Practice Essentials
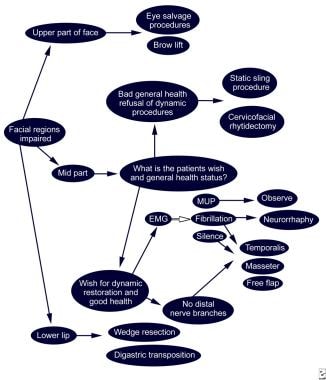
Because speech, mastication, and expression of moods and emotions are based on the ability to move facial musculature—be it voluntary or involuntary—successful treatment of facial nerve paralysis is a vital concern. This article informs the reader about the extracranial etiology of facial nerve paralysis and its current reconstructive options. [1] The diagram below presents a treatment algorithm for facial nerve paralysis according to facial region involvement.
Signs and symptoms of facial nerve paralysis
According to the House-Brackmann classification of facial function, severe dysfunction of the infratemporal facial nerve (grade V in the classification) is characterized by the following:
-
Only barely perceptible motion
-
At rest, asymmetry
-
Forehead - No motion
-
Eye - Incomplete closure
-
Mouth - Slight movement
Grade VI in the classification is total facial paralysis.
Workup in facial nerve paralysis
Computed tomography (CT) scanning and magnetic resonance imaging (MRI) are useful in the diagnosis of injury to intratemporal and/or intracranial affections of the facial nerve.
Electrophysiology can be useful to determine the extent of nerve disruption, possible outcome, and treatment options. [2] Most frequently, the minimal and maximal stimulation test (MST) and electroneuronography (ENog) are used.
Regarding histologic studies, the distal stump of the injured facial nerve undergoes Wallerian degeneration or anterograde degeneration; Schwann cells reveal massive proliferation, thus taking on a phagocytic role and removing myelin and axonal debris.
Management of facial nerve paralysis
Management of synkinesis and hyperkinesis can include botulinum toxin injection. Surgical options in facial nerve paralysis include the following [3] :
-
Direct coaptation
-
Interposition nerve grafting
-
Cross-facial nerve grafting
-
Microneurovascular free tissue transfer
Anatomy
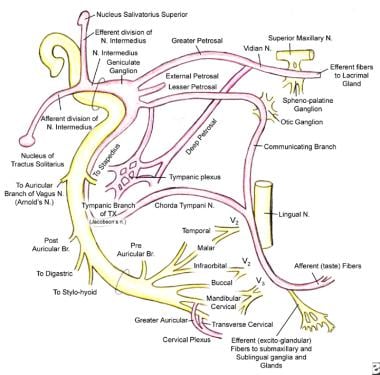
The keystone of successful surgical treatment for facial paralysis, the details of facial nerve anatomy, is recapitulated briefly to review topographic anatomy of the facial nerve and to enable the physician to localize the suspected site of injury. (See the image below.)
The facial nerve (cranial nerve VII) carries motor, secretory, and afferent fibers from the anterior two thirds of the tongue. It originates in the facial nucleus, which is located at the caudal pontine area. Corticobulbar fibers from the precentral gyrus (frontal lobe) project to the facial nucleus, with most crossing to the contralateral side. As a result, crossed and uncrossed fibers are found in the nucleus.
Moreover, the facial nucleus can be divided into two parts: (1) the upper part, which receives corticobulbar projections bilaterally and later courses to the upper parts of the face, including the forehead, and (2) the lower part, the predominantly crossed projections of which supply innervation to lower facial muscles (stylohyoid; posterior belly of digastric, buccinator, and platysma). [4]
Course of the facial nerve
In terms of topography, the facial and intermedius nerves course from the posterior pontine area ventrally, passing through the facial canal together with the vestibulocochlear nerve. All 3 nerves are surrounded by pia mater through their subarachnoid course, with the pia mater thus becoming a common sheath at the internal auditory canal. [5] The inferior anterior cerebellar artery and venous drainage enter the auditory canal together with the facial nerve.
Intratemporally, the facial and vestibular cochlear nerves split, entering the fallopian canal of the temporal bone. Topographically, the further course of the facial nerve is subdivided in 3 segments. The labyrinthine segment, measuring approximately 4mm, extends perpendicular to the temporal bone axis. Initially, the facial nerve runs anterior obliquely, remaining separate from the intermedius nerve and unifying at the next level, the geniculate ganglion. Afferent fibers from the anterior two thirds of the tongue enter the geniculate ganglion with the chorda tympani, as the greater and lesser petrosal nerve emerge from the superior part.
