of doxorubicin, cisplatin, and high-dose methotrexate with or without other chemotherapeutic agents.
Sarcomas are managed with amputation or limb-sparing surgical procedures. Criteria for wide local excision or limb sparing procedures are that the tumor must be suitable for complete resection without sacrifice of major vessels and nerves, or reconstruction using bones grafts or an endoprosthesis must provide limb function equal or superior to the function of a prosthesis.
A limb-sparing procedure is more complex than an amputation. The duration of surgery is longer, infection and pain may be more common, and physical rehabilitation may be more intense with limb-sparing procedure than with amputation. The presumed psychological advantage of limb-sparing procedures versus amputation has yielded conflicting results. When the outcomes of soft tissue sarcoma treated with amputation and those treated with limb-conserving approach are similar, functional or QOL issues may determine ultimate treatment decisions. The location of soft tissue tumor in an extremity does not appear to be a significant variable in QOL or functional outcome. QOL domains vary in the limited published data that are available.
Studies of patients with musculoskeletal cancers
In a study by Davis et al, the Toronto Extremity Salvage score, return to normal living, and scores on the short form 36 (SF-36) were used to compare reports of disability and handicap between patients who had undergone amputations and patients who had chosen limb-preserving procedures with underlying diagnosis of sarcoma of the lower extremity. [36] Disability issues, rather than issues of handicap, were most common in the group who had undergone amputation. On rare occasions, patients adamantly refuse amputation, despite all advice, on the basis of psychological, social, or cosmetic reasons and undergo a limb-sparing procedure instead.
Davis et al also observed a trend toward increased disability among those in the amputation group versus those in the limb-sparing group, with the amputation group having significantly increased levels of handicap. These data suggest that the differences in disability between amputation and limb-sparing patients are smaller than anticipated. The differences may be most notable in measuring handicap.
In a later study, Zahlten-Hinguranage et al investigated QOL and function in 124 patients with lower-extremity sarcoma who underwent amputation or limb-salvage surgery to assess potential differences in subjective treatment outcomes. [37] The results reflect similar QOLs in both treatment groups. However, in contrast to patients with limb-salvage surgery, who described QOL in terms of a high physical performance status in sports and recreational activities, QOL in patients receiving amputation was strongly associated with their social acceptability. High QOL in this group brings into question physicians' expectations regarding the application of time-consuming advanced technical skills for limb-salvage surgery.
Counseling the patient and family
It is imperative to discuss the effect of therapies with the patient as part of the decision-making process. Wound and limb problems are often predictable and preventable and may be reversible when a physical rehabilitation program is included in the patient's care plan. The extent of surgery and the size of the tumor at presentation contribute to additional tissue injury in high-grade sarcoma. The larger the tumor, the greater the volume of radiation needed for treatment. Patients treated with radiation tend to have decreased joint motion, increased edema, and less muscle strength than patients not receiving radiation.
Studies show that chemotherapy does not impose much physical disability on the individual who has chosen limb-sparing surgery, but it does accelerate skin changes from radiation therapy. If the patient is a young child, be aware of leg-length discrepancies. Epiphyseal plates, particularly in the tibia, may be dramatically disturbed when irradiated. If radiation is given at the ankle, differences in shoe sizes may be noted. Scoliosis may also be a secondary effect of unequal leg lengths.
If radiation therapy is to follow wide excision of a sarcoma, involve the rehabilitation staff before surgery. The extent of tumoral invasion, rather than functional considerations, must dictate the extent of muscle-group excision or the excision of individual muscle bundles. However, presurgical discussion of the planned surgical approach gives the rehabilitation specialist some idea of the extent of resection to help advise the patient regarding postoperative function. Throughout radiation therapy, the goal of the rehabilitation staff is to preserve ROM, control lymphedema, and reduce pain. If muscular excision is extensive, strengthening exercises are required before a reasonable functional result can be achieved. Appropriate orthotic devices may be necessary if major nerves were sacrificed.
Even long after radiation therapy is completed, the rehabilitation staff must continue to follow up the patient and repeatedly and periodically assess the patient's functional capacity. Months after the completion of therapy, especially if healing is imperfect, contractures may progress to the point that function is lost. The most important feature to ensure optimal function after wide excision and high-dose radiation therapy is continuous PT during radiation therapy and frequent follow-up visits in the first 18 months after treatment.
Delicate areas, such as hands and feet, especially the plantar surface, are still considered risk areas for limb salvage because of difficulty in applying adjunct radiation therapy to such thin, uneven surfaces. With sophisticated equipment and trained personnel, radiation has been applied successfully to these areas. The following section describes the physical rehabilitation process for patients who received radiation therapy with limb-sparing surgery and several procedures that do not require this adjunct treatment.
Late sequelae of radiation therapy to the extremity
See the list below:
-
Fractures: Radiation affects the integrity of bone, making it osteoporotic and fragile. Caution the patient about risky situations, such as participating in contact sports. Encourage the patient to swim, golf, play tennis, or work on construction. Fracture from cellular and vascular necrosis can substantially delay healing of the bone.
-
Edema: Advise the patient to elevate the leg when possible and to use intermittent compression machines if swelling is severe. Elastic support garments may also help minimize edema.
-
Pain: Both pharmacotherapy and judicious use of physical agents may help in pain management.
-
Wound healing: Problems can result in open wounds that are prone to infection for as long as 2 years. Advise the patient to keep the area clean and protect it with light dressings. Fistulae may occur and carry infection to deep structures. Skin care for the affected extremity should continue for at least 2 years. Recommend light applications of moisturizers (eg, commercial preparations of vitamin E cream, aloe, baby cream). These preparations may be used during radiation therapy only with permission of the radiologist. Scented products have high volumes of alcohol, which dries the skin and defeats the purpose of using a lubricant. Deep massage during application can delay or prevent contracture of the connective tissue in the extremity.
-
Deformity: Deformities or defects can be remedied by external filling of the areas with heat-moldable thermoplastic materials to provide an intact, positive body image.
-
Muscle fibrosis: Sometimes muscle fibrosis is unavoidable and results in contractures that occur long after supervised PT ends. Chronic changes occur in supportive tissues and may be irreversible. These changes can involve fibrosis, bone necrosis, endarteritis, decreased elasticity, and obstruction of lymphatic channels, all of which can cause severe edema, pain, and decreased function.
Cancer of the Upper Extremity
Wide excisions and limb salvage of the upper extremities have not caused great problems in the rehabilitation process, especially in terms of pain, edema, or limitation of strength or motion. Instruct the patient how to gain maximum use of the extremity. Individuals who have had nerve resections and limb-preserving procedures need the most input.
Tumors in the upper extremity are generally smaller than those in the lower extremity, allowing for relatively conservative or limited surgery. Whole muscle groups are removed only rarely. If any muscles or nerves are resected close to the wrist or hand, the hand may become insensate. In this case, the procedure of choice is amputation.
After surgery, morbidity in the upper extremity is less than that in the lower extremity. Suction drainage is shorter, wound infection is less frequent, and radiation is better tolerated most of the time. The anatomic location of the tumor and volume to be treated determine the reaction.
The interdisciplinary services under the direction of PM&R specialists have much to offer patients undergoing a Tikhoff-Linberg procedure. These patients are likely to retain hand function and some elbow function, but they lose shoulder function. Outcomes in this area are clearly superior to those in forequarter or shoulder disarticulation. Furthermore, the Tikhoff-Linberg procedure is minimally disfiguring and associated with only mild to moderate pain and edema. The patient's acceptance of the procedure and its outcome is generally good.
The rehabilitation process begins with a patient orientation program. The patient often views pictures of other patients who have undergone the same procedure that show what they can do postoperatively and what limitations in function are likely. Next, a shoulder mold is fashioned by using the involved shoulder, provided that its contours are not distorted. Heat-moldable material is used. The cosmetic shoulder helps preserve the symmetry and appearance of the shoulder contour and can support a bra strap or heavy overcoat. This cosmesis is the same as that provided after forequarter amputation. In patients in whom the deformity after surgery is minimal, a commercially available shoulder pad may suffice. Use of these devices is optional.
Clothing options for women include blouses with asymmetric, or off-center, closures and decorative scarves to mask the body contour. On the first postoperative day, an arm sling is provided for support and to restrict abduction. Maintain motion restriction until the incision is healed (usually about 2 wk). Control edema, when present, with an elasticized glove or elastic stockinette. At the same time, recommend active maximal hand movement to preserve strength and ROM and to help mobilize edema by means of the pumping action of muscles.
Teaching the patient to be aware of proper head and neck positioning and cervical ROM is initiated during the first postoperative days (or when patient first becomes ambulatory). When permission is given to begin motion, usually at 2 weeks after surgery, recommend active and active-assistive elbow motion within the confines of the sling. At about 3 weeks, remove the sling for passive shoulder ROM and wrist pronation and supination. Discontinue use of the sling after the suture line is healed, but recommend its use for upright activities in which arm support increases comfort.
Joint immobilization for less than 2 weeks results in capsular adhesions that are overcome easily. Immobilization longer than this often results in fixed contracture; advise the patient to avoid immobilization. After the arm is out of the sling, recommend performing full elbow and wrist ROM (eg, flexion, extension, pronation, supination) for several minutes daily.
Advise the patient to perform passive shoulder ROM (eg, flexion, abduction, external and internal rotation) and pendulum exercises for several minutes daily with the help of a family member or healthcare professional. Recommend use of bathroom equipment (eg, grab bars, tub seats) to enhance safety for these patients. Encourage the patient to resume his or her normal daily activities. Advise the patient not to lift more than 20 lb with the arm that has undergone the Tikhoff-Linberg procedure. Modified tennis and even rowing activities can be performed after rehabilitation. Pain and shoulder or arm dysfunctions are not clinically significant management problems. Pain is often controlled with modest analgesia.
Partial or total scapulectomy is performed when tumors involve the scapula or surrounding soft tissue. Removal of all or part of the scapula, including the glenoid fossa, may be necessary. If the glenoid complex is left intact, function of the upper extremity may be close to normal. Removal of the glenoid creates restrictions of arm movement, often actively beyond 90°. Pain and complaints of fatigue at the end of the day are not uncommon. A sling for temporary support may be adequate because dependency increases the risk of edema.
The deltoid muscle mass forms the roundness of the shoulder and moves the upper extremity at the glenohumeral joint. After partial resection, the arm is usually held in a sling until drainage subsides. Do not initiate active ROM at the shoulder until sutures are removed, though external rotation can be started with the arm held at the side. Patient can perform full elbow motions. At the time staples or sutures are removed, have the patient perform active and resistive exercises. No chronic residual problems have been observed in patients with partial deltoid resections.
Vital structures adjacent to soft tissue sarcomas of the axilla often are difficult to define. Proximity to the brachial plexus may be impossible to discern unless the patient has neurologic signs. Proximity of the tumor to the humerus is difficult to identify despite use of sophisticated scanning. The adjacent musculature, such as the long head of the triceps from its origin or the latissimus dorsi as it approaches the axilla posteriorly, may need to be sacrificed. In general, if deep structures are involved, surgery to remove the sarcoma cannot be performed except by means of forequarter amputation or Tikhoff-Linberg procedure.
After excision of a sarcoma in the axilla, keep the arm in a sling until drainage subsides, possibly for more than 2 weeks because this area is intimately associated with major lymphatic channels. If radiation is prescribed, position the patient's shoulder at least 100° abduction/flexion and 75° external rotation, probably one of the most difficult postures to assume without discomfort after surgery. Suggest use of electrical stimulation to decrease pectoral muscle spasm, a great inhibitor to full shoulder ROM and other modalities. Have the patient assume this position when radiation is being given to minimize radiation exposure to the breast and upper arm. PT treatment may be needed twice a day. After the shoulder can be moved about 90°, no problems are generally encountered until the area becomes sensitive to radiation.
Skin breakdown is not uncommon and delays delivery of radiation treatment. Suggest that the patient wear T-shirts made of 100% cotton for absorbency. No deodorants or body creams are allowed unless recommended by the radiation therapist. Recovery of arm motion becomes easier the second and third time radiation therapy is resumed, but, throughout the course of treatment, the program must be repeated. Chronic lymphedema is common. An elastic stockinette or a customized sleeve may be adequate to control swelling. If lymphedema is severe, use of an intermittent compression machine is recommended.
If the brachioradialis muscle must be excised, the elbow should be protected in a splint until closed suction drainage slows and healing is underway. After this occurs, proceed with active ROM to the elbow as tolerated. If radiation must be applied to the antecubital fossa, the tendons of the biceps and brachialis muscles may become fixed. The brachial artery and the median nerve may become enclosed in scar tissue. Damage to any or all of these structures can cause secondary problems, such as an insensate nonfunctioning hand, at the worst, or a weak elbow. With soft tissue sarcoma adjacent to the head of the radius and radial nerve, the elbow is vulnerable over the surgical area. Consider having a protective device made from thermoplastic material, or provide a commercial elbow protector. In rehabilitation, emphasis is on maintaining a functional position during elbow and finger ROM.
Fabricate a dynamic splint with wrist and fingers stabilized in functional position so that finger flexors and interossei can function well in grasping. After the patient completely recovers from surgery and radiation, attempt tendon transfers by using the flexor carpi radialis and thumb stabilizers.
Cancer of the Trunk
Retroperitoneal tumors are difficult to excise and often recur because of problems in attaining negative surgical margins. PT is usually requested in conjunction with adjunct radiation therapy. The femoral nerve is often in the radiation field, resulting in the need to protect and support the quadriceps muscles. Edema is a secondary complication if the inguinal nodes are in the field. Recommend use of support stockings along with elevation of the lower extremities throughout the day.
Buttockectomy is performed with en bloc resection of the gluteus maximus muscle. The surgeon must be careful not to damage the sciatic nerve during surgery. Closure of the incision may be tenuous if large amounts of skin are removed. The patient may complain of difficulty climbing stairs, pain along the incision, and an altered body image. Radiation that includes the buttock disrupts normal sexual functioning and bowel habits.
The physical therapist should encourage strengthening of other hip girdle muscles and provide seat cushions. A custom buttock cosmesis may be fabricated from thermoplastic material to resemble the contralateral buttock. Buttock cosmesis is secured to the undergarments with Velcro. Seat cushions or wedges may be needed for the patient to sit comfortably and provide symmetric weight bearing on the buttocks.
Internal hemipelvectomy may be indicated with a diagnosis of soft tissue sarcoma in the upper thigh and/or buttock or a low-grade sarcoma of the pelvic bones. The sacrum is transected through the neural foramina with resection of the hemipelvis, proximal femur, and, occasionally, bladder, rectum, or genitalia. In cases of an intrapelvic tumor, entering the peritoneal cavity is inevitable in surgery. Stabilization of the pelvis and femur requires prolonged bed rest with skeletal traction to allow for fusion and maintenance of as much leg length as possible. In regard to internal and external hemipelvectomies in postoperative cancer patients, a study by Guo et al determined that about the same percentage of patients were admitted to inpatient rehabilitation but that patients who underwent external hemipelvectomy required longer hospital stays, needed more pain medication, and had more ambulatory issues. [38]
Recommend the use of shoe lifts as soon as bed restrictions are discontinued, usually 3-6 weeks after surgery. Partial weight bearing is allowed on crutches until the remaining pelvis or ilium forms a fibrous union with the femur, which may take as long as 6 months. Emphasize the importance of strengthening the distal muscles and upper extremities with repetitive active exercise against gravity. Sensation generally remains intact, and few patients complain of pain. Variations of this procedure are common, and the therapist and surgeon and/or physiatrist should maintain a close relationship to monitor the patient's progress. At 6 months, the patient can walk on all surfaces with use of only a cane and/or shoe lift to equalize pelvic height if they have leg-length discrepancy.
If the sciatic nerve is sacrificed, motor loss is inevitable. Patients may also have leg anesthesia and a tendency for the skin to become ulcerated with trauma. Recommend use of an ankle-foot orthosis (AFO) to assist with foot clearance. After initial treatments, suggest ankle fusion or a posterior tibialis transfer procedure. Educate patients on proper foot care, choice of shoes, and orthotic application.
Cancer of the Lower Extremity
The thigh is one of the most difficult anatomic areas in which to attain local tumor control without clinically significant morbidity. It has historically been the area most likely to develop soft tissue sarcoma. Tumors discovered in the lower extremity are generally large because they have been masked by bulky muscle tissue. Morbidity involved in irradiating the upper medial thigh and groin potentially is severe. Because of radiation scatter, sexual dysfunction is probable. Chronic lymphedema after irradiation of the lymph-node complex in the groin is frequently observed. Dysfunction and pain of the hip joint are not usual symptoms, but they may be late findings.
For most wide local excisions of the thigh, serosanguineous drainage is prolonged. When drainage is decreased or suction tubing is removed, start ambulation and active exercises in earnest. Suggest use of commercial immobilizers to protect the lower extremity from poor positioning and also to prevent wounds from being inadvertently overstretched, particularly when incisions cross a joint.
A large, soft tissue sarcoma in the anterior thigh group may require excision from the origin to the insertion of the whole quadriceps muscle. This procedure is reserved for high-grade tumors. Low-tumor grades may be excised adequately by removing some portion of the quadriceps.
Radiation treatment is usually not required with formal excision of a muscle group because radiotherapy includes 2 joint spaces and is associated with the highest risk of morbidity. If the patellar tendon were irradiated with 60 Gy rads or more, tendon breakdown would occur over time.
At approximately 2 weeks after surgery, a dual-channel metallic AFO is provided to block dorsiflexion and allow only 5° of plantar flexion. Encourage use of a cane when the patient walks on precarious terrain. The patient should expect to continue using the AFO. The knee can be extended in a brace by locking it in hyperextension and by increasing the lordotic curve. Patients who discard the brace may fall, fracturing the patella, femur, and/or shoulder. Some patients are uncomfortable with the cosmetic appearance of the thigh. To enhance their body image, recommend use of an orthosis or cosmesis fabricated of Pelite to simulate contours of the sound extremity and allow for wearing of contemporary fashions. The orthosis can be suspended by using an elastic wrap (ACE bandage), or it can be held in place with pantyhose.
For patients with soft tissue sarcoma of the medial thigh, excision of an adductor muscle group is required. This procedure is usually followed by irradiation and chemotherapy. After this procedure, prolonged drainage through suction catheters is a common complication. The patient may require bed rest for long periods, sometimes longer than 2 weeks, with the obvious sequelae. The lymph nodes are not removed as in a groin dissection; the medial aspect of the thigh contains major lymphatic channels that are sacrificed with the specimen. Initially keep all motion of the extremity to a minimum. Loosely wrap the area with elastic bandages to help protect the incision. Isometric contractions of the quadriceps seem to increase drainage when performed as part of an exercise program after surgery to this area and should not be recommended.
When allowed, weight bearing is as tolerated, but a cane may be necessary for balance. Custom-measured elastic stockings or commercial support stockings should be applied for the performance of all upright activities. Complaints of motor dysfunction are rare, but edema and pain are common. Educate patients about the importance of leg elevation and avoidance of prolonged sitting. Review techniques of basic skin care, including caution when shaving the legs.
When a tumor is removed from the posterior portion of the thigh, tight wound closure may compromise skin in the area of the popliteal fossa. If adjunct radiation and chemotherapy are required, the incision may open and remain a problem for the first year after treatment, despite active participation in PT. PT is usually interrupted and resumed sporadically as complete wound healing progresses. The patient remains in bed, frequently in a knee immobilizer, until drainage subsides. Teach the patient quadriceps isometrics and ankle ROM exercises. When the incision appears to be healing well, start ROM of the hip and the knee. Initiation of knee flexion may be difficult, but this motion can be accomplished in the side-lying position. Patients have few physical complaints except for stiffness after prolonged sitting and unsteadiness when running.
