Practice Essentials
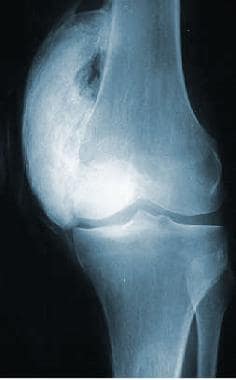
Heterotopic ossification (HO) following spinal cord injury (SCI) was first described by Dejerine and Ceillier in 1918 as paraosteoarthropathy. The ossification process involves the formation of mature lamellar bone, which is indistinguishable from normal bone, in soft tissues surrounding paralyzed joints (see the following image). The bone is not connected to periosteum and becomes encapsulated as it matures.
The pathology is similar to that of fracture callus, except that bone forms in the connective tissue between the muscle planes (histologic findings in neurogenic HO are similar to those in healing fracture callus). HO is also seen after other neurologic insults, such as traumatic brain injury (TBI), stroke, Guillain-Barré syndrome (GBS), and after burn injuries and orthopedic procedures (eg, total hip replacement).
In experimental models of HO formation, ischemia and tissue expression of bone morphogenic proteins have been shown to play important roles. Bone morphogenic proteins likely act on mesenchymal stem cells present in tissue, activating the cells to differentiate into osteoblasts. [1]
The incidence of HO in SCI is between 16% and 53%, depending on the incidence reports from various institutions. Clinically significant HO develops in about 20% of patients with an SCI. [2, 3] Fortunately, only 3-5% of cases involve joint ankylosis. In SCI, HO always occurs below the level of the lesion, most commonly at the hip (70-97%). [3, 4] Other body segments, including the knee, elbow, shoulder, hand, and spine (in decreasing incidence), may also be involved.
There is no known race or sex predilection for neurogenic HO; however, the incidence of neurogenic HO after SCI is lower in pediatric patients than in adults, ranging from 3% to 10%. In addition, spontaneous resorption of the neurogenic HO is frequently seen in pediatric patients. [5]
The risk factors associated with the development of HO in patients with traumatic SCI has been studied in a case control study of 264 patients by Citak et al. According to this study, patients with spasticity, thoracic trauma, complete lesion, pneumonia, presence of tracheostomy, and urinary tract infection had a higher risk of developing HO. [6]
The following image depicts 3 common locations of HO in the hip.
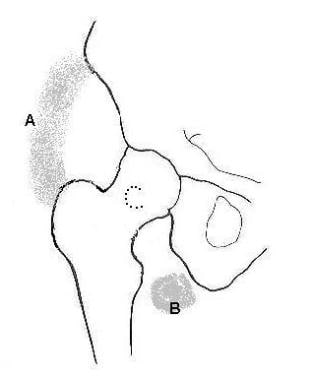 Three common locations of heterotopic ossification around the hip joint. A: Anterolateral/anteromedial location; B: Inferior and medial location; and C: Location around the femoral neck and posterior.
Three common locations of heterotopic ossification around the hip joint. A: Anterolateral/anteromedial location; B: Inferior and medial location; and C: Location around the femoral neck and posterior.
See also Spinal Cord Injuries, Autonomic Dysreflexia in Spinal Cord Injury, Functional Outcomes per level of Spinal Cord Injury, Hypercalcemia in Spinal Cord Injury, Osteoporosis and Spinal Cord Injury, Prevention of Thromboembolism in Spinal Cord Injury, Rehabilitation of Persons with Spinal Cord Injuries, and Spinal Cord Injury and Aging.
Signs and symptoms of heterotopic ossification
The adult patient with neurogenic HO gives a history of progressive loss of range of motion (ROM) accompanied by pain or swelling in the involved area. Localized erythema along with warmth and low- to moderate-grade fever may also be present.
Most pediatric patients present with decreased ROM but are less likely to have physical symptoms.