The tympanic segment of the fallopian canal extends approximately 1cm. [6] Here, the facial nerve runs horizontally at the medial wall of the cavum tympani. The third, or mastoidal, segment extends directly vertical approximately 1.5cm. The stylomastoid branch of the posterior auricular artery provides vascular supply to the facial nerve during its intrafallopian course.
The bony canal-facial nerve diameter is an important clinical ratio, especially considering susceptibility to nerve injury. Most often, the facial nerve takes up approximately 25-50% of the canal diameter. The facial nerve exits the fallopian canal through the stylomastoid foramen, afterward taking its extratemporal course anteriorly, inferiorly, and laterally.
The posterior auricular nerve (innervating postauricular and occipital muscles) branches posteriorly cranial just below the foramen, as do 2 smaller ones to the stylohyoideus and posterior belly of the digastric muscle. The facial nerve runs laterally to the styloid process. The facial nerve then enters the parotid gland between the stylohyoid and digastric muscle. The nerve gives off branches lateral to the external jugular vein, constituting the zygomatic-temporal and the cervicofacial trunks.
A diverse number of classifications of the extratemporal course of the facial nerve are found in literature. One was proposed in 1956 by Davis et al, who investigated the different course patterns of the infratemporal facial nerve in 350 cervicofacial halves.
Innervations
The temporal trunk innervates the following muscles:
-
Frontalis
-
Orbicularis oculi
-
Corrugator supercilii
-
Pyramidalis
The zygomatic division innervates the following muscles:
-
Zygomaticus major [7]
-
Zygomaticus minor
-
Elevator ala nasi
-
Levator labii superioris
-
Caninus
-
Depressor septi
-
Compressor nasi
-
Dilatator naris muscles
The buccal division gives off fibers to innervate the buccinator and superior part of the orbicularis oris muscle.
Mandibular division innervations are found in the following muscles:
-
Risorius
-
Quadratus labii inferioris
-
Triangularis
-
Mentalis
-
Lower parts of the orbicularis oris
The cervical division provides platysma innervation. A "facial danger zone" is known to follow an imaginary line drawn from the lateral canthus to the lateral corner of the mouth and from the zygomatic arch down to the angle of the mandible. The plastic surgeon should keep in mind that the more distal the injury to the facial nerve, the better the chances for spontaneous recovery.
Generally, good reconstructive results for facial nerve repair were reportedly yielded by Terzis et al even when a comparatively small number of axons were regenerated. [8] Terzis found a higher nerve-to-muscle fiber ratio than in other skeletal muscles (1:8, compared with 1:50 in other skeletal muscles).
Pathophysiology
Facial nerve injury can be complete or partial. Generally, partial disruption of axonoplasmal flow reveals a greater chance of complete functional recovery. Loss of motor function can be observed immediately after facial nerve injury. Depending on the affected trunk and localization (proximal or distal), various patterns of motor function loss can be seen and used for primary diagnosis of the lesion site.
Significant muscle fiber decay has been demonstrated when denervation has been present for more than 3 years. [9] Early changes at the cellular level (approximately 1wk after denervation) include chromatin changes and increased mitochondria number, deoxyribonucleic acid (DNA), and satellite cells, thus reflecting the plastic state of denervated muscle.
In addition to clinical and histopathologic findings, it may also be found that parasympathetic functions such as salivation, lacrimation, and taste sensation are impaired.
Etiology
Bell palsy
A common entity of facial nerve paralysis is Bell palsy, which is unilateral and is considered to be of idiopathic etiology. The annual incidence of Bell palsy is approximately 20 cases per 100,000 persons. [10, 11, 12]
A viral etiology (ie, herpes simplex virus, others) has been suspected as a precursor inciting factor. [13]
Bell palsy normally has a sudden onset that is often preceded by facial dysesthesia, epiphora, pain, hyperacusis, dysgeusia, and decreased function of the lacrimal gland. [14]
Ramsay Hunt syndrome
Ramsay Hunt described a syndromic occurrence of facial paralysis, herpetiform vesicular eruptions, and vestibulocochlear dysfunction. [15, 16]
Patients presenting with Ramsay Hunt syndrome generally have a greater risk of hearing loss than do patients with Bell palsy, and the course of disease is more painful. Moreover, a lower recovery rate is observed in these patients. [17]
Medical treatment is equivalent to that for Bell palsy; most often, a combination of steroids and antiviral agents is used. [18, 19]
Lyme disease
Infection with Borrelia burgdorferi via tick bites reveals another etiology of facial paralysis, thereby presenting along with all the symptoms of Lyme disease. Of patients affected with Lyme disease, 10% develop facial paralysis, with 25% of these patients presenting with bilateral palsy. [20]
Bacterial infection
Bacterial infection also may lead to facial nerve paralysis, most often correlated to acute otitis media or externa. Slow-onset facial nerve palsy is observed in patients with cholesteatoma.