Chronic problems that may occur long after medical treatments have been completed are knee flexion and ankle plantarflexion contractures. Institute programs of whirlpool treatments and debridement for slow-healing wounds, serial casting for contractures, and review of stretching exercise techniques. A woman should be discouraged from wearing shoes with excessively high heels. Lateral thigh excisions frequently leave the individual with notable cosmetic and physical deficit, though it is not so limiting as to prevent normal work or social activities.
Bony tumors involving the proximal tibia or distal femur result in limb-preserving procedures with use of the kinematic rotating hinged knee joint or distal femoral replacement. The incision is long and lateral to the patella. Removal of the distal femur or proximal tibia, along with the joint capsule ligaments and muscle, is necessary. The endoprosthesis maintains skeletal continuity and near-normal function of the knee. Lack of knee stability is inherent. Problems associated with the use of this knee joint in growing children are resolved by using an expanding or telescoping device. See the image below.
A bulky dressing and knee immobilizer are applied in the operating room. Because methyl methacrylate is used to hold the endoprosthesis in place, the dressing is only to control swelling and comfort. PT can be started as early as day 1 for quadriceps sets, especially for patients with only femoral replacement. Patients with proximal tibial prosthesis are restricted only from vigorous quadriceps function and knee flexion to protect the attachment of the patellar tendon. Recommend that these patients start gentle active flexion and extension strengthening exercises 4 weeks after surgery. Some patients with a kinematic rotating hinged knee joint may immediately use continuous passive motion (CPM) machines. However, this alternative has not been found to be more beneficial than an active program.
Potential exists for many complications, such as wound infections, edema, and temporary peroneal nerve palsies from over stretching during surgery. Full active ROM is expected, as well as full weight bearing. The rehabilitation process begins with quadriceps isometrics and progresses until the patient can ambulate with use of a cane.
Leg muscles are compartmentalized but not so definitively as the muscles in the thigh. Anterior, posterior, and small lateral compartments exist; the interosseus membrane between the tibia and fibula separates the anterior and posterior regions. The anterior compartment is actually more anterolateral and contains muscles that act as dorsiflexors of the foot.
If surgical excision is necessary, place the patient in a posterior leg and/or ankle splint early after surgery to prevent contracture of the heel cord and overstretching the incision. Place the splint over the surgical dressing for additional protection to the wound and to keep in place while the patient is in bed. Request that the patient use the splint for extended periods, even after discharge. For ambulation, the patient can use a metal double-upright AFO with dorsiflexion assist or a solid plastic AFO if sensation is intact and there is minimal edema. Using knee-high support stockings and wearing low-heeled shoes or high-quartered sneakers is also recommended.
Educate the patient about proper foot care. The peroneal nerve and the peroneus longus muscle that evert the foot are frequently sacrificed, either partially or completely. PT intervention usually consists of stretching of the heel cord and maintenance of ROM with fitting of a custom-made ankle stabilizer, air splint, or metallic AFO.
The gastrocnemius muscle spans 2 joints and joins with the soleus to form the Achilles tendon, which inserts on the calcaneus. These muscles flex the knee or plantarflex the ankle. The gastroc-soleus complex comprises the posterior compartment of the leg. If irradiation is necessary after surgery in the posterior compartment, the knee joint should be spared. Radiation fields are generally directed laterally to spare the skin behind the area. The skin is at high risk of breakdown during treatment.
Secure the lower extremity in a long-leg immobilizer with a posterior splint on the foot. Apply the immobilizer in the operating room to facilitate ease in transferring the patient without disturbing the wound or suction catheters. After 2 weeks, the physical therapist may remove the splints and start gentle ROM to the knee. Partial weight bearing also can be initiated with the knee splint in place. The contralateral shoe may have to be raised temporarily to allow for clearance during swing-through phase of gait.
As healing is ensured, encourage increased weight bearing. The shoe on the involved foot may have to be modified to include a rocker bottom to enable the patient to push-off with greater ease during ambulation. Add an AFO to maintain a neutral ankle if indicated. Recommend daily stretching of the heel cord. If the posterior tibial nerve is excised, sensation is interrupted along the lateral sole of the foot. Other problems do not seem to occur. If radiation causes small fractures at the calcaneus, an ankle stabilizer (eg, hindfoot orthosis) is sufficient to hold the foot in a neutral position.
Musculoskeletal Impairments in Cancer Syndromes and Their Rehabilitation
Approach Considerations
When soft tissue tumors cannot be excised easily and when complications from adjunct radiation are apt to render the foot nonfunctional, excision of 1 or several rays may result in satisfactory foot function. An orthotic device must be fabricated to act as a shoe filler and ankle stabilizer. Modifications may be necessary to accommodate the external sole of the shoe, such as a rocker bottom to enhance push-off during gait or a lateral flare can be added to the outer heel to increase stability at heel strike. If these changes are not made, problems can occur (eg, recurring ankle sprains and/or strains with internal bleeding, metatarsal bone displacement, painful limited ambulation).
Concurrently with the patient's recovery from surgery, initiate an exercise program for strengthening ankle musculature and stretching of the heel.
Many patients with soft tissue sarcomas are ideal candidates for PT. Functional limitations may be a direct result of disease or a result of treatment. Failure to identify these problems in the past has been a barrier to optimal rehabilitation. The obligation of any rehabilitation team is to allow patients to achieve their maximum physical, psychological, social, vocational, and educational potentials.
Anatomy and Pathophysiology
Sites of involvement
Of the estimated 570,280 people who will die of cancer in 2005, almost all will have metastasis. However, certain cancers are more likely than others to spread to bone. [39] These are cancers of the breast, prostate, kidney, thyroid, and lung. In persons with breast or prostate cancer, bone is most often the first site of metastasis.
The upper extremity is the part of the skeleton least commonly involved in metastatic bone disease. The literature suggests a rate of 10-15% for involvement of the upper extremities. The axial skeleton and lower extremities, in particular the hip region, are affected most frequently. Sites of primary tumors associated with bony metastases are breast (73.1 %), lung (32.5%), kidney (24%), rectum (13%), pancreas (13%), stomach (10.9%), colon (9.3%), and ovary (9%). Other tumors often associated with skeletal metastasis are carcinoma of the prostate and multiple myeloma. The vertebral column is involved in 69% of cases, the pelvis in 41%, and the hip region in 25%. Pathologic fractures that require surgical instrumentation occur in 9% of patients who have metastatic bone disease. The femur, pelvis, and humerus are commonly affected in this way.
The most frequently affected parts of the skeleton are the vertebral column, hip, femur, and humerus. Patterns of bone destruction are recognized and have been described as geographic, moth-eaten, and permeative. Geographic destruction consists of large, well-defined lytic areas greater than 1 cm in diameter with a distinct sclerotic rim. Moth-eaten destruction contains small (2- to 5-mm) lytic areas with ill-defined margins. Permeative lesions associated with bone destruction are characterized by multiple, small (1-mm) areas, principally in cortical bone. Geographic destruction is associated with slow growing tumors, moth-eaten with moderately aggressive lesions, and permeative with highly aggressive tumors. Apart from these lytic lesions, osteoblastic and mixed-type metastases should be recognized radiographically.
Tumoral genesis and progression
Metastasis to bone requires both progressive displacement of marrow elements and resorption of bone to allow local progression of the tumor. Bone resorption and intraosseous tumor growth lead to bone pain, which is possibly due to necrosis, inflammation, and elevation of intraosseous pressure. In addition, loss of mechanical strength because of structural damage leads to pathologic fractures, a common problem with metastatic carcinomas. Extensive bone resorption, or osteolysis, by metastatic tumors can lead to systemic hypercalcemia, an additional cause of morbidity and mortality. See the images below.
Metastatic bone deposits initially tend to displace marrow elements in a preferential manner, taking the path of least resistance. For this reason, radiographs frequently appear normal, even with extensive metastatic involvement of bone. Radiographic evidence of bone lysis can require up to 50% loss of mass of the trabecular bone to become readily apparent. Radionuclide bone scans, which depict subtle bony reaction to advancing lesion, are more sensitive method than radiographs for detecting most tumors. This phenomenon is partly from local coupling of bone resorption and formation that occurs between osteoblasts (ie, bone-forming cells) and osteoclasts (ie, bone-resorbing cells).
Manifestations of blood-borne metastasis in bone represent the outcome of a series of interactions between tumor and host. Metastasis involves an intricate and complex sequence of events and is fundamental to the definition of malignancy. An extremely diverse spectrum of neoplastic diseases shares this feature of metastatic capability. The long-standing theory of seed and soil holds that metastasis results both from biologic properties of the malignant cell and conducive host tissue factors. An increasing body of data supports this hypothesis.
Metastasis involves a series of cellular properties that results in specific events, including the following:
-
Cell motility
-
Expression of matrix metalloproteinases (MMPs), which confers ability to degrade extracellular matrix components
-
Ability to cross basement membranes, gain access to vascular or lymphatic circulation, and egress into a remote organ site related to motility and MMP expression
-
Endothelial adhesion mechanisms that facilitate distant vascular or lymphatic escape
-
Chemotaxis conferring target organ selectivity
-
Selective cell adhesion to specific extracellular matrices or cellular components through cell surface receptors such as integrins
-
Ability to induce angiogenesis to support metastatic tumor growth
-
Local invasiveness related to MMPs and other proteases and, possibly, to cytotoxic effects on host tissue
-
Continued uncontrolled growth driven by a variety of molecular mechanisms, including locally secreted host and tumor cytokines
Bone resorption around metastatic cancer foci is predominantly mediated by osteoclasts. Osteoclast differentiation and activation are regulated at the local level by the relative expression of receptor activator of nuclear factor-B ligand (RANKL) and osteoprotegerin (OPG). RANKL and OPG are mainly produced by cells of the osteoblastic lineage. RANKL directly acts on osteoclast precursors and mature osteoclasts through its receptor RANK to increase osteoclast differentiation and activation. RANKL expression is increased around tumors. OPG is a decoy receptor for RANKL. The relative expression of RANKL and OPG osteoblasts and/or stromal cells is regulated by, and mediates, the proresorptive effects of hormones (eg, PTH and 1,25(OH)2 vitamin D3), inflammatory cytokines, and cancer-produced factors (eg, parathyroid hormone-related peptide [PTHrP]).
The complex array of requisite steps for occurrence of this pathologic process suggests multiple potential points of therapeutic antimetastatic intervention. The importance of the appropriate soil is also increasingly evident. Certain cancers have long been known to have a predilection for particular organ distributions of metastasis, such as the tendency to metastasize to bone mentioned above for breast and prostatic cancers. Indeed, different tumors tend to metastasize to specific bony sites, and metastases to distal long bones or small bones of the extremities are extremely rare with most carcinomas. When such acral metastases occur, they are typically associated with lung carcinomas, an observation that suggests some specificity in the metastatic process. Traditional explanations attribute this finding to anatomic factors (eg, vascular and lymphatic distribution), but evidence now indicates that local tissue factors may be a strong determinant.
The osteoblastic component is not neoplastic, but it should be interpreted as a reaction of normal bone to metastatic cancer. Primary tumors of the prostate and GI tract may account for a blastic response. Lytic lesions are frequently observed in metastases of kidneys, melanoma, and breast and lung tumors. Mixed-type lesions are found in metastases secondary to primary tumors of the breast, GI tract, and reproductive system. Although patients with blastic lesions may have serious problems with bone pain, they tend not to have fractures because of the sclerotic nature of the reactive bone within and around metastatic lesions.
Two theories have been proposed to account for the mechanism of progression of lytic lesions in bone:
-
Direct osteolysis of bone by tumor cells
-
Stimulation of host osteoclastic bone resorption by tumor cells
Evidence suggests, however, that both mechanisms may be at work.
Clinical Evaluation
Imaging
Imaging techniques presently available for detection and monitoring of skeletal metastases include conventional radiography, scintigraphy, CT scan, MRI and fluoride ion (F-18) with positron emission tomography (PET) as well as quantitative bone single-photon emission computed tomography (SPECT). Radiographic skeletal survey now is largely obsolete as a screening method for metastases in patients with malignant disease. Bone scintigraphy is the method of choice in most cases, with the exception of patients with multiple myeloma, in whom bone scintigraphy often produces false-negative results. Conventional radiographs demonstrate a high degree of accuracy in differentiating metastatic bone lesions from primary bone tumors.
Bone scintigraphy is an excellent method for the early detection of skeletal metastases, especially when bone lesions remain radiologically occult. Today, technetium-99m–labeled polyphosphonates are preferred for bone scans. Bone scans can depict metastatic lesions 2-18 months earlier than conventional radiographs. Multiple myeloma, leukemia, and lymphoma present the most difficulty for the clinician in terms of diagnosis.
Factors complicating the interpretation of bone scans are trauma, infection, and miscellaneous factors, such as preexistent disease (eg, osteoporosis, rheumatoid arthritis). Finding of a lesion on scintigraphy suggests the need for additional evaluation (eg, CT, biopsy). Discrimination of attenuation on the CT scan is superior to opacity discrimination on conventional radiographs. Changes in the soft tissue are demonstrated well and have clinical implications in defining the extent and operability of tumors. CT may also help in determining appropriate fields for radiation therapy of metastatic lesions.
In the past, angiography was performed to assess both the vascularity and the soft tissue extension of tumors. Enhanced CT had largely replaced angiography in this respect. However, angiography and embolization still are important in the preoperative assessment and treatment of vascular tumors.
MRI provides soft tissue contrast superior to that of CT. Images can be obtained in the axial, coronal, and sagittal planes to clearly demonstrate the extent of the lesion, especially the extent of bone-marrow involvement. Evidence suggests that MRI is sensitive enough to depict the extent of disease more rapidly than isotopic studies can. However, remember that MRI results must be correlated with those of other studies.
Uptake of fluoride ion (18 F) from18 F-fluorodeoxyglucose into bone is 2-fold higher than uptake of technetium polyphosphonates, and blood clearance of fluoride ion is faster than blood clearance of technetium polyphosphonates. Therefore, the bone-to-background ratio of18 F is increased. At present, gamma-camera systems respond better in terms of sensitivity and resolution to the 140-keV photons of technetium than to the 511-keV photons of18 F.
99m Tc bone scanning has been shown to be superior to planar18 F bone scanning. However, with the development of positron emission tomography (PET), high-quality whole-body surveys have become possible with18 F PET. Because of the superior pharmacologic properties of18 F and the high-resolution sensitivity and high lesion contrast of PET without superposition of soft tissue, detection of both osteolytic and osteoblastic metastases in patients with solid cancers may be improved with18 F PET bone imaging. Its role in the diagnostic therapeutic and prognostic value in metastatic bone disease remains to be determined.
History, physical examination, and laboratory studies
Clinical history includes a thorough review of symptoms and profound examination of the patient. Bone metastasis is often associated with pain. Discomfort is often worst at night. In extensive bone disease, multiple and migratory areas of pain are recorded. Diagnostic pitfalls include pain in metastatic disease of the spinal column, which is treated as lumbar-disk disease, and discomfort around the knee from metastasis in the region of the hip.
Important laboratory studies include analyses of blood, enzymes, proteins, and minerals. Perform serum protein immune electrophoresis routinely to exclude multiple myeloma. Hypercalcemia may be found in patients with bony metastases. Other markers are serum alkaline and acid phosphatase, which is elevated in patients who exhibit large lytic lesions and serum prostate-specific acid phosphatase associated with cancer of the prostate. Carcinoembryonic antigen (CEA) is another indicative test, especially in GI tumors.
The question then remains whether bone markers are helpful in the early diagnosis of bone metastases. An older longitudinal study claimed that in 70% of the patients assessed, serial measurements of bone-specific alkaline phosphatase (sBALP) levels correctly identified patients with bone metastases, and that the biochemical diagnosis was made an average 7 months earlier than the assessment based on clinical, radiologic, and isotopic techniques. However, subsequent studies showed that measurements of bone markers are useless to detect bone metastases at a preclinical stage.
Most markers of bone remodeling and particularly those of bone resorption are elevated in patients with established bone metastases. Although these observations strongly suggest that bone markers may have a potential as diagnostic tools in cancer patients, the currently available data do not allow final conclusions regarding the accuracy and validity of any of the presently used markers in the (early) diagnosis of bone metastases. The same applies to the prognostic value of abnormal marker results in patients with malignant tumors.
Surgery
Preoperative staging and diagnosis
Preoperative staging studies also include conventional radiography, bone scintigraphy, CT scanning, and MRI. As mentioned earlier, single photon emission CT (SPECT) with the use of injectable metabolic radioactive tracers may be useful in the clinical diagnostic workup and differentiation of metastatic disease, including skeletal metastasis. One should consider biopsy to confirm metastatic disease in patients with a known primary tumor, to evaluate a lesion shown on conventional radiographs or on bone scintigraphy, and to obtain tissue for special studies. Before biopsy, the surgeon must be aware of the patient's clinical, immunologic, and hematologic condition. Patients can be highly susceptible to infection and hemorrhage. Perform biopsy with great care. For instance, have adequate blood replacement available for patients with carcinomatous metastases of the kidney.
Needle aspiration and cytologic evaluation may be performed to confirm the diagnosis of cancer. However, with skeletal lesions biopsy is preferred. Fluoroscopic guidance is often useful; a radiograph should be obtained to document that the correct area has been sampled. Order bacteriologic cultures and frozen sections to rule out infection and to evaluate reliability of the sample. If the pathologist requires special techniques, take appropriate measures. In cases of excessive bleeding, pack the lesion with Gel-foam or polymethyl methacrylate (PMMA). The site of biopsy always should be in line with the definitive incision.
Surgical techniques
Major progress has been made in surgical management of metastatic skeletal disease over the past 20 years. Many techniques have been developed to treat bone defects. The surgical procedures most frequently performed in tertiary care hospitals include the following:
-
Tumor curettage and cemented osteosynthesis - Intracapsular resection of tumor combined with internal fixation (eg, bone plates, screws, intramedullary rods) and PMMA
-
Tumor curettage and spinal instrumentation - Intracapsular resection of tumor followed by interposition of PMMA or some kind of biomaterial (eg, hydroxylapatite), bone grafting, and spinal anterior and/or posterior stabilization
-
Tumor resection and joint replacement - Used most frequently in the hip region with PMMA for fixation
-
Segmental resection and reconstruction marginal or intracapsular resection of tumor together with a large segment of bone followed by implantation of a custom-made or modular mega endoprosthesis (with PMMA used for fixation purposes) - More common than in the past, especially when reconstruction with ordinary endoprosthesis is impossible because of absence of functional bone remnants
-
Cryosurgery - Used to obtain better margins without resection of great amounts of bone; also useful in treatment of tumor hemorrhage
-
Amputation - Seldom required; not in agreement with goals of palliative treatment, which should be used in these patients
Additional Therapeutic Points of Interest
In addition to the preceding synopsis of different treatments available for patients with skeletal defects due to metastases, a focus on several points of interest can be worthwhile.
Bone grafting
Bone grafting may be ineffective in promoting bony union unless more than 9 months have elapsed since the completion of local radiotherapy, as this is when osteoblastic and chondroblastic properties are regained. Methyl methacrylate improves fixation, allows for early ambulation, and does not interfere with radiation therapy. The resistance of the acrylic cement to compression loads, combined with torque and shear strength of the metallic device, promotes secure fixation.
