Overview
Electrodiagnosis is the field of study that, by employing the science of electrophysiology, uses electrical technology to study human neurophysiology. Neurodiagnostics (NDS), electromyography (EMG), and evoked potentials (EPs) are aspects of electrodiagnosis.
Information needed to answer any questions regarding nerve injury, muscle injury, muscle disease, localization, and prognosis can be obtained through electrodiagnostic testing. This information should help to focus treatment on the exact site of injury.
Interestingly, the information provided by electrodiagnosis is functional and not static, telling the practitioner how the nerve and muscle are functioning. In contrast, a magnetic resonance imaging (MRI) study is a static test that simply provides a picture of anatomy. A relatively benign appearing MRI scan can be obtained from a patient with significant pain. Conversely, MRI scans reveal disk protrusions and herniations in many people who are asymptomatic. Therefore, electrodiagnostic testing can be an important adjunct in diagnosis. [1, 2, 3]
Various specialists perform electrodiagnostic studies. Understanding and performing electrodiagnostic tests is a requirement in the specialty training of physical medicine and rehabilitation (physiatry) residents and is considered an elective in the training of neurology and anesthesia residents.
The American Board of Electrodiagnostics was developed to provide additional certification for physicians who perform electrodiagnostic tests, and this certification is considered the criterion standard for specialty training. The American Board of Neurology also has a subspecialty certification for neurologists with specialty training in electrodiagnosis. [4]
Neurophysiology
Every practitioner should be well aware of the normal neurophysiologic function of the nervous system. Electrical signals are generated in the brain, pass through the spinal cord, and travel into the peripheral nervous system. These signals are carried down the nerve to the synaptic cleft, where a chemical release of acetylcholine crosses the synaptic cleft to create an electrical discharge in the muscle. This electrical signal causes the muscle to contract.
Neurodiagnostic testing bypasses the brain by delivering an electrical charge to the patient. The equipment then is used to measure several aspects of the body's response to that signal to determine whether it is functioning properly. Location and degree of the injury, acuity, prognosis, and (in some cases) specific diagnosis can be determined through electrodiagnostic testing. [5, 6]
Electromyography
EMG was the first electrodiagnostic test to be developed. This procedure involves the placement of a needle into various muscles to record different stages of muscle activity, including rest, minimal contraction, and maximal activity. At rest, normal muscle is electrically silent. Damaged muscle tissue may result in spontaneous depolarization of individual muscle fibers. This abnormal activity can be detected during the needle examination portion of the electrodiagnostic examination.
Primary nerve injuries that are severe enough to create neurotmetic or axonotmetic lesions, which result in Wallerian degeneration of the nerve, demonstrate fibrillation and positive sharp-wave discharges at rest. Fibrillations and positive sharp waves are pathognomonic for nerve injuries. Primary muscle disorders can also be detected by EMG.
Muscle fiber recruitment patterns
With minimal volitional activity, the recruitment patterns of muscle are normal. An individual muscle fiber begins to fire, reaching a threshold of 15-20 Hz, at which point the original fiber recruits a second fiber, which fires up to 15-20 Hz, and so on until there is full recruitment of the muscle.
Determination of recruitment patterns is difficult and requires more modern, computerized equipment; however, recruitment patterns provide critical information for determining the degree of injury and prognosis. Furthermore, this information is helpful in identifying individuals who are malingering. A patient may not be fully cooperative in manual muscle testing for weakness. Determination of the recruitment pattern, however, is not dependent on patient cooperation.
A study by Impastato et al indicated that in patients with traumatic brachial plexus injuries, needle EMG results are prognostic for spontaneous motor recovery. With manual muscle testing for strength conducted at least 1 year postinjury, the investigators found that patients in whom voluntary motor unit potential recruitment was absent at 1-9 months had a poor prognosis for spontaneous recovery. Moreover, as measured on the Medical Research Council scale at mean 1.4-year postinjury follow-up, a strength score of over 3/5 was reached in only 25% of muscles with discrete or severely reduced recruitment. [7]
Testing considerations
One problem with electromyographic testing is that needles are used and the study may be painful; however, new computerized technology allows the use of recording needles so small that insertion feels more like a pinch than like the insertion of a needle. Small-gauge needles can be used, because nothing is injected or aspirated. Seek out electromyographers who have access to current technology and who have board certification, in order to ensure that patients do not suffer more discomfort than is necessary during a procedure.