Noninfectious causes
Noninfectious causes of facial nerve palsy include head trauma affecting the intracranial intratemporal course of the facial nerve or, less commonly, the infratemporal course, as seen in facial blunt or sharp injury. [21]
Iatrogenic injury to the facial nerve most often is seen after cervicofacial rhytidectomies, surgery of the parotid gland, acoustic neuroma resection, or tumor resection at any point along the course of the facial nerve. Therefore, when facial paralysis occurs after surgery, operative exploration must follow if uncertainty exists concerning the intactness of the facial nerve. Due to topographic relations and/or tumor extension, the facial nerve occasionally must be sacrificed voluntarily as part of sound oncologic management.
Tumor of the facial nerve (eg, hemangioma, neuroma) or tumors in the direct vicinity of the facial nerve often are concomitant with facial nerve palsy. In general, gradual onset of paralysis may lead to suspicion of a tumor as the cause. However, several authors have demonstrated a sudden onset of facial nerve palsy in patients with tumors (20-27%). [22, 23]
Summary of causes
The causes of facial nerve palsy are summarized in Table 1, below.
Table 1. Causes of Facial Nerve Palsy in a Review of Medical Literature (1900-1990)* (Open Table in a new window)
Birth |
Molding Forceps delivery Dystrophia myotonica Möbius syndrome (facial diplegia associated with other cranial nerve deficits) |
Trauma |
Basal skull fractures Facial injuries Penetrating injury to middle ear Altitude paralysis (barotrauma) Scuba diving (barotrauma) Lightning |
Neurologic |
Opercular syndrome (cortical lesion in facial motor area) Millard-Gubler syndrome (abducens palsy with contralateral hemiplegia caused by lesion in base of pons involving corticospinal tract) |
Infection |
External otitis Otitis media Mastoiditis Chickenpox Herpes zoster cephalicus (Ramsay Hunt syndrome) Encephalitis Poliomyelitis (type 1) Mumps Mononucleosis Leprosy Influenza Coxsackievirus Malaria Syphilis Scleroma Tuberculosis Botulism Acute hemorrhagic conjunctivitis (enterovirus 70) Gnathostomiasis Mucormycosis Lyme disease Cat scratch Acquired immunodeficiency syndrome (AIDS) |
Metabolic |
Diabetes mellitus Hyperthyroidism Pregnancy Hypertension Acute porphyria Vitamin A deficiency |
Neoplastic |
Benign lesions of parotid Cholesteatoma Seventh nerve tumor Glomus jugulare tumor Leukemia Meningioma Hemangioblastoma Sarcoma Carcinoma (invading or metastatic) Anomalous sigmoid sinus Carotid artery aneurysm Hemangioma of tympanum Hydradenoma (external canal) Facial nerve tumor (cylindroma) Schwannoma Teratoma Hand-Schüller-Christian disease Fibrous dysplasia Neurofibromatosis II |
Toxic |
Thalidomide (Miehlke syndrome, cranial nerves VI and VII with congenital malformed external ears and deafness) Ethylene glycol Alcoholism Arsenic intoxication Tetanus Diphtheria Carbon monoxide |
Iatrogenic |
Mandibular block anesthesia Antitetanus serum Vaccine treatment for rabies Postimmunization Parotid surgery Mastoid surgery Post-tonsillectomy and adenoidectomy Iontophoresis (local anesthesia) Embolization Dental |
Idiopathic |
Familial Bell palsy Melkersson-Rosenthal syndrome (recurrent alternating facial palsy, furrowed tongue, faciolabial edema) Hereditary hypertrophic neuropathy (Charcot-Marie-Tooth disease, Dejerine-Sottas disease) Autoimmune syndrome Amyloidosis Temporal arteritis Thrombotic thrombocytopenic purpura Periarteritis nodosa Landry-Guillain-Barré syndrome (ascending paralysis) Multiple sclerosis Myasthenia gravis Sarcoidosis (Heerfordt syndrome, uveoparotid fever) Osteopetrosis |
* Adapted from May and Klein |
|
Prognosis
In a study of 100 free muscle transfers (93 patients total), Terzis et al demonstrated an overall improved result postoperatively in 94% of the patients, with 80% of patients being ranked as having a moderate or better result compared with the preoperative stage. [24]
The investigators also found that women received higher scores and had an earlier onset of muscle function than men, younger patients had better results than older patients, and patients with a developmental cause of facial nerve paralysis had a better outcome than did patients with posttraumatic facial nerve paralysis. Intraoperative ischemia was not found to correlate with onset of muscle function.
