Practice Essentials
Spinal cord injury (SCI) can exacerbate the physical and physiologic declines—including in the musculoskeletal, cardiovascular, gastrointestinal (GI), pulmonary, and integumentary systems—brought on by the aging process. A number of long-term follow-up studies and many authors have documented the tendency for individuals with SCI to age faster than the able-bodied population does. That is to say, individuals with SCI develop characteristics and medical problems commonly associated with the aging process at a much younger age. [1]
Cushman and Hassett, for example, evaluated people with SCI of 15 years or more and found that 93% had experienced a decline in functional status. [2]
A study by Smith et al found that in adults with long-term disability—specifically, SCI, multiple sclerosis, muscular dystrophy, or post-polio syndrome—the development of chronic comorbid medical conditions is associated with factors such as body mass index, waist circumference, and the existence of another chronic comorbidity. [3]
Physiologic changes
Osteoporosis occurs uniformly in individuals with SCI, with bone loss beginning immediately after the injury. [4] Garland and colleagues described a reduction of bone mineralization as high as 22% in the first 3 months postinjury. [5] It has been demonstrated that there is a continual linear loss of bone that is a function of the time that has passed since the occurrence of SCI. [6]
Soft-tissue changes occur as a result of aging with SCI. The areas over weight-bearing surfaces (the buttocks) experience a loss or thinning of the subcutaneous tissues. This results in thinning of the skin and a loss of elasticity. These effects result in skin that is subject to breakdown and that has more difficulty healing once a pressure sore or ulcer has developed. The rate of decubitus ulcers increases with time after the onset of SCI.
Kocina reported that the incidence of cardiovascular disease among individuals with spinal cord injury (SCI) is over 200% higher than the expected incidence in an age- and gender-matched control population. [7] In individuals surviving 30 years or longer following SCI, nearly 50% of all deaths occur due to premature cardiovascular disease. [8]
Problems associated with gastrointestinal (GI) tract dysfunction increase with age after SCI. Seventy-four percent of patients with SCI develop hemorrhoids, 43% develop abdominal distention, 43% experience autonomic dysreflexia related to the GI tract, and 20% develop difficulty with bowel evacuation.
Declining pulmonary function can result from restrictive disease, obstructive disease, or a combination of these. In SCI, restrictive lung disease occurs as a result of respiratory muscle paralysis. The development of kyphosis, scoliosis, or increasing spasticity can cause further restrictive disease as the individual with SCI ages.
During the first year following the onset of SCI, approximately 15% of patients develop a decubitus ulcer. The rate of decubiti formation increases to 30% by 20 years post-SCI.
Numerous endocrine changes have been described in patients with SCI. The rate of diabetes has been reported to be four times higher in SCI patients than in the general population.
Functional reductions
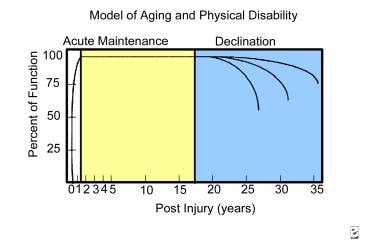
Menter and Hudson developed a model of aging that predicts functional decline according to the duration of SCI. [9] This model consists of the following three phases:
-
Acute restoration phase - The individual goes from having very little functional capability to regaining the maximum amount of functional return during rehabilitation
-
Maintenance phase - In this phase, the individual with SCI can enjoy a relatively stable level of function
-
Decline phase - Results from the degenerative effects of overuse syndromes and the physiologic aging process
Characteristics of Aging
The characteristics of aging have been well defined through the years. Menter and Hudson describe normal aging as involving 3 processes, all overlapping but distinctly different. [9] These processes are (1) the physiologic changes of the body itself, (2) the individual's changing social roles, and (3) self-realization. This article focuses primarily on the physiologic changes in aging.
As individuals age, they experience a variety of new and unanticipated issues. These may include medical, functional, socioeconomic, and support problems. Individuals experience changes and functional declines in all body systems as they age. The rates of decline vary from individual to individual, depending on genetics, body habitus, lifestyle, and general state of health.
Williams and Hadler demonstrated that there is variation in the rate of decline for the different organ systems in the body. [10] A report by Katzman did as well, illustrating the differing rates of decline between the pulmonary system and the peripheral nervous system; the study demonstrated a 60% decline in pulmonary function by age 80 years, as compared with a 15% decline in nerve conduction velocity.