A current worrisome trend is the performance of electromyographic testing by nonphysician health care personnel. The performance of electromyography and the interpretation of electromyograms require technical skill and the ability to integrate the physician's knowledge base of disease pathology with diagnostic acumen. [8]
Nerve Conduction Studies
Nerve conduction studies (NCSs) are an important part of the complete electrodiagnostic exam. [9] In an NCS, an electrical charge is delivered to a peripheral nerve. That charge is carried down the nerve and generates a muscle contraction. A recording electrode is placed on a muscle innervated by that nerve, and information about the impulse can be recorded, including its latency (the time needed for the impulse to travel from stimulus to recording). The distance traveled and the nerve conduction velocity (NCV) can also be computed.
These measures are a sensitive indicator of nerve damage and look specifically at the integrity of the myelination of the nerve. The needle examination and NCSs are key components of a complete electromyographic exam. [10, 11]
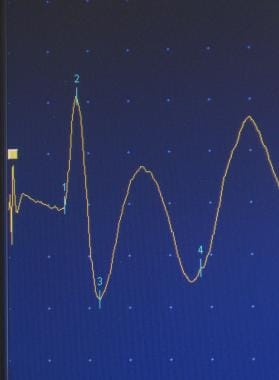
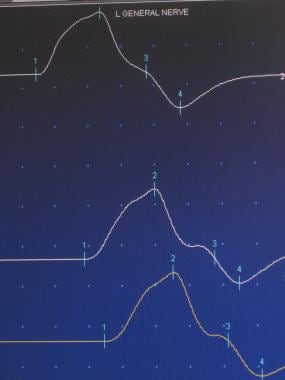
The amplitude of the muscle contraction can be determined and compared to the initial size of the signal, providing information about the number of neurons that are functioning within the nerve. By stimulating the nerve in various places along its course, the practitioner can isolate a specific site of injury. Sensory (first image below) and motor (second image below) nerves may be tested.
In a routine carpal tunnel study, for example, conductions of the median nerve above and below the tunnel may be normal, while conduction across the carpal tunnel itself may be impaired. [12, 13, 14] This test isolates the injury to this specific location. Changes in amplitude often signal an axonal injury.
A multitude of entrapments and injuries can be identified in this manner. In the upper extremity, some of these disorders include brachial plexopathies, ulnar entrapments at the cubital and Guyon tunnels, median entrapments at the carpal tunnel, and radial injuries at the spiral groove in the humerus. [15] In the lower extremity, the most common entrapments include the sciatic nerve at the piriformis, the peroneal nerve at the fibular head, and the plantar nerve at the tarsal tunnel. [16]
Peripheral neuropathies also can be identified and monitored objectively. The type of nerve injury (demyelinating, axonal, or mixed) can aid in narrowing the differential diagnosis. [17] In a study of patients with diabetic peripheral neuropathy, Park and Won reported that NCS variables differ in association with phenotype (normoesthesia, hyperesthesia, or hypoesthesia/anesthesia), as assessed using current perception threshold. The investigators concluded that, while NCSs are an important means of evaluating peripheral nerve abnormalities, their usefulness in the assessment of clinical phenotypes is limited. [18]
With knowledge of neuroanatomy and with measurements from a sample of nerves and muscles from various nerve roots, the clinician can use NCSs to determine where a lesion would need to be located in order to affect those combinations of findings in the extremity that suggest the presence of abnormalities. For example, in a medial cord brachial plexus injury, the ulnar and median nerves are involved along their entire length, with sparing of C5-C6 muscles and the paraspinal muscles. A C7-C8 root injury also affects the median-innervated and ulnar-innervated muscles, but the paraspinals are involved as well. [19, 20]
Remember that a direct relationship exists between nerve diameter and conduction velocity. Larger myelinated fibers (Ia) are the ones measured in NCSs. The smaller fibers (Ib, II, III) are not recorded with standard nerve conduction techniques. Therefore, a small fiber neuropathy may not be detectable by EMG.