Concerning outcome and donor muscle selection, patients in the study with pectoralis minor muscle transplants were found to have an earlier onset of muscle function than did those with gracilis transfers.
Overall, free tissue transfer to the face can be considered a safe and efficient method for the restoration of facial muscle movement.
Patient History and Physical Examination
History
Clinical diagnosis is based on 3 steps: (1) identification of the affected site, (2) underlying etiology (trauma, infectious, neoplastic), and (3) clinical staging (eg, with use of the House-Brackmann scale).
Careful delineation of the history should include onset of symptoms, an evaluation of the quality of associated symptoms, and prior infections and systemic diseases (eg, herpes simplex virus, varicella-zoster virus, neoplasms).
In a study of 353 patients with longstanding facial paralysis (treated with botulinum toxin for 11 years), Salles et al found synkinesis in 196 of them (55.5%), including postparalysis synkinesis in 148 patients (41.9%) and postreanimation synkinesis in 58 patients (16.4%); 10 of the patients had both types of synkinesis. An association was found between postparalysis synkinesis and infection, electrical stimulation, facial nerve decompression, and idiopathic causes, while a relationship was found between postreanimation synkinesis and microsurgical flaps, temporalis muscle transfers, masseteric-facial anastomosis, and transfacial nerve grafting. [25]
Physical examination
A thorough head and neck examination is paramount, with occasional use of tests for salivation, tearing, and taste; these are the first steps in determining the site of injury. Physical examination findings reveal affected facial musculature movement.
Tests for facial innervation include the following:
-
Forehead wrinkling (frontalis muscle)
-
Eye closure (orbicularis oculi muscle)
-
Wide smile
-
Whistling
-
Blowing (eg, buccinator muscle, orbicularis oris muscle, zygomatic muscle)
During the patient's initial consultation, evaluate general muscle status (latissimus muscle, rectus abdominis muscle) for eventual reconstruction.
House-Brackmann scale
Clinically, injury to the infratemporal facial nerve can be classified by degree. Multiple classifications of facial nerve injury are found in the literature. The most frequently used is the House-Brackmann scale, seen in Table 2, below. [26]
Table 2. House-Brackmann Classification of Facial Function [26] (Open Table in a new window)
Grade |
Characteristics |
I. Normal |
Normal facial function in all areas |
II. Mild dysfunction |
Gross Slight weakness noticeable on close inspection May have slight synkinesis At rest, normal symmetry and tone Motion Forehead - Moderate to good function Eye - Complete closure with minimal effort Mouth - Slight asymmetry |
III. Moderate dysfunction |
Gross Obvious but not disfiguring difference between sides Noticeable (but not severe) synkinesis, contracture, or hemifacial spasm At rest, normal symmetry and tone Motion Forehead - Slight to moderate movement Eye - Complete closure with effort Mouth - Slightly weak with maximum effort |
IV. Moderately severe dysfunction |
Gross Obvious weakness and/or disfiguring asymmetry At rest, normal symmetry and tone Motion Forehead - None Eye - Incomplete closure Mouth - Asymmetrical with maximum effort |
V. Severe dysfunction |
Gross Only barely perceptible motion At rest, asymmetry Motion Forehead - None Eye - Incomplete closure Mouth - Slight movement |
VI. Total paralysis |
No movement |
House and Brackmann staged injury from grade 1-6, with different chances of spontaneous recovery. These stages correspond with the pathologic findings of neurapraxia, axonotmesis, neurotmesis, and partial and complete transection of the facial nerve.
A clinical House-Brackmann grade 1 injury refers to neurapraxia, which is the most likely stage for spontaneous recovery.