Radiation therapy
The object of radiation therapy is to destroy malignant cells in the affected area, facilitate union of fracture, and prevent local recurrence at least for a limited period of time. Radiation therapy produces tumor necrosis and softening of bone that can increase the risk of fracture, especially in the first 6-8 weeks; bone does not regain full strength until 6 months after completion of radiation therapy. Over time, stages include degeneration and necrosis of cancer cells, replacement by proliferative fibrous tissue, and aggregation of collagen fiber, which becomes calcified and mineralized, forming bone trabeculae and osteoblastic rimming with woven bone structure maturing into lamellar bone. Recalcification becomes evident within 3-4 months, and normal bone structure may be present 6 months after therapy. Many physicians restrict weight bearing during and for several months after radiation therapy, but this practice varies widely.
Radiotherapy is an effective treatment for cancer and skeletal metastasis. The primary aim of radiotherapy is relief of pain, restoration of function, and arrest of tumor growth. In patients with multiple lesions, use radiotherapy for the most symptomatic areas. A total of 30 Gy in 10 fractions, 3 Gy daily, is recommended for palliative purposes. Sometimes, a single 8-Gy treatment is given to patients with a short life expectancy. Use effective chemotherapy and/or hormonal therapy if available. Because most patients with bone metastases have breast cancer, empirical treatment with tamoxifen or aromatase inhibitors in combination with chemotherapy may be warranted.
Goals and principles of treatment
Primary goals of treatment are relief of pain, restoration of function, and facilitation of nursing care. Remove as much tumor and destroyed bone as possible to eliminate the necessity for a second procedure. Guidelines for treatment of these lesions include risk of failure of fractures to unite, shortened life expectancy of patients, and weakened bone in the vicinity of the tumor. Further criteria for treatment are inadequate reaction of lesion to adjunct therapy, lytic lesion more than 2.5 cm in diameter, and destruction of the cortex exceeding 50% of the circumference.
In case of lesions of the lower extremity in which partial weight bearing is permitted, the rehabilitation physician should be aware of the condition of patients' upper extremities because lesions in these areas may preclude use of walking aids. Nutritional condition of the patient should be optimal. Perioperative antibiotics are obligatory. For vascular lesions, preoperative embolization is advisable. In general, all patients receive chemotherapy and/or radiotherapy before and after surgery to diminish the risk of soft tissue seeding and local recurrence. In major bone defects, especially in the hip region, marginal resection is always performed. Choose the plane of resection 3 cm distal or proximal (knee region) of the radiographically recorded boundaries of the tumor. Follow resection by implantation of a mega endoprosthesis with the aid of cement. With this technique, risk for further bone destruction in the area of bone prosthesis interface can be reduced to a large extent.
In the diaphyseal area of long bones, cemented osteosynthesis with an intramedullary rod and PMMA is preferred to internal fixation with a plate, screws, and PMMA. In the metaphyseal area cemented osteosynthesis after intracapsular resection is often indicated. Provide for extensive vacuum drainage.
After surgery, the patient is prescribed bed rest for 24-48 hours. After this period, a rehabilitation program may be initiated. After drains are removed, the patient can move from a bed to a chair. The patient may commence static resistance exercises and basic ADLs training and transfer training, as well as wheelchair mobility training. The patient then can progress to pregait activity and then gait training activities as tolerated. The aim of this program is to help the patient walk independently with crutches or a walker within 1-2 weeks after surgery. Encourage the patient to become independent in transfers and in basic ADLs with the help of adaptive equipment. Radiotherapy and chemotherapy are typically resumed around 3 weeks after surgery.
Treatment with bisphosphonates
Additional treatment with bisphosphonates has been proven to prevent a number of events (eg, fractures, need for additional radiotherapy), and it may induce sclerosis of lytic bone lesions. Bisphosphonates are effective in treating hypercalcemia and can inhibit osteoclast activity by mechanisms that still are unknown. Inhibition of bone mineralization may be a problem in the long-term but not in patients with cancer. Most bisphosphonates are resorbed poorly and should be given on an empty stomach to prevent binding to calcium salts in food. Advise patient to drink ample amounts of water to prevent local ulceration.
In a literature review, Saad et al concluded that bisphosphonates can mitigate bone loss induced by cancer therapy (specifically, in this review, by aromatase inhibitor therapy for breast cancer and by androgen deprivation for prostate cancer). [40]
Other issues
Monitor bone lesions not amenable to surgery, and treat with great care. For lesions in the region of the spinal column, the prescription of a brace is often justified. Because of tumoral progression and the effects of radiotherapy, the vertebral body may collapse, and a brace generally prevents excessive axial deviation. Orthosis should immobilize 1 level above and 1 below the region of the vertebra with the symptomatic lytic lesion or pathologic fracture. Bracing is inadequate to prevent spinal-cord compression. Bracing is an excellent ancillary means of metastatic spinal pain management. (See the article Spinal Orthotics for further details.)
In other cases, such as diaphyseal lesions of the upper extremity, a brace may reduce risk or symptoms of pathologic fracture. A brace may facilitate use of the upper extremity for functional activities that do not involve weight bearing. In lesions of the lower extremity, orthoses may help control pain-related symptoms, but their ability to afford much stability for pathological fractures is limited. If the upper extremities are devoid of clinically significant lytic lesions, achieve restricted weight bearing with an assistive device for ambulation.
Radiotherapy and chemotherapy affect not only the tumor but also adjacent normal bone, reducing the healing potency of bone. In some cases of metastatic disease of the skeleton, physicians use radioactive isotopes to palliate pain.
Prognosis
For patients with skeletal metastasis, prognostication plays a major role in the conception of therapy. In patients with a short life expectancy, avoid major surgery.
Factors contributing to unfavorable prognosis include the following:
-
Aggressive primary tumor
-
Short recurrence-free interval after primary treatment
-
Radiographic absence of bone sclerosis in metastases before and after systemic therapy
-
Multiple bone lesions
-
Metastatic involvement of more than 1 organ (especially the liver)
-
High overall tumor burden
-
Poor general condition
Survival rate after pathologic fracture varies with type of primary tumor. Patients with carcinoma of the lung rarely survive longer than 1 year and often do not survive 6 months, whereas patients with carcinoma of the thyroid commonly live 5 years or longer. In general, approximately 50% of patients sustaining pathologic fracture survive longer than 6 months and approximately 30% survive 1 year. The ability to manage the primary tumor improves with the use of chemotherapy, radiation therapy, and surgery. A corresponding increase in postfracture survival time necessitates improved surgical methods and development of implants to improve treatment for these patients.
Factors contributing to a more favorable prognosis include the following:
-
Moderately progressive primary tumor (prostate cancer)
-
Long recurrence-free interval after primary treatment
-
Radiographic presence of sclerosis in bone metastases initially and after systemic treatment
-
Solitary bone lesion, preferably of a geographic type
-
Low overall tumor burden (preferably bone only)
-
Good general condition of the patient
Overall cancer diagnosis, ECOG performance status, number of bone metastases, visceral metastases, hemoglobin level, and survival estimate were independent predictors of survival. However, these factors should not prevent a consideration of surgical treatment.
Few studies of QOL have been performed in patients with metastatic skeletal disease. Clohisy et al found the SF-36 to have questionable value in identifying patient characteristics that yielded high QOL scores. [41] Heterogeneity of the patient population and floor effects limited utility of the SF-36 in his cohort. FLIC was somewhat more helpful than the SF-35. Scores of the subscale for physical well-being at 6 weeks were correlated with increased length of survival. See the images below.
 Continuation of the summary of quality-of-life (QOL) studies in patients with metastatic bone disease.
Continuation of the summary of quality-of-life (QOL) studies in patients with metastatic bone disease.
Rehabilitation
Goals
Goals of rehabilitation include relief of pain and improved ambulation and function. The literature about the effectiveness of traditional inpatient measures of patients with a malignancy involving bone is limited. These individuals often have clinically significant loss of mobility and have much to gain empirically from treatment.
Bunting et al examined 58 patients with 62 pathologic fractures at various bony sites. [42] The average length of rehabilitation stay, 37 days, was only slightly higher than that for general patients with fractures. Functional results were mixed. Twenty-six patients achieving independent transfers; 23, independent ambulation; and 27, improved scores for ADLs. A total of 34 patients were discharged home, and 7 to other facilities. The mortality rate was high; 17 patients died. Hypercalcemia and the need for parenteral narcotics were risk factors for death or a poor result from rehabilitation.
In a separate study, Bunting et al found that the risk of fracture during PT among patients in an oncology unit was low, involving only 1 of 54 patients. [43] However, 12 patients did have fractures during hospitalization. (The circumstances were not described.)
Allan et al reported results of periacetabular reconstruction in 25 patients with metastatic disease. [44] Only 50% were living 6 months after surgery. At a mean of 14 months after surgery, all surviving patients had progressed from wheelchair or non–weight-bearing status to restricted weight-bearing ambulation. About 62% of patients were discharged home. Seven of 25 patients died within 6 weeks of surgery and, in retrospect, they were poor surgical candidates. Three patients had diffuse lung metastases, 1 patient had multiple bone metastases, 1 had liver metastases, and 1 had cerebral metastases. As these results emphasized, patient selection is important in considering surgical intervention for metastatic bone cancer.
Summary
In summary, the primary goals of rehabilitation should include relief of pain, improved mobility and function, bone protection, and safety awareness. Maintaining ambulatory function is a major goal for both QOL and for preventing the negative sequelae of immobility (eg, effects on cardiopulmonary function).
A therapist must evaluate the patient's upper-extremity function and coexisting upper-extremity metastases before weight bearing through the upper extremities can be allowed. Special consideration should by given to further bony protection in the presence of multiple metastasis, to the care of plastic reconstructions and/or closures, to donor sites for flaps and/or skin grafts, and to modification of bracing as appropriate to protect the flaps or grafts. Bony resections without reconstruction may warrant bracing for patient comfort and positioning for mobility.
A major emphasis of rehabilitation before and after surgery must be placed on instruction in fall prevention, including optimal body mechanics and exercises to maintain strength and balance. The patient's specific environment and activities must be considered, and any necessary equipment or strategies should be used.
Exercise recommendations for patients with bone metastases both before and after surgery focus on increasing their muscle strength and endurance while maintaining bone-protection strategies. High-impact and high-torsion activities should be avoided. After surgery, ROM activities should be included for the joints above and below the affected area. Also, after surgery, it is reassuring to note that the affected bone is usually far more stable than it was before surgery.
The number of cases of metastatic bone disease is relatively high, and the median survival time from diagnosis is clinically significant in the United States. Patients with this type of disease can appreciate a dramatic positive change in their QOL with PT, which can occur both before and after surgical intervention for pain relief and/or bone stabilization. Although these 2 interventions seem to go together, most patients with metastatic bone disease do not undergo surgery but do receive chemotherapy, radiation therapy, immunotherapy, or hormonal therapy. Patients in these categories may also gain substantial benefits from PT interventions. This possibility illustrates the need for a true multidisciplinary approach to the care and treatment of patients with bony metastatic disease.
Primary and Secondary CNS and PNS Tumors: Neuro-oncologic Issues
Overview
Tumors that start in other organs, such as the lung or breast, and then spread to the brain are called metastatic or secondary brain tumors, and those that start in the brain are called primary brain tumors. Most brain tumors come from cancers that started somewhere else in the body and spread, or metastasized, to the brain. Primary brain tumors can start in any of the different tissues or cells in the brain or spinal cord. Some tumors contain a mixture of cell types. This is an important point because metastatic brain tumors and primary brain tumors are usually treated differently.
The American Cancer Society estimated the diagnosis of 18,500 malignant tumors of the brain or spinal cord (in 10,620 in men and 7,880 in women) during 2005 and the deaths of 12,760 people (7280 men and 5480 women) from these malignant tumors in the United States. This type of cancer accounts for approximately 1.3% of all cancers and 2.2% of all cancer-related deaths. Both adults and children are included in these statistics.
The patient's age, functional status, and grade of tumor are the most important prognostic factors. Treatment selection is often based on these factors. Patients with unfavorable prognostic factors, such as advanced age and poor KPS, are unlikely to benefit from aggressive therapy. However, these factors, particularly KPS, are not good measures of patient QOL.
Nonsurgical and surgical treatment for glioblastoma and astrocytoma are available but have had a limited effect on morbidity and mortality.
To overcome the limitations of the blood–brain barrier and to permit the delivery of a high concentration of drug, the administration of chemotherapy directly into a tumor has been studied. Among the various methods used are polymer wafer implants placed during surgery and implanted catheters. These techniques have the potential benefit of reducing the amount of drug affecting normal cells in the brain and throughout the body and increasing the amount of drug reaching tumor cells. See the image below.
The use of biodegradable polymer impregnated with BCNU (Gliadel) has shown promising results as a component of postsurgical chemotherapy in conjunction with external radiation therapy. Temozolomide (Temodar) has revealed promising results as an adjuvant chemotherapy agent after surgical resection of high-grade glioblastoma and astrocytoma. It has also shown efficacy in cases of recurrent glioblastoma and astrocytoma.
Primary brain tumors are both life-threatening malignancies and neurodegenerative disorders. Anderson et al compared the neuropsychologic profiles of patients with tumor and stroke, matching on the basis of lesion location. The defects were generally most severe and pronounced in the patients with stroke. The authors hypothesized that the different degree of defects for matched lesions was due to tumor growth displacing normal brain tissue. The rate of tumor growth may also be an issue.
Hom and Reitan studied neuropsychologic function in patients with either rapidly growing tumors (glioblastoma multiforme [GBM], anaplastic astrocytoma, metastatic carcinoma) or slow-growing tumors. [45] The effects of rapidly growing malignancies were most pronounced. See the image below.
Research on the cognitive effect of cancer treatment has traditionally focused on cranial radiation therapy, but cognitive impairment can also occur after systemic therapy. These cognitive changes are particularly marked in patients with brain tumor. Systemic therapies may additionally influence health-related QOL by affecting disease recurrence. Surgical therapy is also associated with neuropsychologic effects.
Pringle et al assessed anxiety and depression before and after surgery. They found an overall increase in anxiety and depression in women with tumors in the left hemisphere. This level decreased postoperatively.
Few investigators have adequately addressed the cognitive and psychosocial function of adults with brain tumor. Investigators have generally evaluated only a small number of subjects, using a retrospective designs and/or study-specific questionnaires.
Hahn et al prospectively examined 68 patients. [46] They intentionally enrolled patients early in the course of treatment, before definitive radiation therapy, to avoid having treatment-associated neurotoxicity confound the results. An extensive battery of neuropsychologic testing was performed and correlated with patient and tumor factors to identify patients for whom medical and psychological interventions might improve function and QOL. Patients with left-sided tumors have significantly increased memory loss and decreased verbal fluency and verbal learning. Neuropsychologic testing did not show deterioration in function with the bilateral cranial insult of total-body irradiation. However, this result might have been due to laterality and also due to the degree of insult of the tumor versus low-dose radiotherapy. They also noted that depression was most frequent with left-sided tumors.
Patients with GBM performed worse than patients with other histologic features. Why these data show poor performance in patients with high-grade tumors is uncertain. It may be because of the rapidity with which aggressive tumors progress. Just as patients with stroke generally have more deficit than other patients with tumors, the rapid progression of high-grade tumors likely cause insult in a timeframe that makes recovery more difficult than it is with a slower process.
The authors suggested that many patients can improve their ability to function with cognitive, behavioral, and vocational rehabilitation. Tailored programs for this group of patients should be considered. Patients identified with specific deficits on neuropsychologic testing can be referred for tailored rehabilitation programs that may improve their function, or medical management (eg, with antidepressant or stimulants) can be considered. Although formally screening all patients may not be practical, the suggestion that some simple screening tools may help in predicting the results of strenuous testing may widen the application of testing strategies. See the image below.
The average length of stay for 150 survivors treated with traumatic brain injury during the same period was nearly twice as long as that of patients with brain tumors (4.29 ± 3.31 vs 2.64 ± 1.88 mo). This shortened length of treatment is likely due to less overall cognitive impairment and relatively preserved social skills in patients with brain tumor. As survival times for patients with malignant brain tumors continue to increase, the number of patients who could benefit from this type of intervention is also likely to increase.
In addition to the direct benefit of increased community independence and employment to the patient and family, a societal benefit might also result. The improved QOL and increased financial independence of the patients may decrease their need for services from healthcare providers and decrease their use of social service programs and government programs, such as Social Security Disability Insurance. The additional earnings of patients who are returned to work may eventually repay society for the cost of their rehabilitation services. The authors intend to investigate these issues as they follow up these patients over the long term.
Primary Tumors
Meningiomas
Meningiomas are not strictly brain tumors because they arise from the meninges, the layers of tissue that surround the outer part of the brain and spinal cord. Meningiomas cause symptoms by pressing on the brain or spinal cord. Meningiomas are common; they account for about 25% of primary brain tumors and most spinal cord tumors. They are the most common brain tumor in adults. Their incidence rate increases with age, being highest in people in their 70s and 80s. They are almost twice as common in women. These tumors occasionally occur in families, mostly those with a syndrome of multiple benign tumors of nerve tissue called von Recklinghausen disease. Another risk factor for meningiomas is cranial radiation, particularly in young individuals.
About 85% of meningiomas are benign and can be cured with surgery. Some meningiomas are located dangerously close to vital structures in the brain and cannot be cured with surgery alone. A few meningiomas are malignant and may recur many times after surgery; in rare cases, they may even spread to other parts of the body.
Astrocytomas
Most tumors that arise in the brain itself start in brain cells called astrocytes. These tumors are called astrocytomas. About 35% of brain tumors are astrocytomas. Most astrocytomas cannot be cured because they spread widely throughout the surrounding normal brain tissue. Sometimes astrocytomas spread along the CSF pathways. With rare exceptions, astrocytomas do not spread outside of the brain or spinal cord.
In general terms, astrocytomas are classified as low, intermediate, or high grade. Their grade is based on examining a biopsy specimen (sample of the tumor) under the microscope. The pathologist examining an astrocytoma looks for how closely cells are packed together in the tumor, how abnormal the cells are, how many of the cells are dividing or multiplying, whether abnormal blood vessels are growing in the tumor, and whether some of the cancer cells have broken down or died on their own.
Low-grade astrocytomas are the slowest growing. Intermediate-grade astrocytomas, or anaplastic astrocytomas, grow at a moderate rate. The highest-grade astrocytomas, glioblastomas, are the fastest growing. These make up about two thirds of all astrocytomas and are the most common malignant brain tumors in adults.
Patients with special types of astrocytomas tend to have a particularly good prognosis. These tumors are called noninfiltrating astrocytomas (eg, juvenile pilocytic astrocytomas).
Oligodendrogliomas
These tumors start in brain cells called oligodendrocytes. They spread or infiltrate in a manner similar to that of astrocytomas, and, in most cases, they cannot be completely removed with surgery.
Oligodendrogliomas sometimes spread along the CSF pathways but rarely spread outside the brain or spinal cord. Only about 4% of brain tumors are oligodendrogliomas.
Ependymomas
About 2% of brain tumors are ependymomas. These tumors arise from the ependymal cells, which line the ventricles. Ependymomas may block the exit of CSF from the ventricles, causing the ventricles to become large, a condition called hydrocephalus. Unlike astrocytomas and oligodendrogliomas, ependymomas characteristically do not spread or infiltrate normal brain tissue. As a result, some but not all ependymomas can be completely removed and cured with surgery. Spinal cord ependymomas have the greatest likelihood of surgical cure. Ependymomas may spread along the CSF pathways but do not spread outside the brain or spinal cord.