Moreover, a prospective study by Fowler et al indicated that NCSs have a higher false-positive rate than does ultrasonography in the diagnosis of carpal tunnel syndrome in asymptomatic patients. Assessing hands with a Carpal Tunnel Syndrome 6 (CTS-6) scale score of 0, the investigators found positive NCS test results in 42.5% of hands, compared with 22.5% in ultrasonographic evaluation. [21]
Acute injury
A frequent misconception is that electrodiagnostic testing is not useful in an acute injury. Immediately following complete nerve transection, a complete loss of motor unit recruitment is noted distally (neurotmesis). Even with incomplete nerve injuries (neurapraxia and axonotmesis), decreased motor unit action potential recruitment patterns may be seen acutely.
Within the first 2-3 days, a decrease in motor amplitudes proximally may be noted. Sensory amplitude reductions are noted toward the end of the first week following injury. Slowing of conduction velocity across the site of injury suggests a demyelinating injury. Membrane instability can be detected by needle examination in 1-4 weeks, with proximal muscles showing up the soonest (due to Wallerian degeneration). At 2-6 months, axonal sprouting occurs, which results in increased motor unit action-potential amplitude and prolonged duration.
In a traumatic nerve injury or acute neuropathic process, serial EMG is often performed to assess disease progression or response to treatment, as well as to aid in prognosis.
Evoked Potentials
EPs are electrical signals generated by the nervous system in response to sensory stimuli. EP testing is the application of electrodiagnostic testing to the central nervous system (CNS). [22, 23] These tests are a clinically useful means to do the following:
-
Demonstrate abnormal sensory function when the neurologic examination results do not reveal abnormalities
-
Reveal clinically unsuspected pathology when demyelinating diseases are suggested [24]
-
Determine the anatomic distribution of a disease process
-
Objectively monitor a patient's progress or deterioration over time.
Tests include those on the following EPs:
-
Somatosensory EPs (SSEPs)
-
Visual EPs (VEPs)
-
Brainstem auditory EPs (BSAEPs)
-
Dermatomal EPs
-
Myotomal EPs
Recording electrodes may be either surface electrodes or small subdermal needles placed anywhere along the neuropathway, including the spine and scalp.
SSEPs
SSEP studies are the most widely used EP tests. Stimulation occurs at the extremity, and recordings are made on the scalp, near the sensory cortex. This technique may be used to locate the level of the injury in the nerve root, spinal cord, or brain. In CNS insults, such as spinal cord trauma and stroke, SSEP testing has been helpful in establishing the degree of insult and in determining its prognosis.
SSEPs can also be used to localize demyelinating diseases, such as multiple sclerosis, and root level injuries in cervical and lumbar radiculopathies. [24]
A retrospective study by Devic et al indicated that SSEPs can aid in the diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) when nerve conduction studies (NCSs) fail to reveal peripheral demyelination in patients with other signs of the disease. The study involved 26 patients with clinical signs of CIDP, with SSEPs in 22 of the patients (85%) demonstrating abnormal proximal conduction in sensory fibers; this included 16 of 20 patients (80%) who responded to immunotherapy, considered to be confirmation of CIDP. [25]
In addition, SSEPs can be used to determine the level of coma and to evaluate for brain death. [26] SSEPs are also useful for intraoperative monitoring of patients undergoing neurosurgical procedures.
In 2018, the International Society of Intraoperative Neurophysiology published recommendations regarding the intraoperative monitoring of SSEPs. These included the recommendation that propofol and opioid total intravenous anesthesia be used during surgery to avoid the dose-dependent suppression of polysynaptic cortical SSEPs found with inhalation anesthetics such as nitrous oxide and to facilitate a higher signal-to-noise ratio. The recommendations also specified the use of an adaptive warning criterion consisting of “visually obvious amplitude reduction from recent pre-change values” that clearly exceeds variability, “particularly when abrupt and focal.” [27]
VEPs
In VEP tests, [28] the practitioner uses a photoelectric, checkerboard-pattern flash to stimulate the optic nerve. This pattern is then recorded on the cortex, arriving at the occiput, near the visual centers. The pattern usually takes 100 milliseconds to arrive and is referred to as the P100. Injuries along the optic nerve, including demyelination, result in a delay of the latency and loss of the amplitude of the signal, similar to the results in NCVs.