Axonotmesis is the term for longer compression of the nerve, clinically a House-Brackmann level 2-3 injury, with temporary axonoplasmal flow interruption and subsequent Wallerian anterograde degeneration. Degeneration in axonotmesis is most often incomplete, with more or fewer axons surviving. Thus, partial facial weakness often results.
Neurotmesis is a state of permanent loss of axons further characterized by (partial) demyelinization leading to moderate to severe facial musculature dysfunction. Regenerative impulses may end in facial synkinetic movements, mass movements, or contracture.
Finally, clinical findings in House-Brackmann stage 5 and 6 injuries (partial or complete transection of the facial nerve) are either the retaining of minimal facial musculature movements or complete loss of function (grade 6).
Imaging and Electrophysiologic Studies
Imaging
Computed tomography (CT) scanning and magnetic resonance imaging (MRI) are useful in the diagnosis of injury to intratemporal and/or intracranial affections of the facial nerve, as they may reveal temporal fracture patterns (vertical, transversal, mixed) and edema formation. Under certain circumstances, the facial nerve can be viewed, and swelling or disruption may be seen. [27]
Electrophysiologic studies
Electrophysiology can be useful to determine the extent of nerve disruption, possible outcome, and treatment options. [2] Most frequently, the minimal and maximal stimulation test (MST) and electroneuronography (ENog) are used. These tests are performed with percutaneous stimulation of the facial nerve.
ENog studies are required to determine timing and necessity of surgical intervention (decompression or microneurorrhaphy). [28, 29, 30]
ENog records a compound action potential (CAP), as well as latency after nerve stimulation. Degeneration of 90% or more has been shown to predict poor prognosis without surgical intervention. [31, 22]
A study by Azuma et al indicated that ENoG results can predict the development of facial synkinesis. The investigators reported that study patients who, 10-14 days following the onset of facial palsy, had an ENoG value below 46.5%, were at increased risk of developing oral-ocular synkinesis 12 months after facial palsy first occurred. [32]
Guidelines
The following clinical practice guidelines on facial nerve electrodiagnostics were developed by members of the International Head and Neck Scientific Group and of the Multidisciplinary Salivary Gland Society for evaluation of patients with peripheral facial nerve disorders. [33]
Facial electrodiagnostics should be used in most patients with a facial nerve disorder.
ENoG, and at least 2-channel electromyography (EMG) and blink-reflex testing, should be performed via the facial electrodiagnostic equipment.
One of the standard facial electrodiagnostic tests, ENoG is best employed between 72 hours and 21 days after onset of the lesion and should be utilized and interpreted in combination with EMG.
Most valuably employed 2-3 weeks to 3 months after the onset of a facial nerve injury, EMG can aid in monitoring for regeneration if reinnervation occurs; its utilization and interpretation should be carried out in combination with clinical examinations.
Facial muscle recording should employ a standardized scheme and sequence. A good overview of facial nerve function can be derived from an EMG of the frontalis, orbicularis oculi, orbicularis oris, and zygomaticus muscles on the affected side. If just a portion of the peripheral facial nerve is affected, nEMG is abnormal only in that area.
The standard recording sequence for needle EMG (nEMG) of the muscle of interest is as follows:
- Insertion activity
- Pathologic spontaneous activity at rest
- Activity during voluntary muscle movement
- Synkinetic activity (in case of chronic palsy); the following last steps of nEMG analysis are carried out after all muscles of interest have been evaluated:
- Analysis of the waveform morphology
- Interpretation of the nEMG results
Since reinnervation is detectable approximately 2-3 months before clinical movements, nEMG can predict future recovery through monitoring of spontaneous facial nerve regeneration or regeneration after facial nerve repair.
If ENoG and nEMG are both performed, blink-reflex testing offers only low additional benefit. Allowing stimulation of the facial nerve proximal to the lesion site, blink-reflex testing may be of most use if facial nerve damage within the brainstem is suspected.
Though not currently recommended for routine facial electrodiagnostics, transcranial magnetic stimulation (TMS) may aid in highly selected cases of intratemporal pathology.
Patients with an acute facial nerve disorder are not routinely assessed electrodiagnostically with multichannel surface EMG (sEMG). Only in cases requiring detailed information on muscle activation with mimic expression, or in patients with chronic facial nerve disorder or post-paralytic synkinesis in whom compensatory movement patterns must be analyzed, is this modality recommended. Such information may aid in surgical or medical treatment planning.