Workup in heterotopic ossification
Laboratory studies
Laboratory studies in the diagnosis of HO include the following:
-
Alkaline phosphatase - Serum alkaline phosphatase (SAP) level can be used to detect early onset of HO, because it is a marker of osteoblastic and osteogenic activity that increases with bone deposition [7]
-
Calcium - Serum calcium levels are transiently depressed in HO
-
Creatine kinase - Elevated serum creatine kinase may be associated with a more aggressive course of HO, as well as resistance to etidronate therapy [8]
Imaging studies
Imaging studies that can be used in the diagnosis and evaluation of HO include the following:
-
Radiography
-
Computed tomography (CT) scanning
-
Magnetic resonance imaging (MRI)
-
Ultrasonography
-
Nuclear imaging
Management of heterotopic ossification
Physical therapy
Physical therapists (PTs) work on ROM exercises, which are important in maintaining joint function. Once HO is identified, the ROM exercises should be withheld until the inflammatory signs (eg, warmth, erythema) have subsided.
Occupational therapy
The occupational therapist (OT) works on activities of daily living (ADLs) and functional transfers to compensate for lost ROM due to HO.
Pharmacotherapy
Bisphosphonates prevent the formation of hydroxyapatite crystals, while nonsteroidal anti-inflammatory drugs (NSAIDs) reduce the inflammatory process that precedes the development in HO of the collagenous bony matrix.
Surgical intervention
Orthopedic surgical consultation is recommended for patients with neurogenic HO who require surgical resection of the bone, such as those who have loss of function at a joint.
Surgery is also indicated in those patients with seating problems, skin breakdown, pain, or loss of function. [9]
Etiopathophysiology
Debate continues on whether there is migration of distant mesenchymal cells or transformation of existing mesenchymal cells into osteoblasts. Osteoinductive factors have been studied, including circulating biochemicals and local factors (eg, venous thrombosis, venous insufficiency, decubitus ulcers, edema, tissue hypoxia). None of these factors has been proven to play a pivotal role in neurogenic heterotopic ossification (HO).
Genetic predisposition for neurogenic HO has also not been confirmed.
Patients with limb spasticity have a greater risk of developing neurogenic HO, and patients with extensive amounts of neurogenic HO have severe spasticity.
The pathophysiology of HO involves an inflammatory process, with increased blood flow in soft tissue. There were significantly greater levels of osteoblast-stimulating factors present in the sera of SCI patients with HO. [10] Bone matrix is laid down and mineralized, and this sequence reaches completion in 6-18 months. As noted above, local, systemic, neural, and hormonal causes for the HO process have been hypothesized but have not been proven.
Clinical Features, Diagnosis, and Differential Diagnosis
See the image below.
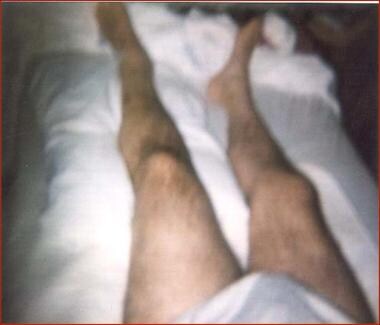 A 54-year-old man with T6 burst fracture due to motor cycle accident, with T6 American Spinal Injury Association (ASIA) Class A paraplegia and left hip dislocation developed left lower limb swelling 4 weeks after the injury. Workup revealed left hip heterotopic ossification.
A 54-year-old man with T6 burst fracture due to motor cycle accident, with T6 American Spinal Injury Association (ASIA) Class A paraplegia and left hip dislocation developed left lower limb swelling 4 weeks after the injury. Workup revealed left hip heterotopic ossification.
The adult patient with neurogenic heterotopic ossification (HO) gives a history of progressive loss of range of motion (ROM) accompanied by pain or swelling in the involved area. Localized erythema along with warmth and low- to moderate-grade fever may also be present. Most pediatric patients present with decreased ROM but are less likely to have physical symptoms.
