Practice Essentials
The first known description of neonatal brachial plexus palsy (BPP) dates from 1779 when Smellie reported the case of an infant with bilateral arm weakness that resolved spontaneously within a few days after birth. In the 1870s, Duchenne and Erb described cases of upper trunk nerve injury, attributing the findings to traction on the upper trunk, now called Erb palsy (or Duchenne-Erb palsy). [1] In 1885, Klumpke described injury to the C8-T1 nerve roots and the nearby stellate ganglion that now bears her name. High-resolution magnetic resonance imaging (MRI) is the best imaging study available for evaluating neonatal brachial plexus palsy.
Many cases of BPP are transient, with the child recovering full function in the first week of life. A smaller percentage of children continue to have weakness leading to long-term disability from the injury. The mainstay of treatment for these children is physical and/or occupational therapy in concert with a regular home exercise program. A select few patients may benefit from surgical intervention in the early stages to improve innervation of the affected muscles. Others benefit from tendon transfers performed later to improve shoulder and (sometimes) elbow function. [2]
Numerous other nonsurgical treatments, including electrical stimulation and botulinum toxin injections, also may prove effective in the treatment of children with BPP. In view of the variability in presentation, treatment options, and outcome measures, a multidisciplinary approach to the care of the infant with BPP is recommended.
Signs and symptoms of brachial plexus palsy
Newborns
The infant with complete BPP (C5-T1) typically lies in the nursery with the arm held limply at his/her side. Deep tendon reflexes (DTRs) in the affected arm are absent, and the Moro response is asymmetrical, with no active abduction of the ipsilateral arm.
In children with total arm involvement, careful examination of the child's eye often demonstrates Horner syndrome (ie, miosis, ptosis, anhidrosis), suggesting injury to the stellate ganglion. [3]
The infant with an upper plexus palsy (C5-C7) keeps the arm adducted and internally rotated, with the elbow extended, the forearm pronated, the wrist flexed, and the hand in a fist. In the first hours of life, the hand also may appear flaccid, but strength returns over days to months.
Older children
The older child with BPP involving the upper trunk typically has difficulty with active shoulder abduction, forward flexion, symmetrical elbow flexion, and forearm supination.
With shoulder abduction, the medial edge of the scapula often can be seen protruding above the shoulder line, a manifestation referred to as Putti sign.
The term "trumpet sign" describes the child's typical pattern of bringing objects to the mouth (ie, shoulder abduction accompanied by elbow flexion).
Posterior subluxation of the humeral head can develop as the internal rotators of the shoulder overpower the weaker external rotators and become contracted.
Mild shortening and atrophy of the limb are observed.
Workup in brachial plexus palsy
High-resolution MRI is the best imaging study available for evaluating neonatal brachial plexus palsy. Plain radiographs can help to diagnose hemidiaphragm paralysis from phrenic nerve involvement and fractures of the clavicle or humerus. Axillary radiographs also should be performed in children who show progressive loss of external rotation, to rule out posterior shoulder dislocation.
Electrodiagnostic studies are used as an extension of the physical examination and can provide data on the severity and timing of the injury.
Management of brachial plexus palsy
Physical therapy
Physical and/or occupational therapy provide the cornerstone of the management of symptoms in a child with BPP. The rehabilitation of children with BPP must begin in infancy to achieve optimal functional returns. For the first 2 weeks, the child may have some pain in the affected shoulder and limb, either from the injury or from an associated clavicular or humeral fracture. The arm can be fixed across the child's chest by pinning his/her clothing to provide more comfort. However, some authors have discouraged this pinning in favor of immediate institution of gentle range-of-motion (ROM) exercises. Parents should be instructed in techniques for dressing the child that avoid further traction on the arm. Often a wrist extension splint is necessary to maintain proper wrist alignment and reduce the risk of progressive contractures.
As the child gets older, bimanual activities (eg, swimming, basketball, wheelbarrow walking, climbing) should be encouraged. A comprehensive therapy program that has been designed and implemented by a pediatric physical therapist is essential for children whose case is being managed conservatively, as well as for children who require surgical intervention. [4]
A comprehensive therapy program should consist of ROM exercises, facilitation of active movement, strengthening, promotion of sensory awareness, and provision of instructions for home activities. Overall goals should focus on minimizing bony deformities and joint contractures associated with BPP, while optimizing functional outcomes.
Severe contractures should be avoidable with consistent therapeutic exercises, including passive and active stretching, flexibility activities, myofascial release techniques, and joint mobilization.
Surgery
In early surgery for BPP, two neurosurgical options (ie, neurolysis vs excision of the neuroma and nerve graft reconstruction) exist. Late surgery for BPP most often involves tendon transfers and/or osteotomies.