Musculoskeletal changes
In the musculoskeletal system, a loss of calcium eventually leads to osteoporosis. Muscles lose strength and coordination, coordination and balance deteriorate, and joint capsules tighten, lose flexibility, and develop contractures. Lean muscle mass is lost and is replaced with adipose tissue. Eventually, a slender athletic build gives way to one that is overweight, with individuals developing a protruding abdomen and a somewhat stooped posture.
Clark and Siebens summarized musculoskeletal changes as consisting of a decrease in muscle mass, muscle fiber size, and the number of myofibrils, along with a reduced concentration of mitochondrial enzymes. [11] These changes occur regardless of an individual's level of activity. Muscle strength declines 20-30% after age 60, and maximum power output (the work rate) declines 45% after the fifth decade. Degenerative joint changes in weight-bearing joints essentially are a universal occurrence by age 60.
Neurologic changes
The central and peripheral nervous systems also experience decline. Starting at age 24 years, neurons in the central nervous system are lost in a slow, steady decay process. Clark and Siebens also reported normal aging to include decreases in short-term memory, a loss of speed and of motor activities, and a slowing in the rate of central information processing. The peripheral nervous system experiences a decline in the connections and speed with which it can conduct messages. These declines result in decreased balance in strength, coordination, and agility.
Between the ages of 25 and 75 years, physiologic changes in the neurologic systems include a greater-than 60% reduction in vibratory sense in the lower extremities and a greater-than 20% reduction in simple reaction time.
Cardiovascular changes
The cardiovascular system loses capacity to pump blood through a decreasing stroke volume, as well as through the decreased ability to maintain vessel tone. Aging is associated with a progressive, gradual increase in systolic and diastolic blood pressures, likely related to a loss of arterial elasticity. An increased incidence of orthostatic hypotension exists, as does a greater incidence of syncope syndromes associated with micturition.
Pulmonary changes
The pulmonary system experiences a decreased compliance or elasticity of the lung tissues, interfering with the lungs' ability to expand on inspiration. In addition, the chest wall loses its flexibility and muscle strength, leading to a restriction of pulmonary function. Vital capacity, maximum voluntary ventilation, expiratory flow rate, and forced expiratory ventilation all decline with aging.
Other systems
In the GI tract, transit time increases, resulting in incomplete absorption of some medications and overabsorption of others; excessive water reabsorption due to the prolonged transit time can lead to a dilated or enlarged colon, rectal fissures, and hemorrhoids. A decreased force and coordination of smooth muscle contraction in the colon is associated with aging.
Endocrine changes include a reduction of hormones, such as human growth hormones and testosterone, responsible for the repair and maintenance of cellular tissues. Reduction in the levels or the effectiveness of insulin and insulinlike growth factor also takes place. In general, as one ages, the cellular-mediated and humeral-mediated immune response systems become less effective.
The integumentary system experiences a loss of subcutaneous supporting and adipose tissues, as well as thinning of the skin with loss of elasticity. Skin tears and bruising become much more common with age.
The renal system loses functional units or glomeruli, which can result in some renal insufficiency. Incontinence is not considered a normal part of the aging process.
Effects of Aging on Activities of Daily Living
Fortunately, all of the body's organ systems have a large functional reserve capacity. For most individuals, the natural decline in these body systems is only a minor nuisance or inconvenience until late in life.
Clark and Siebans report that at age 65 years, men can expect greater than 80% of their remaining life expectancy to be free of disabilities. [11] Additionally, the percentage of adults aged 75-84 years who require assistance with activities of daily living (ADL) is approximately 11%; only 5% of elderly individuals are expected to live in an institution at some point. For persons older than 85 years, only 23% require assistance with some ADL. [12]
In 1999, Kane and colleagues showed that at age 85 years, 55-60% of persons may require assistance with instrumental ADL (IADL). [13]
These levels of independence in ADL are in marked contrast to the level of independence of the average person with spinal cord injury (SCI). The individual with SCI typically is young at the time of injury and, as a result of the SCI, experiences an immediate reduction of some of the functional reserves and capacities that were present. As a result, he/she often requires assistance with ADL.