The average length of time reported between spinal cord injury and diagnosis of neurogenic HO in the adult population is 6 months, which is in contrast to 14 months after injury in the pediatric population. The use of 3-phase bone scanning to detect HO may result in a shorter average reporting time between injury and diagnosis.
Four clinical stages of HO in people with spinal cord injury were described by Nicholas in 1973. [11] Please see the figure below.
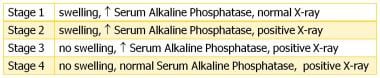 The 4 clinical stages of heterotopic ossification based on data from Nicholas. Ectopic bone formation in patients with spinal cord injury. Arch Phys Med Rehabil. Aug 1973;54(8):354-9.
The 4 clinical stages of heterotopic ossification based on data from Nicholas. Ectopic bone formation in patients with spinal cord injury. Arch Phys Med Rehabil. Aug 1973;54(8):354-9.
Also in 1973, Brooker et al described the classification system for HO around the hip following total hip arthroplasty. [12] See the figure below.
Neurogenic HO around the hip due to SCI occurs more often in the medial region than in the lateral region, with ossified tissues extending from the pubic symphysis to the anteromedial femoral shaft posterior to the femoral neurovascular structures. Ossification is also seen anteriorly involving the iliopsoas and femoral neurovascular structures, laterally within gluteus minimus, and posteriorly extending from the ilium to the posterior femur encasing the sciatic nerve.
Neurogenic HO around the hip due to SCI and TBI has been classified into 4 types by Mavrogenis et al. [13] See the figures below.
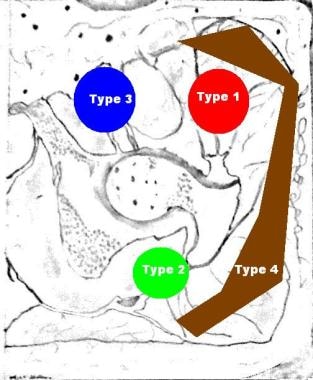 Neurogenic heterotopic ossification based on data from Mavrogenis et al. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. Jun 2012;13(2):69-78.
Neurogenic heterotopic ossification based on data from Mavrogenis et al. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. Jun 2012;13(2):69-78.
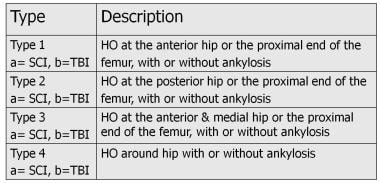 Neurogenic heterotopic ossification based on data from Mavrogenis et al. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. Jun 2012;13(2):69-78.
Neurogenic heterotopic ossification based on data from Mavrogenis et al. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. Jun 2012;13(2):69-78.
Neurogenic HO around the knee due to SCI may occur anteriorly, medially, and posteriorly. Anterior ossification occurs beneath the extensor mechanism, medial at about the medial collateral ligament, and posterior at about the hamstring insertion.
Differential diagnosis
Other conditions to consider when evaluating a patient with spinal cord injury and suspected HO include cellulitis, deep venous thrombosis (DVT), benign effusion, fracture, hematoma , and tumor.
Laboratory Evaluation
See the figure below.
Alkaline phosphatase
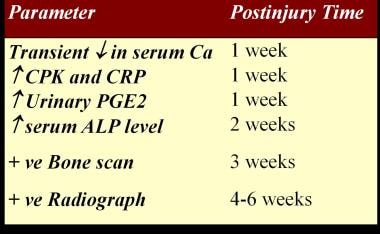
The serum alkaline phosphatase (SAP) level can be used to detect early onset of HO, because it is a marker of osteoblastic and osteogenic activity that increases with bone deposition. [7] With HO, the SAP level rises at 2 weeks, exceeds normal values at 3 weeks, peaks at 10 weeks, and then returns to normal after the HO is mature. However, SAP levels are nonspecific for this condition and levels are also elevated with trauma and fractures. Sensitivity of SAP is also questionable in the diagnosis of HO, and serial SAP levels may be normal in documented cases of HO. [14]
Calcium
Serum calcium levels are transiently depressed in HO.