Pathophysiology
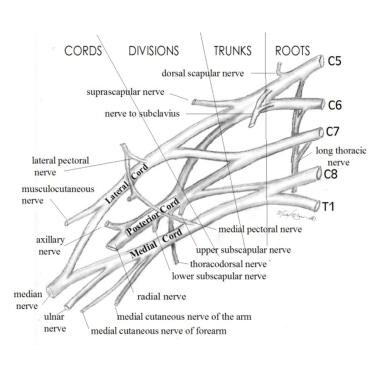
To understand the clinical presentation of brachial plexus palsy (BPP) and provide anticipatory guidance for families affected by the condition, the clinician must first know basic anatomy. As seen in the image below, the brachial plexus consists of nerves (the ventral rami) from C5-T1.
C5 and C6 join to form the upper trunk, C7 travels alone as the middle trunk, and C8-T1 join as the lower trunk. Each trunk divides into anterior and posterior divisions to create the cords, which then subdivide further into branches that supply the muscles of the arm. Injuries of the brachial plexus may be mild, with only temporary sequelae, or devastating, leaving the child with a flaccid, insensate arm.
Severity depends on the number of nerves involved and the degree to which each level is injured. The basic types of BPPs include the following:
-
Erb palsy affects nerves arising from C5 and C6.
-
Upper-middle trunk BPP involves nerve fibers from C5, C6, and C7 levels.
-
Klumpke palsy results in deficits at levels C8 and T1, although many clinicians agree that pure C8-T1 injuries do not occur in infants and may be indicative of spinal cord injury (SCI).
-
Total BPP affects nerves at all levels (C5-T1).
-
Bilateral BPP demonstrates bilateral involvement.
When defining the severity of a peripheral nerve injury, differentiation between neurapraxic, axonotmetic, and neurotmetic lesions is helpful.
-
Purely neurapraxic lesions do not affect the axon itself. These lesions generally are reversible and do not leave sequelae.
-
Axonotmetic lesions involve disruption of the myelin sheath and the axon, leading to degeneration of the axon distal to the injury. The connective tissue across the lesion remains intact. These injuries improve gradually over 4-6 months, depending on the level of the lesion.
-
Neurotmetic lesions are the most severe, destroying not only the axon and myelin, but also the supporting structures across a nerve. As the proximal end of the nerve attempts to regenerate without this supportive connective tissue, a neuroma may develop. The extent of improvement in the patient's condition depends on the ultimate number of nerve fibers that reconnect distal to the neuroma. Muscle atrophy from a neurotmetic lesion begins 3-6 months after injury and by 1.5-2 years is irreversible.
Although the traditional mechanism of injury is lateral neck flexion, the upper rootlets (C5-C7) are 25% as likely to be avulsed as the lower roots (C7-T1). The upper roots (C5-C6), however, are far more likely to be ruptured (88%) because of the anatomy of the transverse processes and the degree of flexibility at that level.
The clinician must also distinguish neonatal BPP from traumatic BPP in older children and adults. The damage in neonates usually results from slow traction injuries, unlike the high-energy shearing type of trauma seen in older individuals. Not only are the latter injuries often more severe, but with similar injuries, infants show a better functional outcome.
This clinical observation is confirmed by Vredeveld and colleagues, who studied 14 infants and 19 adults with surgical evidence of complete avulsion of the C5-C6 roots or upper trunk. [5] Electromyography (EMG) showed normal recruitment of biceps and deltoid in the infants and complete denervation in the older individuals. When C7 also was torn, the infants demonstrated complete denervation. Vredeveld and coworkers attributed this observation to neonatal C7 innervation of the biceps and deltoid that subsequently was lost if the C5-C6 roots were functional.
Epidemiology
Frequency
United States
An incidence of 0.5-4.4 cases of brachial plexus palsy per 1000 full-term births has been reported; however, a review by Gilbert and colleagues reported an incidence of 0.8-1 case per 1000 births. [6]
International
Studies in France and Saudi Arabia have suggested an incidence of 1.09-1.19 cases of brachial plexus palsy per 1000 live births.
Mortality/Morbidity
The incidence of permanent impairment is 3-25%.
The rate of recovery in the first few weeks is a good indicator of final outcome. Complete recovery is unlikely if no improvement is noted in the first 2 weeks of life.
Race
Most studies have not found evidence to support a link between race and the risk of brachial plexus palsy. However, a 2007 study in New York City, by Weizsaeker and colleagues, found that being a member of the black population was independently predictive for Erb palsy. [7]
Sex
Eng and colleagues examined 191 infants with brachial plexus palsy. [8] Nearly half of them (49%) were male, and 51% were female.
Age
Neonatal brachial plexus palsy is noted at birth.
-
Brachial Plexus. Image courtesy of Michael Brown, MD.
-
Mallet classification.