In other body systems, the patient with SCI experiences a more rapid decline, consequently assuming at an unusually early age the above-described characteristics associated with aging. [14]
Musculoskeletal Changes in Spinal Cord Injury
Upper extremity pain is a common problem associated with spinal cord injury (SCI) and is most often due to either peripheral nerve entrapments or overuse syndromes. Subbarao and colleagues reported that more than 70% of individuals with chronic SCI report pain in their upper extremities. [15] Most of these individuals require some sort of treatment for the pain or modification of their activities. The pain has been reported to increase with time after the onset of the injury.
Alijure and coauthors reported that the incidence of peripheral nerve entrapment increases with the number of years following the onset of SCI, [16] and Davidoff and colleagues noted that nearly two thirds of individuals with SCI have compressive neuropathies in the upper extremities, with greater than 50% having median neuropathy and almost 25% having neuropathy bilaterally in the upper extremities. [17]
According to Kirshblum and coauthors, the most common overuse syndromes causing pain in the upper extremities are degenerative joint diseases, rotator cuff tears, rotator cuff tendinitis, subacromial bursitis, and capsulitis. [18] Other musculoskeletal complications include fractures of the lower extremities, which reportedly occur in as many as 6% of individuals, with the most common fracture occurring at the femur. As many as 10% of these fractures result in nonunion.
Nearly all pediatric patients with SCI experience scoliosis (97%), and just under half of all adults with SCI develop the condition.
Osteoporosis occurs uniformly in individuals with SCI, with bone loss beginning immediately after the injury. [4] Garland and colleagues described a reduction of bone mineralization as high as 22% in the first 3 months postinjury. [5]
It has been demonstrated that there is a continual linear loss of bone that is a function of the time that has passed since the occurrence of SCI. [6] This research was conducted on sets of identical twins in which one twin had an SCI and the other was able-bodied.
Individuals with Brown-Séquard–type SCI have been shown to have greater bone loss in the paretic extremity than in the stronger extremity. People with SCI reach the fracture threshold levels of osteoporosis in the proximal femur within 1-9 years postonset of SCI. [19]
Soft-tissue changes occur as a result of aging with SCI. The areas over weight-bearing surfaces (the buttocks) experience a loss or thinning of the subcutaneous tissues. This results in thinning of the skin and a loss of elasticity. These effects result in skin that is subject to breakdown and that has more difficulty healing once a pressure sore or ulcer has developed. The rate of decubitus ulcers increases with time after the onset of SCI.
In addition, Rossier and colleagues, as well as other authors, have described a loss of lean muscle mass that occurs with time. [20]
Bauman and coauthors also have described body composition changes that can be summarized as a loss of lean muscle mass and an increasing percentage of body adipose tissue that occurs as a function of time. [6, 21, 22] Most individuals with SCI experience weight gain that increases with time and that eventually results in a level of obesity that puts significant limitations on the patients' functional capabilities.
Cardiovascular Changes in Spinal Cord Injury
Kocina reported that the incidence of cardiovascular disease among individuals with spinal cord injury (SCI) is over 200% higher than the expected incidence in an age- and gender-matched control population. [7] In individuals surviving 30 years or longer following SCI, nearly 50% of all deaths occur due to premature cardiovascular disease. [8]
A report by Peterson et al found that among the study's subjects, the 5-year incidence of cardiometabolic morbidity was 56.2% in adults with traumatic SCI, compared with 36.4% in adults without SCI. In addition, the hazard ratio for heart failure in the former group was 3.55. [23]
Hypertension is nearly twice as common in individuals with paraplegia as it is in able-bodied controls. Moreover, while approximately 10% of the US population have high-density lipoprotein cholesterol values of less than 35 mg/dL, 24-40% of persons with SCI have levels below this value. [22]
Because of an ineffective distribution of oxygenated blood, individuals with SCI have reduced exercise tolerance. Venous return to the heart is limited because of a decrease in sympathetic tone and a reduction in the muscular pumping action of the lower extremities, leading to pooling.
Homocystine levels have been reported to be elevated in men with SCI, and the levels increase with the duration of SCI. In addition, levels of prostacyclin receptor antibody increase with the duration of SCI; this, in turn, can result in platelet aggregation.