C-reactive protein
A retrospective study of 37 patients with HO indicates that serum c-reactive protein (CRP) is a useful and more specific test than erythrocyte sedimentation rate (ESR) for monitoring the inflammatory activity of HO after SCI. [15, 16]
Creatine kinase
Elevated serum creatine kinase may be associated with a more aggressive course of HO, as well as resistance to etidronate therapy. [8] Elevated serum creatine phosphokinase levels have value in predicting the outcome of HO. [17]
Prostaglandin E2
In animal models, prostaglandin E2 (PGE2) has been shown to induce subperiosteal lamellar bone formation [18] ; PGE2 may be an inducer of bone formation in humans. PGE2 urinary excretion has been measured over a 24-hour period in patients with acute spinal cord injury (SCI), and excretion was shown to increase in patients who developed HO. [19] Excretion continued until the bone reached maturity.
Imaging Evaluation
Radiography
Plain film radiographs detect neurogenic heterotopic ossification (HO) 5-7 weeks after spinal cord injury (SCI), a relatively late finding (see the following image).
CT scanning
Computed tomography (CT) scanning is used to determine the volume of bone needed when planning surgical resection.
MRI
Magnetic resonance imaging (MRI) has been shown to demonstrate an increased T2 signal (edema) in muscles, fascia, and subcutaneous tissue during acute onset of HO. [20]
Ultrasonography
Ultrasonography permits an early diagnosis of HO (before radiography). [21, 22] A typical zone phenomenon that depends on the age of the lesion and the degree of mineralization takes place, characterized by the following:
-
An echolucent zone of surrounding muscle enclosing a broader reflective zone, which in turn surrounds an amorphous, echolucent zone
-
A reflective zone containing foci of echogenic islands, which rapidly become confluent and increasingly reflective due to increased mineralization
This imaging modality is also useful because it can differentiate HO from abscess or deep vein thrombosis (DVT).
Nuclear imaging
Bone scintigraphy is performed using a 3-phase test involving the injection of technetium-99m (99m Tc)–labeled methylene diphosphonate. This procedure permits an early diagnosis of neurogenic HO. [7] The first 2 phases (blood flow and blood pool) are the most sensitive indicators, but they are less specific; the third phase is positive 4 weeks before the appearance of findings on plain radiographs.
The 3-phase bone scan returns to normal as the neurogenic HO matures in 6-18 months after injury. False negative studies can occur, so follow-up studies are indicated for patients with clinically suspicious HO but negative initial bone scans. [23]
Quantitative radionuclide scans compare the ratio of uptake in heterotopic bone versus normal bone. This ratio decreases over time, and a steady state is noted as the bone reaches maturity. [24] This steady state, however, has not been shown to be a good predictor of recurrence of HO.
Physical and Occupational Therapy
Experimental studies in animals suggest that forcible stretching and hematoma induce new bone formation and heterotopic ossification (HO). This conclusion has not been substantiated in humans. There appeared to be some evidence from case reports and animal studies that passive range of motion (ROM), performed gradually over a long period, helps improve ROM without worsening the HOs already present. [25]
Physical therapists (PTs) work on ROM exercises, which are important in maintaining joint function. Once HO is identified, the ROM exercises should be withheld until the inflammatory signs (eg, warmth, erythema) have subsided. Active-assistive ROM (AAROM) should then be prescribed, and gentle passive ROM (PROM) should be initiated for completely paralyzed joints.
The occupational therapist (OT) works on activities of daily living (ADLs) and functional transfers to compensate for lost ROM due to HO. In addition, the OT and PT work on customizing seating systems to minimize pressure over heterotopic bony prominences.