Gliomas
This is not a specific type of cancer. Glioma is a general category that includes astrocytomas, oligodendrogliomas, and ependymomas. About 42% of all brain tumors, including benign ones, are gliomas. Counting only malignant tumors, 77% are gliomas. They are uncommon in children, but their incidence increases with age and peaks in those aged 75-84 years.
Medulloblastomas
Tumors arising from the neurons are rare. Medulloblastomas are tumors that develop from neurons of the cerebellum. They are fast-growing tumors, but they can be treated and are often cured with radiation therapy. Medulloblastomas most commonly occur in children and often spread throughout the CSF pathways.
Metastatic Brain Cancer
Epidemiology
Metastatic brain tumors are the most common intracranial neoplasms in adults and are a notable cause of morbidity and mortality. The prevalence of primary brain tumors is 6.6 cases per 100,000 population, and estimated incidences of metastatic brain tumors have varied from 8.3-11 cases per 100,000 population. The frequency of metastatic brain tumors is thought to be rising because of prolonged survival after primary cancer is diagnosed; this change is a direct result of improvements in early detection and effective treatments.
Individuals with primary lung, breast, melanoma, renal, and colorectal cancers account for most of those with diagnosed brain metastases. Although most brain metastases come from the lung, melanoma has the highest propensity of all malignant tumors to metastasize to the brain.
In a study by Barnholtz-Sloan et al, the incidence proportions of brain metastases for African Americans was significantly higher than those for white patients in terms of lung, melanoma, and breast cancers and was significantly lower for renal cancer. [47] Incidence proportions for colorectal cancer were similar between the rates. Men had incidence proportions of brain metastases similar to or higher than those of women, except for those with primary lung cancer, for which the incidence proportion was significantly higher for women than for men. The incidence proportions of brain metastases was highest for those receiving a diagnosis of primary lung cancer at age of 40-49 years; for those with primary melanoma, renal, or colorectal cancers at age 50-59 years; and for those with primary breast cancer at age 20-39 years.
Pathophysiology
The most common mechanism of metastasis to the brain is hematogenous spread, usually by means of the arterial circulation. More than 70% of patients who develop brain metastases also have primary lung cancers or known metastases to the lung from a nonlung primary cancer.
In the brain, metastases are most commonly are in the area directly beneath the gray matter–white matter junction. Predominance of metastases at this site is due to changes in the size of blood vessels here; narrowed vessels trap emboli. Brain metastases also tend to be common at the terminal watershed areas of arterial circulation, the zones on the border of or between the territories of major cerebral vessels. The distribution of metastases among large subdivisions of the CNS follows roughly the relative weight of (and blood flow to) each area. Approximately 80% of brain metastases are in the cerebral hemispheres, 15% are in the cerebellum, and 5% are in the brainstem.
Brain metastases may be single or multiple. The phrase single brain metastasis refers to an apparent single cerebral lesion; no implication can be made regarding the extent of cancer elsewhere in the body. On the contrary, the phrase solitary brain metastasis is properly used to describe the relatively rare occurrence of single brain metastasis that is the only known site of metastatic cancer in the body. Metastases from colon, breast, and renal cell carcinoma are often single, whereas malignant melanoma and lung cancer tend to produce multiple cerebellar lesions.
Imaging
The best diagnostic test for brain metastases is contrast-enhanced MRI. In the patient who presents with history of systemic cancer and multiple brain lesions, usually little doubt exists about the diagnosis; however, metastases must be distinguished from primary brain tumors (benign or malignant), abscesses, and cerebral infarcts and hemorrhages. Identify patients with single metastases because their subsequent care may differ from that of patients with multiple metastases. When contrast MRI does not substantially help in distinguishing brain lesions, perform stereotactic biopsy.
Experience with MRI has shown that incidence of multiple metastases is higher than previously was believed. About 67-75% of patients have multiple brain metastases at diagnosis. With widespread use of MRI and new improvements in MRI contrast agents and resolution, the proportion of known multiple brain metastases is likely to be even higher in the future.
Diagnosis
Brain metastases may be detected at the same time the primary tumor is diagnosed (ie, synchronous presentation), but diagnosis of the primary tumor most commonly antedates the development or detection of brain metastasis (ie, metachronous presentation). More than 80% of brain metastases are discovered after systemic cancer is diagnosed.
Clinical presentation
Signs and symptoms related to cerebral lesions from metastases vary, and brain metastases should be considered in all patients with known systemic cancer in whom new neurologic findings develop. Most brain metastases are symptomatic; more than 67% of patients with brain metastases develop neurologic symptoms during their illness. Whether onset is gradual or acute, symptoms are seldom specific enough to allow for definite diagnosis. Progressive neurologic dysfunction is usually related to a gradually expanding tumor mass and associated edema or to the development of obstructive hydrocephalus. On occasion, an acute onset may occur secondary to a seizure, hemorrhage into a metastasis, an invasion or compression of an artery by tumor, or a stroke from embolization of tumor cells.
The clinical presentation of brain metastases is similar to that of other mass lesions in the brain. The 4 most common presenting complaints are headaches, focal weakness, cognitive dysfunction, and seizures. In some cases, problems with gait, speech, or visual disturbances may be the sole complaints.
Headaches occur in about 50% of patients with brain metastases; headaches are often mild, diffuse, or bifrontal, having no localizing value. When focal, the headache may be localized at the site of the lesion in up to 70% of cases. Occurrence of early morning headache, thought to be associated with increased intracranial pressure (ICP), is present in less than 50% of patients with cancer who have headaches. Headaches are more common in patients with multiple metastases and with metastases in the posterior fossa than in others.
In the posterior fossa, headache is caused by increased ICP secondary to brain edema or hydrocephalus with traction exerted on pain-sensitive structures, such as the venous sinuses and the dura at the base or back of the skull. Papilledema, the classic hallmark of elevated ICP, is seen now in less than 10% of patients on presentation because of earlier diagnosis associated with neuroimaging. Headaches may become more intense on postural changes or straining and be associated with other symptoms characteristic of increased ICP, such as vomiting, visual blurring, confusion, and (in rare cases) syncope.
Focal weakness is the presenting complaint of 20-40% of patients. Usually gradual in onset, hemiparesis may be subtle, and the patient may not notice it. Hemiparesis usually suggests tumoral involvement of the contralateral hemisphere with compromise of motor context by tumor invasion or, most frequently, by edema caused by a relatively distant lesion.
About 33% of patients complain of problems with memory, mood, and personality changes, whereas as many as 75% report cognitive dysfunction, as evidenced on standard tests of mental status. The slowness with which signs develop, frank denial, or neglect observed with involvement of the nondominant hemisphere may explain the discrepancy in the frequency of signs and symptoms. Seizures occur in about 10% of patients as the first sign of metastases. However, as many as 25% of patients develop seizures in the course of disease. Seizures are usually focal or generalized secondarily after focal onset.
Mukland et al reported on the incidence of neurologic deficits of patients with brain tumors who were treated in an acute inpatient rehabilitation facility. Their patients were retrospectively reviewed; however the population consisted of a heterogenous group with primary and metastatic brain tumors. Nonetheless, their findings differed from the pattern encountered on diagnostic confirmation of metastatic brain disease and primary brain tumors.
The most common deficit was impaired cognition (80%), followed by weakness (78%), visual-perceptual deficit (53%), sensory loss (38%), and bowel and bladder dysfunction (37%). Other problems, in order of decreasing incidence, were cranial nerve palsy, dysarthria, dysphagia, aphasia, ataxia, and diplopia. Thirty-eight (74.5%) patients had 3 or more concurrent neurologic deficits, and 20 (39.2%) had 5 or more deficits. Concurrent deficits among patients with hemiparesis and tetraparesis involved cognition (n = 29), visual-perceptual function, sensation, cranial nerve palsy, and neurogenic bowel and/or bladder.
Treatment
Seizures occur in about 25% of patients with brain metastases and are the presenting complaint in approximately 10%. Whether anticonvulsants should be started in all patients when brain metastases are diagnosed or whether anticonvulsants should be given to only patients who have had a seizure is controversial. Several clinical studies, including 2 small randomized trials, have not shown that prophylactic anticonvulsants reduce the risk of seizures. Because many commonly used anticonvulsants have adverse effects and because only a minority of patients with brain metastases develops seizures, withholding anticonvulsants is a reasonable practice unless (or until) the patient has a seizure.
Corticosteroids serve an important role in the management of acute neurologic symptoms and signs in patients with intracranial neoplasms, as well as in patients with epidural metastasis and peripheral-nerve metastasis. The mechanism of action of corticosteroids is not completely understood, though edema surrounding the metastatic tumors is frequently reduced. Dexamethasone is the preferred corticosteroid because it has minimal mineralocorticoid effect and a relatively low tendency to induce psychosis. More than 70% of patients have symptomatic improvement after starting steroid therapy. Symptoms from generalized neurologic dysfunction or brain edema respond more consistently to steroids than do focal symptoms, such as hemiparesis.
Clinical effects of steroids are noticeable within 6-24 hours after the first dose and reach maximum effect in 3-7 days. Median survival of patients treated with steroids alone is approximately 2 months, though longer survivals have been observed. The usual starting dose of dexamethasone is 4 mg given orally or intravenously every 6 hours. On occasion, patients require doses higher than this. With the stabilization of symptoms and the completion of definitive treatment, the dose of dexamethasone should be gradually tapered over several weeks and then stopped to minimize long-term toxicity. About 10% of patients do not tolerate a reduction in steroids and redevelop signs of brain edema. In these patients, continue the lowest effective dose.
Conventional whole-brain radiation therapy (WBRT) is the most common treatment for patients with CNS metastases. WBRT increases median survival to 3-6 months. Despite relatively unimpressive increase in median survival, radiotherapy is effective at achieving local control of disease in many patients. Data from large retrospective studies show that more than 50% of patients treated with WBRT ultimately die from progressive systemic cancer and not directly from brain metastases. Best results are achieved in patients with KPS of 70% or greater, patients with an absent or controlled primary tumor, patient younger than 60 years, and patients with metastatic spread limited to the brain (true solitary metastasis).
Current typical radiation treatment schedules for brain metastases consist of short 7-15 day courses) of WBRT with relatively high doses per fraction (150-400 cGy per day) with total doses of 3000-5000 cGy. These schedules minimize the duration of treatment, while still delivering adequate amounts of radiation to the tumor.
Radiotherapy has its complications, some of which include the following:
-
Almost all patients have temporary loss of hair, though their hair usually returns 6-12 months after therapy.
-
In the short term, patients may have transient worsening of neurologic symptoms while receiving therapy. Many physicians believe that maintaining patients on steroids during radiotherapy minimizes complications of radiotherapy, though no conclusive proof exists.
-
During initial days of treatment, mild symptoms (eg, nausea, vomiting, headache, fever) are common. This acute reaction may be related to distorted cerebrovascular autoregulation or increased capillary permeability.
-
Rarely, radiation-induced parotitis and loss of taste occur with cranial irradiation.
-
Long-term ill effects of radiotherapy usually are not a notable issue in treatment of brain metastases or in the relatively short survival of these patients. However, reports suggest that more than 10% of long-term survivors (>12 mo) develop symptoms, such as dementia, ataxia, and urinary incontinence.
In these patients, imaging studies show cortical atrophy and hyperdense changes in the white matter. Although pathogenesis of these alterations is unknown, high-dose and/or large-fractionation schedules may be a factor. Therefore, in patients with anticipated long survival, a long course of radiotherapy with small doses per fraction is probably indicated. A reasonable schedule for patients with good prognosis is a total dose of 4500-5000 cGy given in daily fractions of no more than 200 cGy.
Although advantages to surgery can be cited for selected patients, WBRT alone remains the treatment of choice for most patients with brain metastases. Approximately 33% of patients, and nearly 50% of patients in this group are not surgical candidates because of inaccessibility of the tumor, extensive systemic disease, and other factors. Therefore, only about 15-20% of patients with brain metastases benefit from surgical resection; treat the rest with radiotherapy.
Intensity-modulated radiation therapy (IMRT) is the product of advances in the technology of radiotherapy to precisely deliver radiation to the tumor while relatively limiting the dose to the surrounding normal tissues. IMRT combines 2 advanced concepts to deliver 3-dimensional (3D) conformal radiation therapy: inverse treatment planning with optimization by computer and computer-controlled intensity modulation of the radiation beam during treatment. See the image below.
IMRT has been used in the brain where CNS tumors are encased in the cranium. The only factor is patient's movement, which can be minimized by using either an invasive fixation device (Talon; Peacock Sewickley, Pa) or a special reinforced mask (noninvasive immobilization device). The invasive fixation device system uses 2 self-tapping skull screws or sockets attached to the skull. The body of the device is then secured to the screws or sockets and rigidly fixed to support the structure on the treatment table to achieve good patient immobilization. With the advent of IMRT and its capability to simultaneously treat multiple targets with different doses, a new accelerated fractionation scheme was introduced. It is now known as simultaneous modulated accelerated radiation therapy (SMART) boost.
Stereotactic radiosurgery, a method of delivering intense focal irradiation using a linear accelerator (LINAC) or multiple cobalt-60 sources (gamma knife), has been used to treat single and multiple brain metastases. Radiosurgery delivers a highly focused single dose of radiation to a circumscribed area of the brain. The technique causes tissue destruction in the area targeted; therefore, radiosurgery does not replace WBRT, but it may offer a substitute for surgical therapy.
Radiosurgery is limited to lesions smaller than 3 cm in diameter. Many reports of uncontrolled studies of highly preselected patients have been published. Combined results of several reports suggest that radiosurgery prevents or controls local recurrence in 80-90% of treated metastases with about 5-10% risk of radiation necrosis or new neurologic deficits. Prospective clinical trials currently underway are expected to help in determining the role of radiosurgery, both in the primary treatment of patients with single metastases and in the management of recurrent brain metastases.
Use of interstitial brachytherapy, a technique involving placement of radioactive implants in the area of the tumor, has been advocated in selected patients. Implants allow the delivery of high-dose focal radiation to the tumor while minimizing risk of clinically significant radiation exposure of surrounding normal brain tissue because of rapid fall off in radiation intensity at margins of the precalculated target area. The procedure is limited to relatively small metastases in surgically accessible regions of the brain.
Preliminary data suggest that brachytherapy may be effective in selected patients with brain metastases. The major complication of brachytherapy is radiation necrosis, which may appear as an expanding mass months after treatment. Biopsy is often required to differentiate tumor necrosis from recurrence; steroids, and surgical resection occasionally helps to reverse neurologic symptoms secondary to radiation necrosis. The frequency of this complication varies with the amount of radiation administered.
Along with radiosurgery, brachytherapy may be an additional treatment option for patients with unresectable metastases or who previously received maximal doses of WBRT. However, the role of brachytherapy in management of brain metastases has yet to be determined.
Chemotherapy has been used in the treatment of brain metastases from a variety of primary tumors. However, results have generally been unimpressive. Although small uncontrolled studies of patients with certain highly chemosensitive tumors (eg, breast cancer, small-cell lung cancer, germ-cell tumors) have been reported, chemotherapy is not usually the primary therapy for most patients, and it is seldom the only therapy. Given the present data, chemotherapy to small, asymptomatic brain metastases that are known to be chemosensitive seems reasonable. If progression occurs with the administration of chemotherapy alone, definitive treatment with surgery or radiation may be indicated.
Accumulating evidence suggests that chemotherapy may have a role in treatment of carefully selected patients with brain metastases. However, the efficacy of chemotherapy in managing brain metastases has not been demonstrated conclusively. Most systemically administered chemotherapeutic agents that have been proven effective against systemic cancer are ineffective against cerebral metastases from the same cell population. Delivery of waferlike formulations of chemotherapeutic agents has been studied mostly in primary brain tumors, such as glioblastomas and astrocytomas. However, they may show promise in the treatment of metastatic tumors.
All patients need careful follow-up care, no matter what type of initial treatment they receive for brain metastases. No standard has been set for the frequency of follow-up neuroimaging studies after treatment. MRI or CT is clearly indicated any time after therapy if patients develop new neurologic symptoms.
For patients treated with surgery, contrast-enhanced MRI should be performed within 5 days of surgery to detect residual disease. This test is important, especially if the option to forego postoperative radiation therapy is being considered. If residual disease is present, patients should obviously be given WBRT or IMRT. For all patients treated with WBRT, follow-up images should be obtained at regular intervals after treatment. In general, it takes about 6 weeks after WBRT for definite change to appear on images; therefore, patients usually do not need imaging immediately after they complete radiotherapy. A reasonable schedule of follow-up imaging is at 2 months after the completion of the last therapy (either WBRT or surgery) and then about every 3-4 months for the first year. The interval between scans then can be extended gradually so that asymptomatic patients undergo imaging only once per year.
Leptomeningeal Metastasis in Patients with Systemic Cancer
Leptomeningeal metastases, also known as meningeal carcinomatosis or neoplastic meningitis, develop when cancer cells spread into the CSF of the subarachnoid space, which bathes the brain, spinal cord and spinal nerve roots. Like parenchymal brain metastases, leptomeningeal metastases are most commonly caused by breast cancer, lung cancer and malignant melanoma. [48, 49, 50, 51, 52, 53] See the images below.
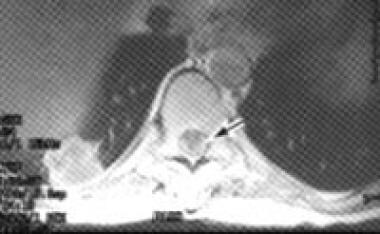 Axial, contrast-enhanced, T1-weighted magnetic resonance imaging (MRI) scan of the spine in a 33-year-old woman with breast cancer who developed low back pain, leg weakness, and numbness. Superficial nodular enhancement around the spinal cord (arrow) is consistent with leptomeningeal metastases.
Axial, contrast-enhanced, T1-weighted magnetic resonance imaging (MRI) scan of the spine in a 33-year-old woman with breast cancer who developed low back pain, leg weakness, and numbness. Superficial nodular enhancement around the spinal cord (arrow) is consistent with leptomeningeal metastases.
The exact incidence of leptomeningeal metastases is unknown, but autopsy data suggest an overall incidence of 5-8% in patients with systemic cancer. In most patients, these metastases are a late manifestation of progressive, widespread disease. Leptomeningeal metastases should always be suspected in a cancer patient with neurologic signs and symptoms indicating dysfunction in more than one anatomic site in the nervous system (eg, seizures, diminished leg reflexes).
The spine is affected in 75-80% of patients with leptomeningeal metastases. Spinal signs and symptoms include neck or back pain, asymmetric reflexes or extremity weakness. Pain is often prominent and can manifest as neck stiffness, localized spinal tenderness or radicular discomfort that radiates from the spine into an arm or leg. Weakness is also common and usually affects both legs in a lower motor neuron pattern (ie, flaccid tone, loss of reflexes, atrophy, fasciculations and negative Babinski sign). More than one half of patients with leptomeningeal metastases have cranial nerve palsies. Common symptoms include diplopia, visual loss, dysphagia, hearing loss and facial numbness.
The brain is affected in approximately one half of patients with leptomeningeal metastases. The most common symptoms are headache, cognitive changes, gait abnormalities, seizures and nausea and vomiting. Mental status and cognitive changes often occur as the leptomeningeal tumor grows over the cerebral hemispheres, causing bilateral cortical dysfunction.