In a large cross-sectional survey of men aged 65 years and older, researchers compared veterans who have spinal cord injury (SCI) (n=794) with veterans (n=13,528) and the general population (n=6105) to determine how often cardiovascular and metabolic conditions appeared in these groups. Diabetes and myocardial infarction were prevalent in older men, and similarly in men with SCI. The odds for coronary heart disease were much lower for veterans with SCI than both comparison groups, while cases of stroke were more widespread in those with SCI. [24]
Gastrointestinal Changes in Spinal Cord Injury
GI complications in acute or chronic spinal cord injury (SCI) are numerous. Following chronic SCI, colonic compliance and motility decrease. Electromyographic studies have shown that basal colonic myoelectrical activity is higher in persons with SCI than it is in controls.
Problems associated with GI tract dysfunction increase with age after SCI. Seventy-four percent of patients with SCI develop hemorrhoids, 43% develop abdominal distention, 43% experience autonomic dysreflexia related to the GI tract, and 20% develop difficulty with bowel evacuation. Following the onset of injury, the prevalence of GI complaints increases with time. Sixty percent of tetraplegic patients require assistance with managing their bowel routine, as do approximately 16% of paraplegic patients.
The functional ability to manage neurogenic bowel incontinence can change as the individual ages or gains weight. The frequency of GI complications and problems is greatest in individuals aged 60 years or older or whose injuries are at least 30 years old. Frisbie and colleagues reported a higher-than-expected incidence of colorectal cancer in these patients. [25] A greater frequency of bowel dysfunction is reported in individuals with SCI whose injuries are at least 5 years old.
Pulmonary and Integumentary Changes in Spinal Cord Injury
Pulmonary
Declining pulmonary function can result from restrictive disease, obstructive disease, or a combination of these. In spinal cord injury (SCI), restrictive lung disease occurs as a result of respiratory muscle paralysis. The higher the level of SCI, the greater the restrictive impairment.
Dicpinigaitis and coauthors described an obstructive airway disorder that can best be called hyperactive airway disease, in individuals whose SCI occurs at T6 and above. [26]
The development of kyphosis, scoliosis, or increasing spasticity can cause further restrictive disease as the individual with SCI ages.
Sleep apnea reportedly occurs in 40% of individuals with SCI. The frequency of obstructive sleep apnea increases with age. Of those individuals with SCI who experience sleep apnea, only 25% have been found to be obese. The long-term use of baclofen may be associated with the development of obstructive sleep apnea.
Integumentary
During the first year following the onset of SCI, approximately 15% of patients develop a decubitus ulcer. The rate of decubiti formation increases to 30% by 20 years post-SCI. Sitting tolerance decreases as the person becomes more susceptible to this complication. [22]
Endocrine Changes in Spinal Cord Injury
Numerous endocrine changes have been described in patients with spinal cord injury (SCI). The rate of diabetes has been reported to be 4 times higher in SCI patients than in the general population. After glucose tolerance testing in SCI patients, 22% were found to have diabetes, with an additional 34% having impaired glucose tolerance.
Bauman demonstrated abnormally low levels of human growth hormone and testosterone in individuals with SCI. [21] Low levels of these hormones can result in a reduced capacity for cellular repair and can lead to a reduced capacity for maintaining lean muscle mass and strength. In addition, low levels of these hormones can prolong healing and soft-tissue repair following injuries.
Another study by Bauman et al indicated that age-related reductions in testosterone levels are greater in men with SCI than in able-bodied males, with low total serum testosterone occurring at an earlier age in men with SCI and with age-related decreases progressing at a faster rate. The study involved 243 men with chronic SCI, aged 21-78 years, 46% of whom were found to have low total serum testosterone levels. The decade-by-decade prevalence rate of low total serum testosterone between the third and eighth decade of life in men with SCI was as follows: 15%, 39%, 50%, 53%, 58%, and 57%. These prevalences were higher than those reported for able-bodied men. Moreover, men with SCI had an annual age-related decline in levels of total serum testosterone of 0.6%, compared with 0.4% for the general male population. [27]
Functional Decline
As previously mentioned, a number of long-term follow-up studies and many authors have documented the tendency for individuals with spinal cord injury (SCI) to age faster than does the able-bodied population. That is to say, individuals with SCI develop characteristics and medical problems commonly associated with the aging process at a much younger age. Some authors have provided assistance in helping to determine when these changes may occur.