A P100 at greater than 102 milliseconds is considered beyond the reference range in most laboratories. In cases of multiple sclerosis, for example, abnormalities in the VEP are often the first indicator of the disease process. Optic chiasm tumors also produce recordings suggesting abnormalities. Visual acuity may be determined in infants with suspected visual disturbances.
BSAEPs
With BSAEPs, the clinician uses an auditory click to stimulate the cochlear nerve. The response is then recorded over the cortex. Because of the multiple signal generators in the brainstem, injuries along this nerve can suggest localization of the lesion to various sites along the nerve route. Five basic waveforms are recorded—for the cochlear nerve, the cochlear nucleus, the superior olivary complex, the midpons, and the upper pons—and interwave times may indicate pathology at a waveform’s anatomic location. BSAEPs may be used to help evaluate for brain death, demyelinating disease, and midbrain tumors.
Dermatomal and myotomal EPs
Dermatomal and myotomal EP studies are much more technically difficult to perform and should not be considered routine procedures. Stimulation occurs along the dermatome or myotome, with recordings at the cortex. These tests have demonstrated greater specificity than have SSEPs and are used frequently in clinical research.
EPs in surgery
Because EPs are conducted along the spinal cord, their use has been expanded to the operating room, where they provide valuable information for the spinal surgeon. [29, 30, 31, 32]
In scoliosis surgery, for example, a 0.5% incidence of spinal cord injury has been recorded nationally during the surgery. Spinal cord injury requires that the patient be reanesthetized and the spinal hardware removed. Unfortunately, the damage usually is permanent. With intraoperative use of SSEPs, this risk can be reduced 100-fold. While the patient is asleep and the surgery is progressing, the electromyographer performs repeated SSEP studies. If something happens that interrupts the signal, the electromyographer can inform the surgeon immediately, and ideally the problem can be corrected before permanent injury occurs. [33]
A study by Matsuoka et al indicated that in cervical spine surgery, false-positive results from intraoperative monitoring of muscle motor evoked potentials (EPs) can be reduced by using alarm criteria for both facial motor EPs and muscle motor EPs rather than just the criterion for muscle motor EPs alone. The investigators found that a false positive rate of 6.3% occurred when the facial and muscle alarm criteria were considered together, versus 21.5% when the muscle criterion alone was used. [34]
Cost
No discussion of electrodiagnosis would be complete without the consideration of cost. As in most technologic procedures, the cost of the equipment and the necessity for specialty training elevate the cost of performing electrodiagnostic testing. In the 1990s, routine studies cost $700-$1000. Like most other procedures, electrodiagnostic studies have been reduced in cost allowable by health care reform. Medicare allowable rates now limit payments generally to $200-$300, depending on the extensiveness of the study.
The real question, however, is whether the information gathered through electrodiagnosis changes the way the patient is treated. Does the test make the treatment more effective? In many practices, there is no question that it does. By localizing the lesion and determining the degree of injury, the clinician can objectively identify the etiology and focus efforts on the actual anatomic problem.
Questions & Answers
Overview
What is the neurophysiology of electrodiagnosis?
What is the role of electromyography (EMG) testing in electrodiagnosis?
What are the disadvantages of EMG testing in electrodiagnosis?
What is the role of nerve conduction studies (NCSs) in electrodiagnosis?
What is the role electrodiagnostic testing in an acute injury?
What is evoked potential (EP) testing in electrodiagnosis?
What are the types of evoked potential (EP) tests in electrodiagnosis?
What is the role of somatosensory evoked potential (SSEP) studies in electrodiagnosis?
What is the role of visual evoked potential (VEP) tests in electrodiagnosis?
What is the role of brainstem auditory evoked potential (BSAEP) tests in electrodiagnosis?
What is the role of dermatomal and myotomal evoked potentials (EPs) in electrodiagnosis?
What is the role of evoked potentials (EPs) during surgery?
What is the cost of electrodiagnostic testing?
-
Sensory nerve action potential.
-
Motor nerve action potential.