The diverse mechanisms by which leptomeningeal metastases produce neurologic dysfunction include the following: elevation of ICP; direct invasion into brain, spinal cord or nerves; obstruction of penetrating meningeal arterial vessels, and metabolic alterations.
The most important diagnostic tests are lumbar puncture and contrast-enhanced MRIs of the brain and/or spine. In selected patients, it may be prudent to rule out a mass lesion with an MRI before lumbar puncture is performed. If the MRI is unequivocally positive for leptomeningeal metastases, lumbar puncture may be unnecessary. CSF cytologic studies are positive in 45-50% of patients after 1 lumbar puncture and in more than 90% of patients after 3 lumbar punctures.
Contrast-enhanced MRI of the brain and/or spine demonstrates meningeal tumor in 30-50% of patients. The disrupted blood-CSF barrier leads to enhancement of neoplastic meningeal vessels, which appear as a thin rind or as multifocal nodules over the brain or spinal cord. Enhancement in a pattern consistent with the patient's clinical findings is often considered sufficient evidence of leptomeningeal metastases to justify initiation of treatment, even if results of CSF cytologic studies are negative. Communicating hydrocephalus can also be a sign of leptomeningeal metastases because meningeal tumor often impairs CSF pathways.
Leptomeningeal metastases are treated with a combination of radiation therapy and intrathecal chemotherapy. Radiation therapy can be directed at the entire neuraxis, at symptomatic sites, or at areas of enhancing bulky disease. Focal radiation therapy to the brain or spine is generally recommended for patients with symptomatic disease. Intrathecal chemotherapy is given by lumbar puncture or with a ventricular access device such as an Ommaya reservoir. Use of an Ommaya reservoir may help provide uniform drug concentrations throughout the neuraxis.
The survival rates of patients with leptomeningeal disease with and without treatment, because such a poor prognosis, has implications on rehabilitation.
The functional deficits, which result from leptomeningeal disease, are variable. The disease can rapidly progress, with functional decline. With limited survival in such a population, it is important to identify specific rehabilitation goals, which should include training of the family and identifying durable medical equipment. If such patients are admitted to acute inpatient unit for aggressive rehabilitation, the length of the stay tends to be shortened. Readdress the overall clinical course, including need for any further treatment or even appropriateness of hospice in some patients, may be appropriate.
Metastatic Disease of the Spinal Column
Most patients with systemic cancer develop skeletal metastases, and the spine is most commonly involved. Spinal metastases are present in 40% of patients who die of cancer. Autopsy studies have shown that distribution of spinal metastases parallels the bulk of the vertebrae; therefore, the lumbar spine is most often affected, followed by the thoracic and cervical segments. However, in clinical practice, symptomatic spinal metastases most often involve the thoracic spine (70%), followed by the lumbar segments (20%) and the cervical segments (10%).
Secondary spinal tumors most often originate from primary tumors of the breast, lungs, and prostate, reflecting both the prevalence of these cancers and their propensity to metastasize to bone. About 10% of patients with symptomatic spinal metastases present with no known primary lesion.
Symptomatic spinal metastases produce a characteristic clinical syndrome beginning with pain, followed by weakness, sensory loss, and sphincter dysfunction. Local back or neck pain is the earliest and most prominent feature in 90% of patients. Palpation or percussion over the posterior spine at the affected level usually elicits local tenderness. Associated radicular pain distribution indicates irritation of segmental roots. When movement aggravates local back or neck pain, which is then relieved by immobility, suspect spinal instability. If pain has a severe, burning, or dysesthetic quality, intradural extramedullary metastases are the likely cause. Pain caused by spinal metastases may be present for as long as 1 year and is often initially attributed to arthritis, back strain, or a slipped disk. An acute onset of back or neck pain in a patient with cancer means spinal metastasis until proven otherwise.
Pain may be present up to 1 year (average, 4 mo) before more blatant manifestations of spinal cord compromise manifest. Rates at which spinal cord compression develops vary; however, once established, weakness, sensory loss, and sphincter dysfunction progress to complete and irreversible paraplegia unless timely treatment is undertaken.
MRI is the imaging modality of choice for spinal tumors, including spinal metastases. The spine may be evaluated in various planes, and the entire spinal column can be visualized in sagittal cross-sections. Patterns of extradural metastases can be identified, including an isolated level of focal disease, multiple levels of contiguous involvement, or multiple levels of disparate tumor foci. MRI with gadolinium enhancement permits identification of typical intradural extramedullary drop metastases typically found along the cauda equina nerve roots and also reveals any intramedullary metastases. Coronal, sagittal, and transverse reconstructions from MRI provide important information concerning location and geometry of secondary spinal tumors and demonstrate integrity of adjacent vertebral bony elements, all of which are essential for planning optimal treatment.
Treatment
Corticosteroids alleviate pain acutely and improve neurologic function. Promptly administer corticosteroids to patients with clinical manifestations of metastatic epidural compression confirmed by diagnostic imaging or when strongly suspected on clinical grounds, pending confirmation with diagnostic imaging. The dosage of dexamethasone, the most commonly reported corticosteroid used, remains controversial. Although some authors recommend 4 mg 4 times a day, laboratory studies have shown a dose-related benefit with dexamethasone leading to clinical use of loading dose of 100 mg followed by 24 mg 4 times a day. A common approach uses 10-100 mg dexamethasone give intravenously and immediately, followed by 4-24 mg 4 times a day. Larger doses are reserved for patients with profound or rapidly progressive neurologic injury, and low doses, for patients with mild or equivocal signs.
Steroid administration is usually continued throughout radiation therapy at a tapering dose. One useful tapering schedule is a reduction in the dose by approximately one third every 3-4 days (eg, 16 to 12 to 8 mg). A trial of escalated dose followed with a taper may be attempted if tapering is not tolerated and neurologic deterioration occurs. Consider the toxicities of high-dose corticosteroids.
Decisions regarding the level of therapeutic intervention involve factors relative to the patient's prognosis, as well as to the extent of disease in the spine. Radiation therapy is the treatment of choice for most cases of spinal cord compression because no overall difference in neurologic outcome has been observed when patients are treated with radiation therapy or with surgery plus radiation therapy. The response to radiation therapy alone is reported to be 80%. This rate of response with radiation therapy alone represents improvement of motor dysfunction in 49%, with stabilization of clinical status in an additional 31%.
The extent of an epidural mass, like paravertebral involvement, influences response to radiation because of the size of the tumor and presenting neurologic injury. Patients presenting with complete spinal cord block generally have residual neurologic impairment after radiation therapy greater than that of patients with partial block because of irreversible spinal cord injury. Neurosurgical intervention is indicated to establish a diagnosis, to remove a tumor that is compressing the spinal cord and resulting in neurologic symptoms and/or intractable pain, or to treat disease that persists or recurs in a previously irradiated area. Surgical intervention is often used for radioresistant tumors, such as melanoma, and for stabilization of the spine.
Parameters of response involve pain and functional status. These parameters may influence and/or predict survival. One study reported that pretreatment functional status is maintained in more than 90% of surviving patients for average of 3 years after radiation therapy. More than 40% of patients who could ambulate before and after radiation therapy for spinal cord compression survived for 1 year, and 20% of this group survived for 3 years after treatment. In contrast, only 30% of patients who were nonambulatory at presentation, and 7% of nonambulatory patients were alive at 1 year after treatment.
Techniques treat spinal cord compression with radiation account for factors of radiation dose and treatment volume. Although a variety of schedules are used, the most common is 3000 cGy administered in 10 treatments (300 cGy per treatment) to the area of the spinal cord compression. In radiobiologic terms, this regimen is approximately equivalent to administering 3600 cGy by using 200 cGy per treatment in a conventional radiation schedule.
Use of an abbreviated course of radiation is considered advantageous in patients who have severe pain because they often have other intervening medical problems. If tumor involves long segments of the spine or if radiation can exit through viscera (eg, stomach), 3500 cGy is administered in 14 treatments to improve patient tolerance. This radiation schedule delivers a dose to the spinal cord that is radiobiologically equivalent to 3850 cGy if administered at 200 cGy per treatment.
The radiation portal typically is 8 cm wide, centered at the midline of the spine. In general, the radiation portal includes the area of spinal cord compression, plus a margin of 2 vertebral levels above and below the region involved by metastatic disease.
Hyperfractionation is a radiation treatment schedule that exploits the radiobiologic principle of repair of normal tissues between radiation fractions. Two small radiation doses are given on each treatment day; typically a minimum interval of 6 hours separates each dose of 100-120 cGy per fraction. During the hour interval between radiation doses, normal tissues undergo repair of the radiation effects. This process of repair of normal tissues allows safe administration of higher total doses of radiation to normal tissues such as the mucosae and skin; however, repair of damage to the spinal cord is lower, and experiments have shown that more than 8 hours is needed for the spinal cord to complete repair of radiation injury.
Radiation tolerance of the spinal cord is reduced by 0-15% when the interval between radiation actions is decreased from 24 to 6-8 hours. Unlike with the skin and mucosae, hyperfractionation does not spare the spinal cord from radiation injury.
Radiation-induced myelopathy results from dosage to white matter and vasculopathies, which represent 2 mechanisms of radiation injury. Damage to white matter is associated with diffuse demyelination and swollen axons that can be focally necrotic and that can have associated glial reaction. Vascular damage has been shown experimentally to be age dependent, and it can cause hemorrhage, telangiectasia, and vascular necrosis.
Six major types of injury have been shown experimentally to result from radiation to the spinal column. Five of these occur in the spinal cord, and 1, in the dorsal root ganglia. The most severe spinal lesions, all of which are from vascular damage and result in neurologic dysfunction, include white matter necrosis, hemorrhage, and segmental parenchymal atrophy. Clinically, these types of lesions occur 12-50 months after radiation therapy. They can manifest as Brown-Séquard syndrome or as transverse myelitis.
The extent of neurologic loss runs the gamut from incomplete to complete. The 2 least severe spinal lesions include focal fiber loss and scattered white-matter vacuolation resulting from damage to glial cells, axons, and/or the vasculature. These least severe sequelae are seen with low total doses of radiation and are least likely to result in neurologic dysfunction. In the dorsal root ganglia, radiation damage includes intracytoplasmic vacuoles and loss of neurons and satellite cells, which can affect sensory function. These findings are distinct from demyelination of the posterior columns associated with the self-limiting Lhermitte syndrome. This pattern of spinal cord injury occurs early on during the first 12-20 weeks after radiation therapy and usually resolves within 1 year.
Meningeal thickening and fibrosis can also be observed after irradiation, but the clinical significance is unknown. Ependymal and nerve-root damage from radiation is rare. Refinement in surgical strategies, including elaboration of the posterolateral approach and anterior avenues for spinal decompression, together with evolution of spinal stabilization procedures, has improved outcome for patients undergoing surgery for spinal metastases. See the image below.
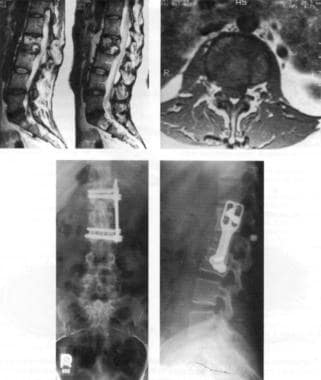 Multiple myeloma in association with disease of the spinal column and compression of the spinal canal necessitating surgical decompression and stabilization.
Multiple myeloma in association with disease of the spinal column and compression of the spinal canal necessitating surgical decompression and stabilization.
This, in turn, has lent support to the concept of de novo surgery for secondary spinal tumors. Furthermore, operating on tumors minimizes risk of wound complications primarily; the risk can be as high as 30% with surgery through an irradiated tissue bed.
Indications for surgery in patients with symptomatic spinal metastases include the following:
-
Failure of radiation therapy
-
Uncertain diagnosis
-
Pathologic fracture dislocation
-
Rapidly progressing or far advanced myelopathic symptoms and signs
Prompt radiation therapy is imperative to maintain neurologic integrity. At present, pretreatment deficits resulting from spinal cord compression are the most important predictors for posttreatment function. Overall, less than 50% of patients regain lost functional capacity. More than 95% of patients with lung cancer and spinal cord compression retain their ability to ambulate, but only 19% regain their ability to ambulate after radiation therapy for epidural disease. Normal bladder function is maintained in 30% of patients, but approximately 50% require catheterization before and after radiation therapy. Recovery of neurologic function also depends on the rate at which symptoms progress, the radiosensitivity of the tumor, and the extent of epidural mass. A response is most likely in paraparetic patients with radiosensitive tumors, such as multiple myeloma, and germ cell and lymphoproliferate tumors.
Treatment of spinal cord compression involves a delicate balance of delivering a sufficient dose of radiation to kill the tumor without further injuring the spinal cord. The ceiling of response, defined as maintaining pretherapeutic level of ambulation and motor function, is considered 80% with irradiation.
In 2005, Patchell et al found that direct decompressive surgery plus postoperative radiotherapy is superior to radiotherapy alone for patients with spinal cord compression caused by metastatic cancer.
Neuro-oncology Issues of the Peripheral Nervous System
Patients with cancer syndromes can present with disorders of the peripheral nervous system (PNS). Disorders may be related directly or indirectly to the physical presence of tumor. Chemotherapy, metabolic derangements, poor nutrition, and comorbid medical conditions also place the patient at risk for PNS impairments. Secondary impairments can generate clinically significant disability and contribute to handicap by virtue of neuropathic pain or loss of strength or sensation.
Paraneoplastic disorder refers to disorders associated with cancer, but they are not attributable to metastatic disease or to nutritional, infectious, metabolic, or treatment-related toxic abnormalities. See the image below.
Work, most notably by Posner and colleagues, has provided strong support for the concept that some of these neurologic disorders result from an immunologic process initiated against tumor antigens that are also present on normal neural tissue. [54] These syndromes often appear before development of symptoms from the tumor itself. Therefore, despite the rarity with which these syndromes occur, identification of patients with these particular clinical syndromes provides the clinician an opportunity to diagnose certain cancers at an early stage. Such early diagnosis is now possible with the aid of specific serologic tests.
Paraneoplastic disorders affecting peripheral nerve, nerve roots, and ganglia, presynaptic and postsynaptic elements of the neuromuscular junction and muscle have all been described.
Peripheral neuropathies occurring as a paraneoplastic phenomenon involve predominantly the sensory or motor fibers, or they may be mixed sensorimotor disorders. Subacute sensory neuropathy is a prototypic paraneoplastic neurologic disorder. The syndrome presents as paresthesias involving the hands and feet, which are often painful, and then progresses over a few weeks to involve the entire body and sometimes the face. All sensory modalities are affected to an equal degree. Although strength is less affected than other aspects, patients are often unable to walk because of sensory ataxia. In addition, 50% of patients have coexistent involvement of other portions of the nervous system because of the same paraneoplastic and/or degenerative process.
Other areas frequently involved are the cerebral cortex (eg, limbic encephalitis), the cerebellum (eg, subacute cerebellar degeneration), and the brainstem (eg, brainstem encephalitis). This combination of different areas involved by the same process has led to use of the inclusive term paraneoplastic encephalomyeloradiculoneuritis. This syndrome is seen most often in patients with small-cell carcinoma of the lung, but it also may be seen with those with Hodgkin disease or other neoplasms. Some patients have antibodies to specific tumor antigens that are also expressed in certain nerve cells.
Classification and Description of Peripheral Neuropathies in Patients with Cancer
Chronic slowly progressive sensorimotor polyneuropathy of mild-to-moderate severity is the most common form of neuropathy encountered in the general population. Defined systemic illness (eg, diabetes, vasculitis), toxin, nutritional disorder, or other specific etiology often is not identifiable. Some of these neuropathies are familial, so careful analysis of family history is critical to evaluation. Chronic sensorimotor polyneuropathy is also the most common form of neuropathy encountered in patients with cancer. Although cancer and neuropathy may be coincidental illnesses, most authors consider chronic neuropathies without other underlying illness in the setting of cancer as paraneoplastic disorders. The mechanism responsible for this paraneoplastic phenomenon is not understood.
Chronic sensorimotor polyneuropathy is most often observed in association with lung cancer. According to electrophysiologic criteria, as many as 50% of patients with lung cancer have evidence of peripheral nerve dysfunction. Most patients are asymptomatic. About 5% of patients with cancer have clinically significant neuropathies, most of which fall into this category. Neuropathy may antedate diagnosis of cancer by years, and the course of neuropathy does not appear to reflect evolution of cancer or its response to treatment. Data from prospective study of neuropathy in patients with small-cell lung cancer suggested that progression of neurologic disorder may be related to the degree of weight loss. Electrodiagnostic studies of patients usually show evidence of axonal loss; however, as with most chronic neuropathies, electrical features of both axonal degeneration and demyelination are often observed together.
Chemotherapy-associated Peripheral Neuropathy
Vinca alkaloids
Of the vinca alkaloids, vincristine has the greatest potential because of its efficacy and relative lack of myelosuppression, cardiac toxicity, or nephrotoxicity, but it is the most neurotoxic. The axonal neuropathy caused by vincristine is usually recoverable, but it may produce great morbidity and can limit clinical use or the drug. The earliest symptoms of vincristine neuropathy are usually myalgias, distal paresthesias, and decreases in ankle jerks, though jaw cramps and other muscle cramps can occur shortly after administration of the agent.
Symptoms typically appear insidiously, but patients with cranial neuropathies associated with vincristine intoxication often develop symptoms rapidly. Ptosis and ophthalmoplegia, causing visual disturbance, may be of such abrupt onset as to mimic brain stem stroke, particularly if these symptoms are accompanied by nausea and ataxia. Paralysis of the recurrent laryngeal nerve can occur, causing stridor, which reverses on drug withdrawal. Symptomatic recovery of vincristine neuropathy may take as long as 40 months, but usually patients are not incapacitated if the dose is kept under 12 mg.
The vinca alkaloids frequently affect small C-fiber dysfunction and dysautonomia. Hemodynamic consequences of autonomic dysfunction appear to be dose-related and consist primarily of orthostatic hypotension, although plausibly a component of the syndrome of inappropriate antidiuretic hormone (SIADH) seen with vincristine stems from altered autonomic function. Other phenomena seen in patients affected by vincristine dysautonomia include reduced GI motility, abdominal colic, impotence, and urinary retention. Abnormal cardiovascular reflexes are commonplace in patients undergoing vincristine therapy. Exercise great care using tricyclic antidepressants, neuroleptics, and diuretics, as they may worsen autonomic function.
Older patients are at greatest risk for symptomatic vincristine neuropathy, and individual doses greater than 2 mg are not tolerated as well as others. The vinca alkaloids poison microtubules by interacting with microtubular protein tubulin, inhibiting the mitotic-spindle movements necessary for cellular reproduction. In vivo experiments have demonstrated clear disruption of both neurofilament and microtubular physiology within hours of nerve exposure to vincristine. The microtubular system of the peripheral nerve, essential to axonal transport, is injured from vincristine "as an innocent bystander." The pace of insult to the nerve has an important role in expression of neuropathy. No experimental studies for vincristine have been designed to determine optimal dose schedules to minimize neuropathic changes.
Taxanes
The taxoids paclitaxel (Taxol) and docetaxel (Taxotere) have been introduced for cancer chemotherapy. [55] Like the vinca alkaloids, the taxoids are plant-derived poisons of the mitotic spindle apparatus.