Pharmacotherapy
A systematic review of therapeutic interventions for heterotopic ossification (HO) in spinal cord injury (SCI) concluded that pharmacological treatments of HO post SCI had the highest level of research evidence supporting their use. [26] Nonsteroidal anti-inflammatory drugs (NSAIDs) demonstrated greatest efficacy in HO prevention when administered early after an SCI, while bisphosphonates were the intervention with strongest supportive evidence once HO had developed. Bisphosphonates prevent the formation of hydroxyapatite crystals. NSAIDs reduce the inflammatory process that precedes the formation of the collagenous bony matrix.
Bisphosphonates
Analogues of pyrophosphate act by binding to hydroxyapatite in bone matrix, thereby inhibiting the dissolution of crystals and blocking the formation of hydroxyapatite crystals. Bisphosphonates prevent osteoclast attachment to the bone matrix, as well as osteoclast recruitment and viability.
Etidronate disodium
When Finerman and Stover treated patients with SCI with etidronate disodium for 12 weeks, starting at 20 mg/kg/d PO for 2 weeks and then 10 mg/kg/d PO for 10 weeks, the final incidence of HO in the etidronate disodium group was reduced and the amount of HO laid down was smaller. [27]
In subsequent research, patients given higher doses of etidronate—administered intravenously (300 mg/d x 3d), followed by 20 mg/kg taken orally for 6 months and started early (before radiographic evidence of HO was apparent)—showed a significant reduction in the incidence of HO. [28] Etidronate disodium also prevents the recurrence of neurogenic HO that has been resected in patients with spinal cord injury.
Etidronate is a relatively safe drug. Gastrointestinal (GI) symptoms are the most common adverse effect (eg, nausea, diarrhea, abdominal distress), but these effects can be limited if the daily dose is split into several doses.
Pamidronate
A newer bisphosphonate, pamidronate, may have pronounced beneficial effects in high-risk patients with established HO who are undergoing excision surgery, but the timing of dosing has not been established. [29]
Alendronate
A study by Ploumis et al suggested that alendronate may aid in the prevention of HO in patients with SCI. The study found no significant difference in the development of HO between patients with SCI who received oral alendronate (125 patients) and those who did not (174 patients). However, the investigators did find a tendency toward abnormal serum alkaline phosphatase levels in patients with HO, as well as a tendency toward normal alkaline phosphatase levels in patients who took alendronate. Thus, they suggested that alendronate may somehow indirectly discourage HO. [30]
Nonsteroidal anti-inflammatory drugs
The mechanism of action behind the NSAIDs is probably the inhibition of prostaglandins and related inflammatory substances during the initial phase of osteoid formation. Indomethacin was the most common drug studied. [31] Banovac et al studied the use of indomethacin during the first 2 months after SCI and have shown it to be effective in preventing HO. [32]
In this population of patients with SCI, that is already at risk for GI bleeding, there is obviously a relative contraindication to NSAID use.
Surgical Intervention
Orthopedic surgical consultation is recommended for patients with neurogenic heterotopic ossification (HO) who require surgical resection of the bone, such as those who have loss of function at a joint, or if other complications exist.
Surgery is also indicated in those patients with seating problems, skin breakdown, pain, or loss of function. [9]
Traditional thought has been that the surgery must be delayed until the bone scan ratio is at steady state and the serum alkaline phosphatase level returns to normal, which is usually 12-18 months after injury.
However, several investigators have published good results that were achieved with early wedge resection of HO that had not reached maturity. [33] Similarly, a study by Genêt et al suggested that, contrary to previous assertions, the recurrence risk for HO after SCI is not affected by the size of the HO around a joint or by early excision (< 6 mo) of the ossification. The investigators based their contention on the perceived paucity, contradictions, and heterogeneity of past studies on the topic and on results culled from 357 patients (539 operations) at their own facility. [34]
Etidronate disodium and/or radiation therapy is warranted after surgery to prevent the recurrence of neurogenic HO. [35, 36, 37]
Prognosis, Complications, and Prevention
Prognosis
No direct mortality is associated with neurogenic heterotopic ossification (HO). Morbidity is associated primarily with loss of range of motion (ROM) and the consequent loss of joint function.