Facial nerve mapping (FNM) can aid in surgical planning in complex cases, allowing more information on the general course of peripheral facial nerve branches to be obtained.
Histologic Studies and Other Tests
Histopathologic changes in the injured facial nerve include those in the distal part of the transected facial nerve and those found in the proximal part of the facial nerve. The distal stump undergoes Wallerian degeneration or anterograde degeneration: Schwann cells reveal massive proliferation, thus taking on a phagocytic role and removing myelin and axonal debris.
Moreover, Schwann cells release certain growth factors that lead the regenerating axons to the distal stump. The basal lamina of the distal stumps' Schwann cells provides scaffolding for the regenerating axonal growth cone from the proximal part of the facial nerve. However, when the distance between the 2 nerve stumps is too great, anterograde degeneration of the distal stump overweighs and finally results in its decay.
Several histopathologic findings can be attributed to the regenerative activity in the affected facial nerve. A DNA increase at the cellular level is observed, and regenerating nerve fibers build filopodia, which in its whole volume forms the axonal growth cone. These axonal growth cones advance with motile elements to the distal stump, guided by the proliferating Schwann cell scaffold formed by the distal stump.
Additional tests
For suspected intracranial or infratemporal injury, always perform a Schirmer test of tearing to assess lacrimal gland function.
Testing with different aromatic agents is needed to determine the integrity of afferent impulses from the anterior two thirds of the tongue.
Botulinum Toxin Therapy
Management of synkinesis and hyperkinesis can include botulinum toxin injection. This technique yields good results in the control of these sequelae of reinnervation procedures but must be repeated approximately every 3 months. Usually, 5-10 units are injected initially to control eyebrow spasm, and an additional 10-20 units are injected into the zygomaticus muscle and then repeated with an adapted dose as needed. [34]
Surgical Options and Overview
When surgical intervention is planned, the surgeon must remember that informed consent and preoperative consult are imperative to the physician and patient. Also, the physician must inform the patient that his or her face will never be symmetrical or have a normal balance. The patient's facial appearance is impaired mainly by loss of muscle tone on the affected side, but it is also influenced by severe contraction on the opposite, healthy side. [35, 36]
Options include direct coaptation, interposition nerve grafting, cross-facial nerve grafting, and microneurovascular free tissue transfer. [3]
If direct anastomosis of the facial nerve stumps is impossible, use an interposition nerve graft. Donor nerves for this procedure are the ansa hypoglossi, sural nerve, and medial cutaneous antebrachial nerve. Use of these nerves as donor nerves for either interposition grafting or cross-facial nerve grafting is described extensively in the literature. [37]
Balancing and adjustment procedures
After these dynamic facial reanimation procedures (active motion restoration), balancing and adjustment procedures are performed to give the face the final desired symmetry. These operations are static procedures, thus providing the face with more symmetry and balance at rest. Because of different patient opinions on further operations, these finishing steps should be made following mainly the patients' own desires of symmetry.
Examples of these ancillary "touch-up" procedures are operations on the depressor anguli oris muscle group, enhancement of the nasolabial fold, and static eye procedures, such as upper eye lifting, static sling placement, and partial cervicofacial rhytidectomy.
Follow-up
During all operative stages, the importance of clinical follow-up care cannot be overemphasized. For example, in cross-facial nerve grafting, use the sign of Tinel (paraesthesia when tapping on the regenerated end of the graft) for monitoring the nerve regeneration along the graft. For the monitoring of microneurovascular tissue transfer in the postoperative period, the method described by Fasching and Van Beek (ie, the placement of electrical monitoring devices into the grafted muscle) can be used. [38]
May et al used thermocouple placement about the anastomosis site. [39] However, these techniques are not available in all centers and require a second operation to remove the implanted devices.