In contrast to vinca drugs, taxoids cause microtubular aggregation. Paclitaxel at doses of 200 mg/m2 produces mild-to-moderate sensorimotor neuropathy in most patients, but, at higher doses, neuropathy can be severe. Severe orthostatic hypotension from autonomic neuropathy has been seen at this dose range. When paclitaxel was combined with cisplatin in a careful study of patients with ovarian cancer, 95% developed dose-related sensory and/or motor axonal polyneuropathy. Patients with preexisting neuropathy were found to be at greatest risk. From anecdotal reports, docetaxel may be less neurotoxic than paclitaxel. Sensory symptoms predominate early in taxoid neuropathy.
Platinum-based compounds
Cisplatin causes peripheral neuropathy initially reported only in subjects receiving high doses of the drug. The minimal dose that caused neuropathy progressively decreased as experience with the agent increased. Success of cisplatin in therapy of certain solid tumors prompted development of related compounds iproplatin, ormaplatin, and carboplatin, with which less clinical experience has been accrued, though carboplatin clearly produces the least neurotoxicity.
Sensory symptoms predominate early in the course of cisplatin neuropathy with patients most frequently noting distal paresthesiae. In studies that included detailed clinical and neurophysiologic testing, symptoms of neuropathy were common at cumulative doses of approximately 300 mg/m2 and virtually universal at cumulative doses greater than 500 mg/m2. In these studies, the earliest clinical sign of cisplatin neuropathy was reduced vibratory sense with diminished ankle jerks following shortly thereafter.
Motor symptoms have been reported less frequently in cisplatin neuropathy than for vincristine neuropathy. Weakness with electrophysiologic evidence of denervation may be found later in the clinical course; however, cramps are not rare and may occur early. Motor deficit contributes less to the incapacitation of patients than sensory ataxia does, even more than with vincristine neuropathy than with cisplatin neuropathy. Patients with early objective severe weakness should be evaluated for other causes, such as thiamine and vitamin B-12 deficiency, paraprotein neuropathy, and thyroid disorders.
In early studies of platinum neuropathy, the autonomic nervous system appeared to be spared injury. Autonomic symptoms may be overlooked or may be mistaken for another condition because of other causes. Although not as prominent in patients undergoing treatment with vinca alkaloids or taxoids, autonomic dysfunction occurs in cisplatin neurotoxicity, affecting particularly cardiovascular reflexes. This development is particularly important, given the marked emetogenic nature of cisplatin chemotherapy, which increases risk for orthostatic hypotension.
Risk factors for cisplatin neuropathy include preexisting neuropathy, but patient age, history of alcohol use, or diabetes mellitus may all play a role. Increased age is positively correlated with severity of symptoms of neuropathy, and diabetes mellitus is a risk factor as well. Dosing schemes with frequent schedules or continuous infusion cause less neuropathy for a given dose than schedules with high bolus doses of cisplatin.
Oxaliplatin, unlike other platinum compounds, has demonstrated activity against colorectal cancer. It is unique in that it produces both an acute sensory neuropathy that is transient and completely reversible, as well as a chronic sensory neuropathy that is cumulative and resembles the neurotoxicity of cisplatin. The acute phenomenon is common, occurring in about 85-95% of patients treated with oxaliplatin.
Symptoms consist of distal or perioral paresthesias or dysesthesias, pharyngolaryngeal dysesthesias with difficulty swallowing and breathing, and, in rare cases, muscular contractions of distal extremities. All can occur during or immediately after an infusion, and they are usually mild and completely reversible within a few hours or days. Exposure to cold is a trigger for these acute manifestations; patient avoidance of cold is recommended.
The chronic phenomenon is a dose-limiting toxicity of oxaliplatin. It occurs less frequently than acute neurotoxicity, it is similar to that of cisplatin in that it is purely sensory in nature, and its intensity increases with cumulative doses. In contrast to acute neuropathy, it cannot be triggered by exposure to cold. Symptoms reflect impairment of peripheral nerve function and include pronounced distal dysesthesias and persistent paresthesias of the extremities.
Several other antineoplastic agents have been implicated in central and peripheral neurotoxicity. Because cancer itself can cause neuronal damage, the abundance of reported incidences of neurotoxic adverse effects in the chemotherapy literature is not surprising. See the images below.
Thalidomide
Initially developed as an agent for insomnia and later removed from the market due to its harmful fetal effects in pregnant women, thalidomide has had resurgence over the past few years as a treatment option for patients with relapsed or refractory myeloma. It is also being studied in newly diagnosed myeloma patients. Doses of less than 50 to 800 mg/day have been used in patients with myeloma.
Thalidomide can produce a primarily sensory neuropathy, especially in the distal limbs. This is generally mild but may progress with continued therapy. Rates of incidence and severity are difficult to quantify for thalidomide-induced neurotoxicity. Data from a study suggested that both may be dependent on cumulative doses but only when such doses are relatively high (>20 g). More than one half of the patients in this study developed or had a worsening of preexisting peripheral neuropathy.
Suramin
Suramin has antineoplastic effects in the treatment of adrenocortical carcinoma, ovarian cancer, malignant thymoma, non-Hodgkin lymphoma, and renal cell cancer. However, the precise mechanism of its anticancer activity is unknown. Neurotoxicity is a dose-limiting adverse effect.
Suramin seems to cause a dose-dependent distal axonal sensorimotor polyneuropathy and a subacute demyelinating polyradiculoneuropathy similar to Guillain-Barré syndrome (GBS). Distal axonopathy is most common and manifests with distal numbness and paresthesias. Diminished light touch, pain, and vibratory sensation are noticeable on examination. Mild weakness of toe extensors and diminished ankle reflexes may be seen. The neuropathy is reversible with the discontinuation of suramin.
The subacute demyelinating polyradiculoneuropathy is relatively severe and associated with increased drug exposure. Peak plasma concentrations of greater than 200 mc/mL on more than 25 days per month, total cumulative dose more than 157 mg/kg over 8 weeks, or a cumulative dose of greater than 48,000 mg/hr/L were associated with an increased incidence of severe demyelinating neuropathy in 1 study.
Initial symptoms are distal-limb or face paresthesias followed by diffuse, symmetric, proximal more than distal weakness and areflexia. Symptoms may continue to progress over 2-2.5 months. Approximately 25% of patients require ventilatory assistance. CSF protein content is elevated in some cases. Discontinuation of the drug may be followed by deterioration for as long as 1 month. Some patients recover completely 1-2 months after the drug is discontinued. Plasma exchange has been tried in an uncontrolled fashion, with mixed results.
Etoposide
Etoposide is used in the treatment of lymphoma, leukemia, small-cell lung cancer, and testicular cancer. A moderate-to-severe distal axonal sensorimotor polyneuropathy occurs in 4-10% of patients. Severe autonomic dysfunction can result in orthostatic hypotension and gastroparesis. The neuropathy gradually improves after discontinuation of the drug.
Cytarabine
Cytarabine is used mainly in the treatment of hematologic cancers, often in combination with other agents. [56] Usual therapeutic doses do not typically cause PNS toxicity. However, there have been several reports of the development of a severe sensorimotor polyneuropathy, resembling GBS, after high-dose cytarabine therapy. Increased CSF protein has been demonstrated in some of these cases. In the reported cases, histopathologic and electrophysiologic studies vary, with some suggesting a primary axonal etiology and others suggesting acquired demyelination. Brachial plexus neuropathy has also been reported. Whether the latter represents a chance association or a true effect of cytarabine is unclear.
Bortezomib
Another agent that has been evaluated in the treatment of relapsed or refractory myeloma is bortezomib. This agent has a novel antineoplastic mechanism of action in that it reversibly inhibits the activity of cellular proteosomes, which have an essential role in regulating concentrations of certain intracellular proteins. Bortezomib appears to produce a primarily peripheral, dose-dependent sensory neuropathy.
The exact incidence and severity of bortezomib-induced neurotoxicity is difficult to determine because it is a relatively new agent with limited available trial data, and because many patients have preexisting neuropathies on enrollment. A phase II trial of bortezomib in relapsed, refractory myeloma reported a 31% incidence of neurotoxicity of any NCI Common Toxicity Criteria (NCI-CTC) grade, with most patients having neuropathy of less than a grade 3.
A variety of potentially neuroprotective agents have been studied, and only a few demonstrate limited success. An extensive amount of literature is focused on the use of tricyclic antidepressants and anticonvulsants in the treatment of painful diabetic neuropathy. Although it seems logical to assume these drugs have similar effects for all types of neuropathy, the present authors know of no trials have been conducted to evaluate their efficacy in patients with chemotherapy-induced neuropathy. Several other drugs have demonstrated an ability to prevent or alleviate the neurotoxic effects of chemotherapy, but for some, subsequent evaluations have yielded conflicting results; therefore, their effectiveness is debated. See the images below.
Amifostine has shown benefit in the prevention of cisplatin-induced neurotoxicity. However, a study conducted in patients receiving a regimen containing cisplatin and paclitaxel or a regimen containing carboplatin showed no benefit in the ability to prevent neurotoxicity of amifostine. The dose and schedule of amifostine varied between these studies, reinforcing the need for standardization among studies analyzing the usefulness of these agents.
Glutamine is an amino acid that may reduce the severity of peripheral neuropathy associated with high-dose paclitaxel. Infusions of calcium and magnesium just before and on completion of oxaliplatin administration have demonstrated benefit in the prevention of the acute neurotoxicity related to this agent. Methylene blue administered either orally or intravenously after the onset of ifosfamide-induced neurotoxicity appears to shorten the duration of adverse neurological symptoms. Although each of these agents has shown some benefit in the prevention or treatment of CIN, none are currently approved by the US Food and Drug Administration (FDA) for this indication.
Patients infrequently have dysesthetic pain late in the course of peripheral neuropathy or even during recovery. Severe sensory disturbances result in sensory ataxia and can be debilitating. Signs and symptoms of peripheral neuropathy commonly worsen for as long as 6 months after treatment and substantially improve at 12 months with continued gradual improvement occurring for as long as 48 months. Theoretical concern that cisplatin neurotoxicity may be irreversible has not been borne out for most patients.
The Lhermitte sign, in which patients complain of electric shock sensations on neck flexion, occurs in patients with cisplatin neuropathy and is believed to be associated with myelin loss in the dorsal columns similar to that occurring in multiple sclerosis or radiation myelopathy. However, scant pathologic evidence exists to support this contention. Concomitant and marked proprioceptive loss observed in these cases suggests that either posterior columns or large fibers are affected, consistent with the finding that somatosensory evoked potentials are the most sensitive physiologic means of detecting cisplatin changes.
The Lhermitte sign may develop as long as 5 months after cisplatin treatment. Cautious therapy may be continued without adverse effect even after patients demonstrate the Lhermitte sign, but clinicians should bear in mind that, in one case, cervical myelopathy was reported after cisplatin and etoposide chemotherapy of small-cell lung cancer. The myelopathy affected cervical dorsal columns and sensory and motor neurons, but not pyramidal tracts, and was heralded by the Lhermitte sign.
Brachial Plexopathy
Metastatic involvement of the brachial plexus is documented in 1-5% of patients with cancer referred for neurologic consultation, but the exact incidence has not been defined clearly. About 70% of these patients have breast or lung cancers. Pain is the typical presenting symptom, antedating other signs by weeks or sometimes months. The pain, often severe, and it may involve the shoulder diffusely. Most characteristically, it extends along the inner aspect of the arm and ulnar side of the forearm and hand. Weakness and paresthesias are found in more than 70% of patients in a distribution corresponding to the portion of the plexus (eg, C8-T1 root levels). In the remainder, the entire plexus is involved. Idiopathic, postinfectious, and familial brachial plexopathies predominantly involve the upper portions of the plexus (eg, C5-7 root levels). Radiation injury often involves the entire plexus.
Additional involvement near the cervical spinal canal is common. Epidural extension, often through the neural (intervertebral) foramen, is seen in at least one third of patients with neoplastic brachial plexopathy. Homer syndrome or tumoral involvement of the entire plexus (eg, C5-T1 root levels) is associated with an increased incidence of epidural extension. CT and MRI are helpful in demonstrating abnormalities in the brachial plexus, but they do not always help in distinguishing neoplastic involvement from postradiation fibrosis. Surgical exploration is required for diagnosis in a few patients. Antineoplastic therapy, commonly focal radiation, may be beneficial for pain control, but recovery of neurologic function is uncommon. Because of this and because of the high frequency of coexisting spinal epidural involvement, early diagnosis is essential to limiting neurologic morbidity from brachial-plexus metastases.
Lumbosacral plexopathy from neoplasia is most commonly related to colorectal cancer, but sarcoma, breast cancer, lymphoma, cervical carcinoma, and a variety of less common pelvic and retroperitoneal tumors may involve the plexus. Direct extension of tumor from adjacent soft tissue or bone is the mechanism of involvement in 75% of cases. Like brachial plexus invasion, pain is by far the most common initial symptom, occurring in 70% of patients.
Patients often describe a combination of local pelvic and/or sacral discomfort and pain radiating into the leg. Pain is often present for weeks or months before other neurologic signs and symptoms become apparent. Sensory disturbance, weakness, and reflex loss involve the lower (ie, sacral root levels) and upper (ie, lumbar root levels) portions of the plexus with approximately equal frequency. Involvement of the entire plexus occurs in about 20% of patients. In less than 10%, involvement is bilateral. In bilateral cases, the lowest sacral roots from each side are involved as they exit the sacral foramen in close proximity. Low sacral metastases may cause incontinence and impotence without epidural extension.
Myelography demonstrates epidural extension in almost 50% of patients with lumbosacral plexopathy. Both CT and MRI may be useful in demonstrating tumor involvement of the lumbosacral plexus. CT is helpful for detecting bone abnormalities in the sacrum and for imaging the paravertebral spaces. MRI improves demonstration of the epidural space. As with brachial plexopathy, treatment of lumbosacral plexopathy, when initiated after onset of sensory and motor deficits, rarely produces neurologic recovery. Most often, radiotherapy arrests progression of deficits and provides some pain relief.
Disorders of the Neuromuscular Junction Disorders in Patients with Cancer
The cardinal feature of disorders affecting the neuromuscular junction and muscle is weakness. Weakness typically is symmetric and most apparent in proximal muscle groups (ie, shoulders, pelvic girdle). Difficulty working with the arms above the head, as in combing the hair, and difficulty rising from a seated position or climbing stairs are common symptoms. In addition to distribution of weakness, features that distinguish these disorders from peripheral neuropathies and other causes of weakness are preservation of reflexes and absence of sensory symptoms or signs.
The other major symptom of neuromuscular junction and muscle disease is fatigue. Excessive fatigue and exertional intolerance are extremely common complaints in patients undergoing treatment for cancer. For most patients, this complaint can be attributed to cardiopulmonary, hematological, toxic, or metabolic derangements; CNS or psychological complications of disseminated cancer; or its treatment. In absence of such an explanation, the complaint of excessive fatigue should direct attention to the possibility of muscle or neuromuscular junction disorder. Fatigue is a property of normal muscles. Weak muscles fatigue more readily than normal muscles. Dramatic fatigability on repetitive strength testing is characteristic of myasthenia gravis, whereas enhancement with repeated contraction sometimes is observed in Lambert-Eaton (myasthenic) syndrome (LEMS). See the images below.
 Effect of isometric exercise on the compound muscle action potential (CMAP) for a patient with Lambert-Eaton (myasthenic) syndrome (LEMS).
Effect of isometric exercise on the compound muscle action potential (CMAP) for a patient with Lambert-Eaton (myasthenic) syndrome (LEMS).
 Repetitive compound muscle action potential (CMAP) with stimulation and response in a patient with Lambert-Eaton (myasthenic) syndrome (LEMS).
Repetitive compound muscle action potential (CMAP) with stimulation and response in a patient with Lambert-Eaton (myasthenic) syndrome (LEMS).
LEMS is a disorder of neuromuscular transmission that manifests clinically as weakness, particularly involving the proximal muscles. Initial complaints are usually related to weakness of the pelvic girdle, with difficulty climbing stairs or rising from a seated position. Some patients notice transient improvement in strength after initial attempts at exertion. Unlike in myasthenia gravis, ocular involvement is rare in LEMS. Bulbar and respiratory impairment is uncommon. Many patients complain of diffuse muscle aches and of symptoms related to cholinergic autonomic dysfunction, particularly dry mouth, constipation, and difficulty with urination.
Examination shows diminished tendon reflexes; however, demonstration of increase in a reflex after brief voluntary contraction of the muscle is a particularly valuable sign. EMG is a valuable addition to clinical examination in the diagnosis of LEMS. Compound muscle action potential is reduced, but low-frequency repetitive stimulation or brief voluntary contraction results in a transient increase in size of the potential. This finding is highly characteristic, and, before the development of diagnostic tests demonstrating antibodies to presynaptic calcium channel, EMG was the best diagnostic test.
LEMS is frequently associated with small-cell carcinoma of the lung found in about 50% of patients. It has also been reported in association with renal cell carcinoma, lymphoma, pancreatic cancer, and breast and ovarian carcinoma. The neoplasm is often found at an early stage, and, in fact, neurologic manifestations may precede demonstration of neoplasm by months or, in rare cases, years. The longest observed interval between the onset of neurologic symptoms and the diagnosis of associated neoplasm is 4 years. Most patients with LEMS are older than 40 years with a mean age at presentation of 54 years.
Antibodies directed against the P/Q-type voltage-gated calcium channels, which are located at presynaptic nerve terminals, are seen in more than 92% of all patients with LEMS. In 70% of patients with LEMS and malignancy, antibodies to N-type voltage-gated calcium channels are also detected. These are associated with postganglionic sympathetic and parasympathetic cells. The autoimmune attack depletes calcium channels, resulting in impaired influx of calcium into the nerve terminal, which leads to impairment of neurotransmission at the synaptic junction. Cells of small-cell lung cancer express P/Q and N-type calcium channels.
Treatment of underlying neoplasm may result in improvement of neurologic symptoms. Symptomatic treatments with agents that promote release of acetylcholine are beneficial for both cancer- and non–cancer-related LEMS. The agent of choice is 3,4-diaminopyridine. Plasmapheresis and immunosuppressive therapies are also effective though the results are modest with regard to symptomatic improvement and electrodiagnostic testing.
Myasthenia gravis
The prevalence of myasthenia gravis is 5 cases per 100,000 population. In 95% of patients, autoantibodies directed against postsynaptic acetylcholine receptors are present in serum. These antibodies diminish the availability of muscle acetylcholine receptors and, thus, decrease depolarization of the postsynaptic membrane necessary to produce muscle contraction. The disease is characterized by fluctuating weakness and excessive fatigue.
Ptosis and diplopia from extraocular muscle weakness are the most common presenting symptoms. In 15% of patients, the process is limited to the extraocular muscles. Also found are various degrees of weakness in the proximal part of the arm and leg. Bulbar symptoms, including dysphagia, dysphonia, and aspiration, along with respiratory muscle weakness, are the most morbid aspects of the illness. Myasthenia gravis is associated with thymoma in about 10% of patients. These tumors are usually benign lymphoepithelial T-cell neoplasms that are sometimes invasive locally but rarely metastasize. Another 70% of patients have thymic hyperplasia.
All patients with myasthenia gravis should undergo CT of the chest. For many patients, thymectomy is recommended as a therapeutic procedure, with or without thymoma. Immunosuppressive treatments (eg, corticosteroids, azathioprine), plasma exchange, and anticholinesterase medications are the major therapeutic options, along with thymectomy.