If prophylactic measures are not taken, surgically resected neurogenic HO has a high rate of recurrence.
A study by Franz et al indicated that while HO in SCI may not affect patients’ motor recovery, it can significantly reduce functional recovery. Motor recovery in SCI patients with HO was comparable to that of controls, who had SCI but no HO. However, in late-stage SCI, functional recovery in the presence of HO, as assessed using the Spinal Cord Independence Measure (SCIM), was about 47% less than that of controls. [38]
Complications
Complications may include the following:
-
Joint ankylosis
-
Skin breakdown over the area of neurogenic HO - This is a significant sequela over the sites of bone formation and an indication for surgical resection of the HO
-
Peripheral nerve entrapment - This has been documented as a possible complication of HO; the ulnar and femoral nerves are most frequently involved, and in such instances, entrapment can result in further neurologic loss of function in incomplete injuries; computed tomography (CT) scanning is useful for planning surgical resection of an entrapped peripheral nerve
-
Deep vein thrombosis (DVT) from compression of the veins by the neurogenic HO
-
Pain is another complication of HO in patients with neurologically incomplete spinal cord injury (SCI)
Prevention
Durovic et al conducted a randomized controlled examining the effect of pulse low-intensity electromagnetic field (PLIMF) therapy as a prophylaxis for HO in individuals with SCI. [39] Both the control and treatment groups received range-of-motion and exercise therapy. Individuals in the treatment group (n=14) also received 4 weeks of PLIMF therapy a mean of 7 weeks after injury. The study demonstrated significant differences in the incidence of HO between the 2 groups (P =.04). None of the individuals in the treatment group developed HO, while HO progressed in 33% of individuals in the control group as measured by Brooker grades and radiographs.
Patient Education
Patient education, a lifelong process for individuals with spinal cord injury (SCI), should include the possible complication of heterotopic ossification (HO). If this condition develops, patients need to be informed thoroughly about the condition and the various means of treatment. A range-of-motion (ROM) program needs to be presented to the patient and family members to prevent a loss of motion, contractures, and a possible loss of function.
-
Extensive heterotopic ossification at the medial aspect of the left knee.
-
Three common locations of heterotopic ossification around the hip joint. A: Anterolateral/anteromedial location; B: Inferior and medial location; and C: Location around the femoral neck and posterior.
-
A 54-year-old man with T6 burst fracture due to motor cycle accident, with T6 American Spinal Injury Association (ASIA) Class A paraplegia and left hip dislocation developed left lower limb swelling 4 weeks after the injury. Workup revealed left hip heterotopic ossification.
-
The 4 clinical stages of heterotopic ossification based on data from Nicholas. Ectopic bone formation in patients with spinal cord injury. Arch Phys Med Rehabil. Aug 1973;54(8):354-9.
-
Timeline for diagnostic tests in spinal cord injury heterotopic ossification.
-
Neurogenic heterotopic ossification based on data from Mavrogenis et al. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. Jun 2012;13(2):69-78.
-
Neurogenic heterotopic ossification based on data from Mavrogenis et al. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. Jun 2012;13(2):69-78.
-
Brooker classification of heterotopic ossification around the hip.
Tables
What would you like to print?
- Practice Essentials
- Etiopathophysiology
- Clinical Features, Diagnosis, and Differential Diagnosis
- Laboratory Evaluation
- Imaging Evaluation
- Physical and Occupational Therapy
- Pharmacotherapy
- Surgical Intervention
- Prognosis, Complications, and Prevention
- Patient Education
- Show All
- Media Gallery
- References