Menter and Hudson developed a model of aging that predicts functional decline—as a result of the many changes that have been discussed—according to the duration of SCI. [9] This model consists of the following 3 phases:
-
Acute restoration phase
-
Maintenance phase
-
Decline phase
The acute restoration phase occurs immediately following SCI, with the individual going from having very little functional capability to regaining the maximum amount of functional return during rehabilitation. This phase is followed by the maintenance phase, which varies in length of time; in this phase, the individual with SCI can enjoy a relatively stable level of function. This period is followed by a predictable functional decline, which results from the degenerative effects of overuse syndromes and the physiologic aging process (see graph below).
A number of studies have validated the occurrence of this functional decline, which may begin as early as 10-15 years postinjury or as late as 20 years postinjury, depending on the patient’s particular set of circumstances. As noted before, Cushman and Hassett evaluated people whose SCI was at least 15 years old and found that 93% had experienced a decline in functional status by the time of the evaluation.
One important thing to keep in mind is that not everyone who has SCI ages in the same way or at the same rate. A large number of factors are important to consider, including genetics, lifestyle, level of injury, age, weight, health history, level of supportive care, level of SCI, and comorbidities.
The age of the individual at the onset of SCI is an important consideration in estimating the duration of SCI before functional decline begins. Kempt and his colleagues at Rancho Los Amigos developed a model for this factor. [28] Younger individuals whose age at SCI onset is during or prior to adolescence may enjoy a maintenance phase of 20 years prior to experiencing functional decline, whereas individuals who are aged 55 years at the onset of their SCI may only have 5-7 years of relatively stable functioning status prior to experiencing a decline.
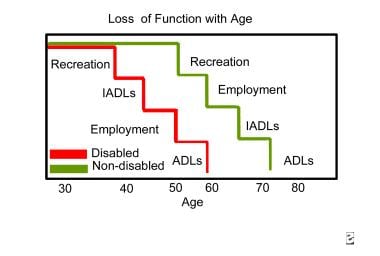
Thomlinson described functional declines in major life areas, such as recreation, independent ADL, and employment, in the disabled population, as compared with the general population. [29] In each of these major life areas, the functional decline was experienced at a much earlier age in the disabled population than in the nondisabled population.
Health care professionals providing care to individuals with SCI need to be aware of the effects of age-related changes on their patients. They must implement services to limit their patients' functional decline to the greatest extent possible or else must provide services or equipment to these individuals in a proactive way to replace lost function or to limit the effects of the functional decline. [30]
Further discussions on these issues can be found in A Guide to Rehabilitation, [31] Life Care Planning and Case Management Handbook, [32] and Spinal Cord Injury Desk Reference: Guidelines for Life Care Planning and Case Management. [33]
Activities of Daily Living
A study by Liem and colleagues noted that with each passing decade, the odds that a person with spinal cord injury (SCI) will need more assistance with ADL increases 42%. [34] The same study demonstrated that in a group of people who were 20 years post-SCI, 33% reported needing more assistance with ADL at 3-year follow-up. The ADL most affected were bathing, transfers, and dressing.
Another researcher found that the IADL with which people with SCI needed increased help included household chores, shopping, and meal preparation. The most commonly cited reasons for needing more assistance with ADL or IADL are greater weakness, increased pain, and increased weight. In addition, the cumulative changes brought on by the number of years post-SCI had a greater effect on the need for assistance with ADL than did an individual's chronologic age. The graph below depicts loss of function with age.
-
Model of aging and physical disability.
-
Loss of function with age. ADL means activities of daily living; IADL means instrumental activities of daily living.
Tables
What would you like to print?
- Practice Essentials
- Characteristics of Aging
- Effects of Aging on Activities of Daily Living
- Musculoskeletal Changes in Spinal Cord Injury
- Cardiovascular Changes in Spinal Cord Injury
- Gastrointestinal Changes in Spinal Cord Injury
- Pulmonary and Integumentary Changes in Spinal Cord Injury
- Endocrine Changes in Spinal Cord Injury
- Functional Decline
- Activities of Daily Living
- Show All
- Media Gallery
- References