Autoimmune myasthenia gravis has been reported in a small number of patients after allogeneic bone marrow transplantation (BMT) in the setting of chronic graft versus host disease (GVHD). Symptoms are generally present 2 years or longer after transplantation, often when immunosuppressants used for GVHD are discontinued. Thymomas are not found. Most described patients have had acetylcholine receptor autoantibodies, with evidence indicating that engrafted donor cells are responsible for antibody production.
Iatrogenic disorders
Toxic and metabolic myopathies probably are underrecognized in patients receiving treatment for cancer. Glucocorticoids are implicated most frequently. Mild-to-moderate symmetric shoulder and pelvic-girdle weakness with preserved reflexes is considered typical. Sensory deficits and pain are not found. The disorder may be related to dose and duration of treatment, but neither of these parameters is well defined. Synthetic fluorocorticosteroids (eg, dexamethasone) are incriminated more often than nonfluorinated drugs such as prednisone.
Laboratory investigations are of limited value in diagnosing this condition. Muscle-enzyme results (ie, for creatine kinase [CK], aldolase) are normal. Results of EMG and muscle biopsy are either normal or show nonspecific abnormalities. Therefore, diagnosis of steroid myopathy is clinical, based on exclusion of other causes of weakness. Observation of improvement after discontinuation of corticosteroid is probably the best evidence in support of the diagnosis. However, because discontinuation is often impossible, dose reduction may be the best option.
Paraneoplastic disorders
A generalized necrotizing myopathy can develop as a presumed paraneoplastic syndrome. Patients can present with an acute or insidious onset of symmetric progressive proximal weakness. Some patients complain of myalgias. The serum CK level is typically elevated 8-100 times normal. Unlike in other paraneoplastic syndromes, the underlying malignancy is typically diagnosed simultaneously. The most common associated malignancies are GI adenocarcinomas and small- and non–small-cell carcinomas of the lung. Biopsy findings are similar to those found in dermatomyositis except for the presence of pipestem capillaries and the absence of perifascicular atrophy. Patients with these findings have a poor prognosis, and their survival has usually been limited to a few months. Some patients reportedly benefit from corticosteroids.
Neuromuscular Complications of BMT
BMT is performed for the treatment of leukemia, lymphoma, aplastic anemia, breast cancer, and other solid organ malignancies. Many patients develop peripheral neuropathies in association with BMT as a result of the toxic effects of chemotherapy, radiation, or infection, particularly with herpes varicella zoster virus. BMT has also been associated with a number of autoimmune neuromuscular complications, specifically myositis, myasthenia gravis, GBS, and chronic inflammatory demyelinating polyneuropathy (CIDP). Most of these autoimmune complications are seen after allogeneic transplantation and the development of GVHD. GVHD has features associated with a variety of autoimmune disorders, raising the possibility that these neuromuscular complications are themselves rare manifestations of GVHD.
Inflammatory myopathies are a rare but well-recognized neuromuscular complication of GVHD. Most cases demonstrate pathologic findings consistent with polymyositis, although dermatomyositis has been described. Many of the reported cases were associated with clinical and biopsy evidence of a concurrent fasciitis and synovitis, with the latter resulting in joint contracture. The myositis and the associated features typically respond to corticosteroid treatment.
Myasthenia gravis is another well-recognized complication of chronic GVHD in BMT recipients. On clinical examination, patients are indistinguishable from those with typical autoimmune myasthenia. Antibodies to acetylcholine receptor were positive in reported cases, and a decrement is seen with low-frequency repetitive nerve stimulation. Donor B lymphocytes seem to be responsible for the antibody production, possibly triggered by antigenic differences in acetylcholine receptors between donor and recipient. Patients respond to treatment with pyridostigmine, prednisone, and azathioprine.
Acute and chronic demyelinating polyneuropathies have been reported in BMT patients. Many patients with severe demyelinating neuropathies have had GVHD. Some cases of GBS have been attributed to chemotherapy or infection and have improved with plasma exchange or intravenous immunoglobulin (IVIG). In other patients, the neuropathy responds to increased immunosuppressive therapy and resolution of the GVHD.
Dermatomyositis and polymyositis in adults are associated with increased incidence of cancer. Incidence of cancer in these patients is 10-15%. The relative risk of cancer is increased 1.7-3.4 times as determined in a population-based cohort study. The highest risk is found in women with dermatomyositis. In this subgroup, risk of ovarian cancer is increased 17-fold; however, unlike many other paraneoplastic neurologic syndromes, myopathies may be associated with a variety of malignancies. Most common associations are with breast, lung, ovarian, colorectal, gastric, and pancreatic neoplasms.
Cancer Fatigue
Fatigue in patients with cancer, often referred to as cancer-related fatigue (CRF), has been defined by the National Comprehensive Cancer Network (NCCN) as a persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning. See the image below.
CRF is the most prevalent unmanaged symptom reported by patients being treated for cancer, and it affects 70-100% of patients receiving cytotoxic chemotherapy, radiation therapy, stem cell or marrow transplantation, or treatment with biologic response modifiers. Fatigue is a persistent, distressing symptom in 17-40% of patients who have completed treatment, and it is a clinically significant symptom in more than 75% of patients with metastatic disease.
Fatigue in patients with cancer is a complex and multifactorial phenomenon that may have a variety of causes and contributing factors. The exact mechanisms involved in its pathophysiology are unknown.
Fatigue may be caused by the malignancy itself or by cancer treatment and treatment-related anemia. Physiologic factors known to contribute to CRF are cachexia, deconditioning, and high levels of certain cytokines (eg, interleukin-1, interleukin-6, tumor necrosis factor-alpha). Psychosocial factors contributing to fatigue include anxiety, depression, and insomnia. Fatigue is also associated with high levels of other symptoms, especially pain.
Several studies indicated that cancer of the lung, GI, urogenital, and hematologic systems provoked the highest levels of fatigue. Patients with leukemia, non-Hodgkin lymphoma and testicular cancer had the most intense fatigue levels before cancer treatment as compared with individuals with breast, GI, prostate cancer or melanoma. Patients who had non-Hodgkin lymphoma described a fatigue that was more incapacitating than that of patients with breast cancer, and more distressful and depressing than those with prostate cancer.
Patterns of fatigue during the course of cancer treatments vary according to the type of treatment. Fatigue typically rises sharply after intravenous cytotoxic chemotherapy to a peak 48-72 hours later and drops to near-normal levels 3 weeks later, with a smaller peak occurring on days 10-14 with some regimens. Studies have not shown substantial increases in fatigue during successive infusions.
During radiation therapy for breast cancer, fatigue levels typically increase linearly over time to a maximum intensity during the fourth week of treatment and then plateau. Levels of fatigue after radiation therapy return to normal in most patients within 3 weeks to 3 months but are most likely to persist at high levels after chemotherapy.
The NCCN has developed guidelines for the evaluation and treatment of CRF on the basis of available research findings and clinical experience (see the Web site of the NCCN for the most recent guidelines). This multidisciplinary panel of experts in CRF developed an algorithm in which patients are screened regularly for fatigue by means of a brief screening instrument and are treated according to their level of fatigue and clinical status. The algorithm includes phases of screening, primary evaluation, intervention according to 3 levels of clinical status, and reevaluation.
The guidelines recommend that screening for the presence and severity of fatigue occur at the patient's initial contact with an oncology care provider, at appropriate intervals (including the follow-up period after treatment ends), and as clinically indicated. If the patient reports the presence of fatigue during screening, the fatigue should be quantified for future comparison.
Although a variety of valid and reliable research instruments are available to measure the multiple dimensions of fatigue, many are lengthy and burdensome for patients with CRF. The guidelines recommend measuring the intensity of fatigue by using a brief clinical instrument such as the 0-10 rating scale commonly used to measure pain. On the scale, 1-3 is generally considered to be a mild level of fatigue; 4-6, moderate; and 7-10, severe. Although moderate levels of fatigue may cause distress and a reduction in activity level, severe fatigue levels are accompanied by a marked decrease in the ability to work and perform other ADLs.
An essential component of the focused history is an assessment of treatable factors that are known to commonly contribute to fatigue. The factors identified by the NCCN practice guidelines panel are pain, emotional distress, sleep disturbance, anemia, nutritional status, activity level, and comorbidities. The guidelines recommend that these factors be assessed and treated as a first step in managing fatigue.
Rehabilitation of Primary and Secondary CNS and PNS Tumors
Rehabilitation of Patients with CNS and PNS Tumors
Investigation with regard to the efficacy of rehabilitation for patients with primary brain tumors has been limited. [57, 58, 59, 60, 61, 62, 63]
Sherer et al provided preliminary support for the use of treatment approaches originally developed for patients with traumatic brain injury with patients with primary malignant tumors of the brain. [64] Compared with the typical outcome of such patients, their patients had favorable outcomes in terms of community independence and employment. Gains made during treatment were generally maintained during follow-up studies performed an average of 8 months after discharge. This finding is relevant because previous studies have shown that similar patients often have a decline in functioning over time. Furthermore, these improvements in QOL were made with a relatively brief intervention and at relatively low cost. The average cost and length of treatment was notably less than that for survivors of traumatic brain injury who were treated in the same program.
Subgroup analysis revealed evidence of gains in the Functional Independence Measure (FIM) and in the Disability Rating Scale (DRS) for patients primarily with less malignant brain tumors as a result of participating in acute inpatient rehabilitation. Follow-up revealed that the patients maintained functional gains at 3 months after discharge.
Of interest, Mukand et al found no significant difference in gains in FIM score between patients with metastasis (ie, 18.6) and those patients with primary brain tumors (ie, 19.8). [65]
In their 1993 survey of 30 caregivers of patients with brain tumors, Meyers and Boake reported a clear pattern of concerns that appears to differ from concerns of other medically ill populations. [66] The most salient problems patients with brain tumors faced were lack of energy; inability to perform usual activities around the home (ie, paying bills, making repairs); social isolation; lack of sexual activity; general slowing of behavior; and problems with reasoning, memory, and concentration. In contrast to other medically ill populations, caregivers of patients did not endorse certain problems with brain tumors as being worthy of concern. These nonproblems included depression, ability to perform basic ADLs (eg, dressing, eating), ambulation, and speaking and being understood. Therefore, it appears that neurobehavioral problems have the largest effect on QOL of patients with tumors and their families.
Two studies enrolled a heterogeneous group of cancer syndromes with subanalysis of brain tumor patients. Marciniak et al reported significant gains across all domains of the FIM. [67] They included patients who presented with metastatic disease, as well as patients receiving radiation therapy. Medical complication rates, and transfer rates back to acute care hospitals, were higher than those of inpatients without cancer syndromes.
Cole et al retrospectively examined 200 patients with cancer diagnoses who had been admitted to an inpatient rehabilitation facility. [68] FIM data were analyzed according to motor and cognitive subscore categories. All groups of cancer syndromes (ie, hematologic, lung, GI, genitourinary, intracranial, breast, gynecologic, miscellaneous) demonstrated significant gains in FIM motor scores. All groups except patients with intracranial neoplasms and advanced terminal disease had gains in cognitive scores of the FIM.
In 3 studies that addressed functional outcomes in patients with primary and secondary brain cancers, investigators did not separate intrinsic brain cancers from metastatic brain cancers. Primary brain tumors may be classified according to histologic grade. The 3 most common primary tumors were glioblastoma, meningioma, and astrocytoma. Unlike metastatic lesions, they may originate at sites other than the cerebral hemispheres, cerebellum, and brainstem. Patients with low-grade gliomas tended to have relatively long survival, namely, 5-8 years. Patients with high-grade gliomas tend to have a short survival time, averaging 9-18 months with treatment.
Huang et al reported a comparative analysis of patients with brain tumors versus patients with stroke (ie, cerebrovascular disease). [69] They scrutinized admission, discharge, FIM D-A, and FIM efficiency data. They separated motor function into mobility and ADLs and documented FIM cognitive scores. No statistically significant differences were encountered in most of the subjects of the 2 groups.
Two studies specifically addressed patients with brain tumors compared with patients with traumatic brain injury. The study conducted by O'Dell et al (1998) was limited by a small sample. Nonetheless, results were consistent with those from the earlier study by Huang et al.
In a later study by Huang et al, patients with brain tumor underwent comparative analysis with patients with traumatic brain injury. Careful matching and data collection confirmed results of O'Dell et al. [70] FIM subscores were similar for the 2 groups on admission, except for those on the cognitive subscale. Patients with traumatic brain injury had low scores. No significant differences were noted in the discharge FIM scores. FIM changes D-A demonstrated more marked differences in patients with traumatic brain injury for ADLs and mobility but not cognition. Length of stay was greater for patients with traumatic brain injury compared with patients with brain tumors. Discharge disposition revealed that patients with traumatic brain injury had greater rates of institutionalization than those of patients with brain tumors.
Rehabilitation and Outcomes for Patients with Malignant or Epidural or Spinal Cord Compression
In a study by Catz et al, the overall recovery rate was impressive. [71] About 51% of patients had no effective movements. Data from all studies seem to agree that ambulatory function at time of diagnosis is the most important criterion for the outcome of ambulatory function.
They used Frankel grades A, B, and C, with total or partial paralysis and nonfunctional movements that cannot be used for daily activities. About 57% of subjects who had nonfunctional movements (Frankel grade C) and full or substantial neurologic recovery, with their conditions upgraded to grades D or E. However, recovery varied according to the severity and etiology of spinal cord lesion (SCL) and to the patient's age at rehabilitation. As in other studies of traumatic and nontraumatic SCL, recovery was inversely related to the severity of the original neurologic deficit as measured with Frankel grades. The initial Frankel grade, which was the principal predictor of recovery in previous research, was also a major factor that was predictive of recovery in this study.
Nontraumatic spinal cord lesion (NTSCL) is a heterogenic group comprising etiologies that may have different tendencies for neurologic recovery irrespective of other factors that may affect recovery. Patients with benign tumors and those with degenerative spine disease (usually after decompressive surgery) had the highest tendency for neurologic recovery, whereas patients with multiple sclerosis had the lowest recovery rate.
Young age at rehabilitation positively affected recovery, but this effect was insufficient to promote recovery from a nonfunctional to a functional state. Neither gender, nor lesion level, nor the decade in which rehabilitation took place affected the neurologic recovery. This implies that recovery depends on the injury that damaged the spinal cord more than on any of the other potentially affecting factors that were studied.
Neurologic recovery rate was generally higher than that achieved by previously examined patients with traumatic spinal cord lesions (TSCLs) during rehabilitation. The NTSCL advantage was most prominent for admission grades A and B, less so for admission grade C. This and the fact that NTSCL recovery from initial grade D was not significantly different from that of any recovery from initial grade A are probably due to a ceiling effect in patients with NTSCL.
In a study by Hirabayashi et al, factors that affected survival after surgery for metastatic spinal tumor included pretreatment neurologic status and tumoral histology. [72] The most significant factor influencing survival was the primary tumor site, as determined from the overall clinical history. Bone marrow [myeloma], thyroid, or prostate findings were favorable and indicated improved neurologic outcome and survival. Unfavorable histologic origins were the lung, GI tract, or unknown primary sites. The type of histology determines radiosensitivity, aggressiveness, and response to cytotoxic chemotherapy and dexamethasone.
Postoperative ambulatory function was also a significant factor in the survival of their patients. Influence from this factor had not been considered in previous reports. The process by which postoperative neurologic status affects survival is not entirely known. However, increased susceptibility to intercurrent infections, immobility-related problems such as development of decubitus, and overall deterioration in paraplegic patients with cancer all undoubtedly contribute to a shorter survival time. Previous studies have shown that poor survival is correlated with these posttreatment clinical factors. Data from other studies have confirmed that patients with spinal cord injury have reduced life expectancy with both the level and severity of neurologic lesion being important determinations.
In their study, Hirabayashi et al focused on postoperative ambulation time as a parameter concerning QOL. Survival time of patients who could walk after surgery was longer than that of patients who could not walk after surgery. No difference in survival or ambulation time was observed between patients who were and were not ambulatory before surgery. Survival of patients who were ambulatory postoperatively was significantly longer than that of nonambulatory patients in general. For those with metastases from the prostate, liver, and lung in particular, ambulation time after surgery was correlated with survival.
However, most studies reveal that pretreatment ambulatory function is the main determinant for posttreatment gait function. Therefore, symptoms of even minor signs of spinal cord compression should lead to supplementary radiological investigations. Survival time is short, especially in nonambulatory patients, and it can be improved only by restoring gait function in nonambulatory patients by immediate treatment. When spinal cord compression is diagnosed, the patient by definition has disseminated disease, which alone may indicate a shorter survival. Therefore, by early diagnosis, it is possible to keep the patient in an ambulatory state, which makes the survival time more valuable than it otherwise would be.
The prognostic variables in the inpatient acute rehabilitation setting may be more challenging to identify and reproduce. Guo et al did not find a prognostic factor that helped identify a subgroup who survived longer than the others and who would therefore benefit from longer rehabilitation. [73]
Five studies have been performed to examine rehabilitation outcomes for patients with metastatic spinal cord injury compared with patients with traumatic spinal cord injury.
McKinley et al found a pattern of less severe neurologic impairment in subjects with nontraumatic spinal cord injury than in those with traumatic spinal cord injury. [74] Individuals with nontraumatic injury were most likely to present with paraplegia than with tetraplegia and with motor incomplete lesions than complete lesions. In fact, no subject with nontraumatic spinal cord injury had tetraplegic-complete lesions. Both the location of spinal involvement and insidious onset of nontraumatic spinal cord injury are believed to contribute. Earlier studies showed that the most common locations for tumorous invasion of the spinal cord are in the thoracic region. Likewise, lumbosacral involvement is most common in vertebral spondylosis. Patients with nontraumatic spinal cord injury were older (>50 y) and included more women than the other group.
Despite the older age of patients of nontraumatic spinal cord injury, individuals were able to achieve significant gains in FIM during inpatient rehabilitation. In addition, 90% of patients from both groups were discharged home.
Several notable subgroup differences were observed between patients with nontraumatic spinal cord injury and those with traumatic spinal cord injury. Individuals with tetraplegic-incomplete nontraumatic spinal cord injury had a length of stay shorter than that of their counterparts. This finding may reflect an early FIM plateau resulting from the patients' relatively high motor FIM scores at admission, which, in turn, reflects the fact that subjects with nontraumatic spinal cord injury were often in the motor-incomplete categories (ie, American Spinal Cord Injury Association [ASIA] grade C or D), instead of sensory incomplete (ASIA grade B).
Note paraplegic nontraumatic spinal cord injury lower motor FIM scores at discharge and FIM change and FIM efficiency compared to traumatic spinal cord injury subjects. These data suggest that subjects with nontraumatic spinal cord injury in the older group improved less and achieved less independence than those with traumatic spinal cord injury. Similar lengths of stay between these groups highlight the continued importance of rehabilitation for patient and family training and for ongoing medical issues.
In a follow-up study, McKinley et al reported that patients with neoplastic spinal cord injury had significantly shorter rehabilitation LOS than those with traumatic spinal cord injury. [75] No significant differences in admission FIM scores were observed; however, patients with spinal cord injury who developed neoplasms had FIM scores on discharge that were significantly lower than the FIM scores of patients with traumatic spinal cord injury. FIM efficiency scores were similar, and no significant cognitive subscores of FIM were noted between the groups. In both groups, most patients were discharged home.
In a study by Partsch et al, the median survival of patients was 11 months after the onset of neurologic symptoms. They expected the type of the tumor and the level of the lesion to be the key factors determining prolonged survival. After completion of statistical analysis by using 2 approaches, the FIM score at admission proved to be the most reliable predictive variable. The FIM score reflects the patient's general clinical situation. Therefore, the indication for rehabilitative efforts should be based on the patient's clinical status at presentation. Tumor type and level of lesion should also be considered, but they were less potent than the FIM score as indicators of survival. The distribution of tumor types (less aggressive in women than in men) may explain the prolonged survival observed in women.
In a study by Eriks et al, patients with epidural metastatic SCC were admitted to a spinal cord unit for less time than patients with an SCC due to other causes. [76] Patients stayed in the spinal cord unit for an average of 104 days (6–336 d). One of the most important findings was that some patients with epidural metastatic SCC survived for a long time. One year after discharge from the spinal cord unit, 52% of the patients were still alive. The average survival was 1472 days (395–6600 d).
The group of patients who survived 41 year after discharge differed in some ways from the group who survived to 1 year after discharge. Their functional progress was greater (7.4 vs 2.5 points on the Barthel score). The discharge destination differed between the groups. Patients who survived longer than 1 year after in-patient rehabilitation were discharged home more often than patients who survived less than 1 year (82% vs 64%). Therefore, it can be concluded that patients with a prolonged survival (>1 y after inpatient rehabilitation) live longest and benefit most from inpatient rehabilitation.
Rehabilitation of Neurologic Impairments of the PNS
Rehabilitation principles used in neuromuscular disease may be extrapolated and customized to the patient's cancer. Guo and Shin have summarized these principles. [77]
Instruction in compensatory strategies provides patients with the means to remain mobile and perform self-care activities. Patients are taught to modify their posture and alter body mechanics to capitalize on the strength of preserved muscle groups. Compensatory strategies are wide ranging and may be targeted at bed mobility; transfers (from supine to sitting position or sitting to standing position); static sitting and/or standing; or ambulation.
Provision of appropriate assistive devices is critical to successful therapy and integral to many compensatory strategies. Assistive devices to enhance mobility in patients with paresis commonly include various types of canes, walkers, and crutches. Assistive devices for household and self-care tasks are designed to permit independent performance of ADLs and instrumental ADLs (IADLs). Reachers allow patients to retrieve objects that would otherwise be inaccessible. Dressing and bathing aids minimize the amount of force and coordination required for these self-care activities. Therapeutic exercise is generally integrated with instruction in compensatory strategies and proper use of assistive devices to develop a composite intervention geared toward maximal autonomy.
Orthotics can be helpful in enhancing stability and safety for patients with motor deficits. Bracing strategies can be applied to protect and stabilize joints controlled by weak muscles, to maintain joints in positions of function, and to compensate for lost motor function. Truncal orthotics can be cumbersome and are generally reserved for cases of symptomatic vertebral metastasis.
Upper-extremity orthotics can help patients with paresis or plegia involving the distal extremity to grasp and manipulate objects. For example, a patient with weakness in the dominant hand due to a C8-T1 plexopathy from metastatic cancer can enhance function through the use of a universal cuff. This orthotic allows the patient to hold objects for feeding, grooming, and hygiene despite the absence of strength in the finger flexors or hand-intrinsic muscles. Orthotics can also be used to maintain the wrist in extension so that the thumb and digits will oppose each other for functional pinch. A balanced forearm orthotic supplements weak shoulder abductors and forward flexors in patients with preserved distal-extremity strength.
Lower-extremity orthoses enhance joint stability and muscle function for safe ambulation. AFOs allow patients with anterior tibialis weakness to clear their feet during the swing phase of gait, as with a drop foot. Knee orthoses prevent buckling in patients with motor deficits affecting the quadriceps. Knee-ankle-foot orthoses extend distally to encompass the ankle if there is associated weakness of the ankle dorsiflexors. Orthotic options should be explored for any patient with clinically significant motor deficits irrespective of their prognosis, because function can be substantially improved.
Equipment for functional restoration can be prescribed to alter patients' environments and compensate for lost motor capacities. Mobility can be restored through the use of wheelchairs and scooters. These optimize community mobility, and minimize social isolation. Ramps can be purchased, allowing wheelchair-dependent patients independent access to their homes. A plethora of equipment has been devised to facilitate ADLs performance. Patients can bathe and toilet with greater ease and safety if appropriate tub seats and commodes are provided. Hoyer lifts allow the transfer of patients with extensive motor impairments by a single caretaker. They may be critical for home discharge. Home modifications fall under the general rubric of restorative equipment. Ramps, lifts, and railings may be added to homes, when feasible, to greatly enhance patient safety and independence.
Motor and sensory deficits may coexist secondary to plexopathy or peripheral neuropathy. Therefore, optimal therapy integrates motor and sensory reeducation techniques appropriate to each patients' unique disability. Physical therapists work on static posture as well as on transfer and gait training, encouraging patients to rely on visual rather than sensory or proprioceptive cues. Patients are taught to use assistive devices, usually canes or walkers, during ambulation and stair climbing to compensate for reduced sensory input. Assistive devices may also be required to broaden patients' base of support and enhance their stability.
Tactile input received by means of an assistive device held in the upper extremity can supplement for diminished lower-extremity proprioceptive acuity. The development of safety awareness to protect insensate extremities is a prominent dimension of therapy. For upper extremities with severe sensory deficits, a sling may be required to prevent trauma to the arm. Orthotics can also be extremely helpful in protecting sensation-deficient extremities. If proprioceptive deficits are severe, orthoses can maintain affected joints in functional alignment for ambulation or grasp. Exercises to enhance patients' preserved proprioception may be useful in augmenting function.
Occupational therapists help patients to overcome deficits in distal-upper-extremity sensation, allowing them to execute the many fine motor tasks required for self-care. Reliance on visual rather than tactile feedback is stressed for hand positioning and task sequencing. Patients are taught compensatory strategies to monitor the amount of pressure exerted during activities requiring pinch and grasp. Many modified utensils for performing ADLs are available with ergonomic alterations that neutralize sensory deficits. Providing patients with such devices is perhaps the most beneficial intervention in restoring autonomy with home and self-care. Cutlery, cups, can openers, combs, buttonholers, and toothbrushes are but a few examples of the many items available.
Neoplastic polymyositis/dermatomyositis (PM/DM) have been associated involvement of other organs (heart, lung, and joints) and complications of therapy (infections, osteonecrosis, and osteoporotic compression fractures) that affect disability status aside from the proximal weakness that characterizes the syndrome. Evaluation before designing the rehabilitation program involves determination of disease stage, impairments, and functional limitations.
Muscle weakness usually begins proximal in hips, then affects the shoulders and anterior neck muscles. The clinical course of PM/DM is varied; some patients experience an acute fulminant illness lasting a few years, others a remitting and relapsing course or a chronic stable course. Adults with PM or DM can recover completely or have residual mild-to-moderate weakness and fatigue that is amenable to rehabilitation therapy. Responsiveness to therapy in PM and DM cannot be predicted at the outset.
In reviews by Forrest and Krivickas, early phase rehabilitation therapy aims to preserve muscle function, prevent disuse atrophy, preserve ROM, and prevent contractures with passive stretching exercises. Severity of the clinical problems in the acute stage is related to the severity and distribution of muscle weakness. If weakness is confined to the shoulder and hip-girdle muscles and if 50% of normal strength is preserved, patients require minimal assistance with ambulation and ADLs.
Rehabilitation therapy should focus on work amplification, pacing physical activities, gait training, use of assistive devices to reduce fall risk, and instruction for active and passive daily stretching exercise routine to preserve ROM and prevent contractures. With more widespread involvement of a muscle group and with profound weakness, patients lose functional independence and require substantial assistance with eating, grooming, transfers, dressing, and bathing, and they are nonambulatory.
Weakness of the neck muscles limits bed mobility, transfers, and sitting endurance and is easily assisted with a soft collar. Neck-muscle weakness predicts swallowing dysfunction due to weakness of the oropharyngeal muscles that may cause aspiration and pneumonia. Dysphagia occurs in 30-60% of patients with DM. Patients with such weakness should undergo swallowing evaluation by a speech pathologist to determine the need for implementation of aspiration precautions. They normally have reflex swallowing in response to the oral bolus stimulating the base of the tongue and palatopharyngeal regions. With weakness of the skeletal muscles of the oropharynx, propulsion of the bolus through the pharynx into the esophagus is weak and uncoordinated, resulting in aspiration and nasal regurgitation when swallowing is attempted. Techniques that may reduce the risk of aspiration pneumonia include upright seating with slight neck flexion and chin tuck with double swallowing.
Weakness of the respiratory muscles can be monitored by measuring tidal volume with a bedside spirometer each day. Intercostal muscle weakness reduces thoracic expansion during inspiration, leaving the patient dependent on diaphragmatic excursion to produce adequate ventilatory volumes. The seated position provides the maximal diaphragmatic respiration. If the diaphragm muscle becomes weak, ventilatory assistance will be necessary, initially with negative pressure ventilation (cuirass, iron lung, rocking bed) and with bilevel positive airway pressure (BiPAP) or tracheostomy if it progresses.
Assistive devices and adaptive strategies are recommended to compensate for muscle weakness, fall risk, ADLs dependency, and reduced endurance. Patients with hip-girdle weakness and difficulty arising from a chair are helped with an elevated seat cushion, lever-controlled booster seat, raised toilet seat, shower chairs, and hand held shower extensions. For those with arm and shoulder weakness, long-handled reachers and tooth, hair, and back brushes are useful.
With distal muscle weakness, various jar and door openers and large-handled utensils and tools are useful. Upper- and lower-extremity orthotics help stabilize joints in position for optimal function. A wrist splint with a volar shank supports the wrist and augments grip strength. With quadriceps weakness, a short leg brace with 5° of plantar flexion produces an extension moment at the knee, increasing knee stabilization. However, a brace with dorsiflexion to support a weak ankle will create a flexion moment at the knee and increase fall risk if the quadriceps is weak. Canes are useful primarily for balance. Walkers may maintain ambulation if upper extremity strength is sufficient. Motorized wheelchairs or scooters are important for maintaining community ambulation.
Patient and family education regarding the variability in clinical course, the elements of chronicity with unpredictable remissions and exacerbations, and the importance of compliance with medication and rehabilitation programs are needed for a realistic understanding of the disease process.
Prognostication is difficult because of the variability in disease severity and responsiveness to treatments. Referrals for vocational rehabilitation should be made when it becomes clear that certain functional deficits are likely permanent. Referral to rehabilitation medicine, occupational therapy, and PT should be made early to design the long-term multimodal therapies that will have to be continuously revised according to the patient's condition. Fatigue is a distressing component of PM/DM that requires thorough patient education regarding strategies for energy conservation and work simplification techniques to maximize functional level. Psychological support is usually needed for the family and patient because of the effects on home life, work capability, sexual function, and social life.
Response to Exercise in Patients with Myositis and/or Myopathy
The controversy regarding the benefits and hazards of exercise in patients with inflammatory myositis or myopathic diseases is generally based on the unsubstantiated belief that strength training exacerbates inflammation or induces further muscle fiber damage and further damages muscles. These beliefs are based largely on several observations of responses to vigorous exercise in normal individuals. Acute high-intensity exercise is associated with transient leukocytosis that can increase endomysial inflammation. Eccentric (lengthening) exercises cause muscle pain and elevate CK levels with focal areas of endomysial inflammation. Levels of cytokines are elevated for as long as 5 days after exercise and could increase muscle fiber destruction. In contrast, exercises emphasizing concentric activation of muscles can increase dynamic strength without damaging muscle fibers.
Patients with weakness often have limited aerobic capacity (reduced maximum oxygen uptake, or VO2 max) caused by intercostal muscle or diaphragmatic weakness compounded by deconditioning due to inactivity, systemic disease, or cardiopulmonary complications. Research into vigorous, progressive strengthening and reconditioning exercise programs by patients with myopathies and dystrophies has been published; however, the data generally have not been incorporated into rehabilitation or neuro-oncology publications.
Three types of therapeutic exercise are prescribed: ROM or stretching (passive or active), strengthening (isometric, isotonic, isokinetic), and aerobic conditioning.
The exercise program is typically modified to progress from passive to active-assisted (when muscle strength is less than 3/5), to active (when muscle strength is sufficient to move through the full ROM), and to progressive-resistive exercises (when strength is sufficient to lift 1-2 lb).
Strengthening exercises are described in the context of the type of muscle contraction involved and the type of external resistance applied against movement. Type I and type II fiber hypertrophy increases the cross-sectional diameter of the exercised muscle after a few months. An isometric muscle contraction does not produce movement of the joint and therefore does not risk muscle or tendon injury. Isotonic (ie, dynamic) exercises involve muscle shortening (concentric) or muscle lengthening (eccentric) while overcoming an external force (weight). The typical exercise prescription includes 20 repetitions of contractions 3 times daily. Concentric muscle contraction exercises overcome external resistance while shortening the muscle (curls). Slower concentric contractions generate more force than fast ones. Eccentric muscle contraction overcomes an external force while the muscle is lengthening and can generate more force than concentric contractions.
Eccentric activation of the muscle can disrupt the muscle cell membrane (elevation of CK levels) and may be associated with early and delayed muscle soreness, therefore should be avoided in patients with already inflamed muscles. Data from high-intensity exercise physiology studies in healthy subjects used to justify exclusion of strength training exercises included the following:
-
The immune response to high intensity acute exercise in healthy individuals is characterized by a transient leukocytosis (lymphocytes and polymorphonuclear cells) and an increase in natural killer cells and cytotoxic T lymphocytes that could theoretically increase endomysial inflammation.
-
Eccentric (lengthening) exercise can cause focal endomysial inflammation, myalgias, and increase in CK levels, suggesting increased or worsened myopathic features.
-
Levels of cytokines, including interleukin-1 beta, are elevated as long as 5 days after exercise and can enhance muscle-fiber destruction or exacerbate cancer related fatigue.
Literature about the effects of therapeutic exercise for myasthenic syndrome and myasthenic gravis are scant. Case reports and series reveal promising results without deleterious effects in controlled stable disease.
Burnham and Wilcox indicated that a low- to moderate intensity aerobic-exercise regimen can be a safe and effective means to improve aerobic capacity, body composition, flexibility, QOL, and other measures (energy, fatigue, anxiety) in patients who survive cancer. [78] Furthermore, the exercise regimen was well tolerated, and exercise adherence was excellent. These findings make a strong case for incorporating low- or moderate-intensity exercise in the rehabilitation of survivors of cancer.
With regarding to fatigue, the management may be categorized by clinical strategic approach.
Energy-conservation strategies
Use sleep-promoting, cognitive-behavioral strategies if patients have insomnia.
Exercise strategies
Implement a monitored exercise program to reduce fatigue as appropriate.
Coping strategies
Keep communications open so that patients can express the presence and effect of fatigue. Communicate to patients before treatment that fatigue is normal, usually transitory, and not necessarily a symptom of treatment failure.
Provide patients with information about the likelihood of cancer-related fatigue and available therapies. Use attention-restoring activities, such as walking in the park, or pleasant distractions.
Self-care strategies
Teach ROM exercises before breast surgery to strengthen muscles and improve mood state. Educate patients on the proper use of pain medication and tranquilizers. Encourage patients to engage in modest recreational activities 20-30 minutes a day, about 3 times a week to prevent attentional fatigue. Promote group activities to increase social support and personal growth.
Information-seeking strategies
Create information packets and teaching materials that address the specific needs of people living with cancer-related fatigue.
Supply written instructions, advice, and information to patients to address cancer-related cognitive impairment due to fatigue.
Schedule the patient appointments with consistent healthcare professionals whenever possible to improve correctness of perceptions, detect problems, and assess treatment compliance.
Other
Bruera et al showed that patient-controlled methylphenidate improved fatigue, a number of other symptoms, and overall QOL; patients also chose to continue treatment for an additional 4 weeks. [79] Their findings suggest that patients had consistent improvement in their fatigue level throughout the day. Fatigue intensity before breakfast reflects fatigue before the first daily dose of methylphenidate. These findings suggest that methylphenidate had a cumulative effect during the day. Methylphenidate had a remarkable effect on the overall QOL scores. However, because it appears to affect many symptoms, establishing the relationship between fatigue and overall QOL improvement was not possible given the small sample. Randomized controlled trials are required to further characterize this response.
-
Breast cancer. Transverse rectus abdominis muscle (TRAM) flap.
-
Breast cancer. Transverse rectus abdominis muscle (TRAM) flap.
-
Surgical rehabilitation in head and neck cancer.
-
Internal components of an electrolarynx.
-
Diagram of a speaking tracheostomy valve.
-
Diagram of a tracheoesophageal puncture anatomic procedure.
-
Multiple myeloma in association with disease of the spinal column and compression of the spinal canal necessitating surgical decompression and stabilization.
-
Metastatic lesion of the proximal femur requiring implantation of a femoral prosthesis.
-
American Cancer Society: 2005 statistics.
-
American Cancer Society: 2005 statistics.
-
American Cancer Society: 2005 statistics.
-
Surgical rehabilitation in head and neck cancer.
-
Summary quality-of-life (QOL) studies in patients with metastatic bone disease.
-
Continuation of the summary of quality-of-life (QOL) studies in patients with metastatic bone disease.
-
Mechanisms and mediators of metastasis to bone.
-
Mechanisms and mediators of bone metastasis.
-
Functional Independence Measure (FIM) scores in primary versus metastatic brain disease.
-
Intensity-modulated radiation therapy (IMRT) for a brain tumor.
-
Drug delivery and administration techniques for glioblastoma and astrocytoma.
-
Glioblastoma multiforme (GBM) before and after surgical therapy.
-
Axial, contrast-enhanced, T1-weighted magnetic resonance imaging (MRI) scan of the spine in a 33-year-old woman with breast cancer who developed low back pain, leg weakness, and numbness. Superficial nodular enhancement around the spinal cord (arrow) is consistent with leptomeningeal metastases.
-
Tumor diagnoses associated with leptomeningeal metastasis.
-
Prognosis and leptomeningeal metastases.
-
Diagnostic and treatment algorithm for leptomeningeal metastatic disease.
-
Options for chemotherapy for leptomeningeal metastatic disease.
-
Differential diagnosis of peripheral neuropathy in the patient with cancer.
-
Chemotherapy and peripheral neuropathy in the patient with cancer.
-
Grading scales for chemotherapy-induced neurotoxicity.
-
Dose adjustments to reduce the risk of chemotherapy-associated peripheral neuropathy.
-
Prevention of peripheral neurotoxicity in the patient receiving chemotherapy for cancer.
-
Paraneoplastic syndromes.
-
Effect of isometric exercise on the compound muscle action potential (CMAP) for a patient with Lambert-Eaton (myasthenic) syndrome (LEMS).
-
Repetitive compound muscle action potential (CMAP) with stimulation and response in a patient with Lambert-Eaton (myasthenic) syndrome (LEMS).
-
Algorithm for cancer-related fatigue.
Tables
What would you like to print?
- Cancer Rehabilitation
- Breast Cancer and Rehabilitation
- Systemic Effects of Cancer-related Deconditioning
- Rehabilitation for Head and Neck Cancer
- Cancer of the Musculoskeletal System and Its Rehabilitation
- Musculoskeletal Impairments in Cancer Syndromes and Their Rehabilitation
- Primary and Secondary CNS and PNS Tumors: Neuro-oncologic Issues
- Rehabilitation of Primary and Secondary CNS and PNS Tumors
- Show All
- Media Gallery
- References