Surgery for Acute Facial Nerve Palsy
Acute facial nerve palsy (injury not older than 1y) must be subclassified as acute nerve injury secondary to direct trauma or injury due to facial surgery (inadvertent transection or sacrifice for oncologic reasons)
Thoroughly evaluate the patient presenting with trauma to the facial nerve for the possibility of immediate reconstruction. The patient may need to undergo emergent surgical exploration in cases of penetrating trauma. However, the surgeon must decide if direct anastomosis of the proximal and distal stump is possible (microneurorrhaphy) or if interposition nerve grafting is necessary. [40]
Direct coaptation
Perform direct coaptation of the injured stumps using microsurgical technique and without undue tension to minimize scar formation, which can hinder axonal regeneration. [41] Fascicle sutures are theoretically possible, but no evidence supports the superiority of this technique compared with the epineural suture. [42] However, synkinesis, facial spasm, and mass movement remain frequent complications in the rehabilitation of the facial nerve [43]
Interposition nerve grafts
If tension-free coaptation cannot be performed, consider the use of an interposition nerve graft. [44] The great auricular nerve often can be used. This nerve is harvested using an incision made in an imaginary line drawn from the mandibular angle posterior to the mastoid tip. The great auricular nerve mainly provides sensation to the postauricular and the posterolateral cervical area.
If, for any reason, the great auricular nerve cannot be harvested or if the length is not sufficient, use the sural nerve as a donor nerve for interposition. The sural nerve supplies the lateral aspect of the calf with sensation and usually is harvested by several small incisions cranial from approximately 1cm posterior to the lateral malleolus. Its major advantage vis-à-vis the great auricular nerve is its length, as up to 35cm can be harvested easily.
Other options include the ansa hypoglossi and the medial cutaneous antebrachial nerve. The ansa hypoglossi often is used when a combination of parotidectomy and neck dissection is performed. In this case, no new skin incision is needed, and the oncologically sacrificed distance of the facial nerve can be adapted exactly to the donor nerve harvest length (ansa hypoglossi). Also, the transfer of partial ansa hypoglossi nerve to the facial nerve can be performed with good results. A partial nerve transfer can reduce donor nerve complications (difficulties with speech and mastication). [45, 46]
In conclusion, direct nerve repair using neurorrhaphy techniques yields better results than interposition nerve grafting. [47] Regenerative impulses yield an axonal length gain of approximately 1mm/day; muscle tone and movement is regained approximately 6-9 months after grafting.
Surgery for Chronic Facial Nerve Paralysis
Clinically, facial nerve paralysis is considered chronic when its onset or the time of injury dates back more than 1 year. Two types of procedures are used in the surgical treatment of chronic facial nerve paralysis: dynamic and static reanimative procedures.
Dynamic techniques include all surgical procedures that enable the patient to actively move facial or grafted muscles; static techniques include operations performed to optimize symmetry and reduce complications (eg, lip wedge resection, sling placement, partial cervicofacial rhytidectomy, upper lid blepharoplasty, lateral canthopexy).
When considering surgical intervention in chronic facial paralysis, carefully evaluate the patient's remaining potential for spontaneous recovery by electromyography (EMG), MST, and ENog.
EMG evaluation
As indicated in the algorithm in the image below, EMG testing can reveal polyphasic motor unit potentials (MUP), which are expressions of regenerative processes of the facial nerve. [2] Direct coaptation of the proximal and distal facial nerve stumps or interposition nerve grafting should not be performed at this time. In this case, close follow-up study of nerve regeneration with clinical and technical methods (physical examination, Tinel sign, ENog, MST) is the therapeutic strategy of choice.
EMG percutaneous testing can reveal fibrillation potentials. These potentials resemble the permanent denervation of the facial nerve; in this case, coaptation of the distal and proximal stump is indicated. If the stumps cannot be approached easily intraoperatively, farther proximal preparation can be performed to gain more length for the coaptation of the facial nerve. Cable grafting (interposition nerve grafting using either the greater auricular nerve or sural nerve) also may be necessary in some patients and is used when the coaptation would be under significant tension. [48]
Silence on the EMG usually indicates long-term denervation. In this case, coapting the stumps is not successful, and innervated free tissue transfer or muscle transposition (temporalis, masseter, gracilis muscle) is indicated. The performance of microneurovascular tissue transfer has 2 major advantages in this scenario; ie, the possibility of voluntary facial musculature movement and volume gain in case of loss of muscle volume after surgery.
Static procedures
If the patient is not compliant enough to undergo several operative procedures, as is often necessary in microneurovascular tissue transfer or cross-facial nerve grafting, offer static procedures, such as sling placements (temporalis sling, Gore-Tex sling) or cervicofacial rhytidectomies to improve static symmetry of the face. The same decision should be made if the patient is in bad health or at higher risk (eg, diabetes, old age, multimorbid state).
In the upper third of the face, a frequent problem is lack of eye closure and ectropic lower eyelid; placement of upper-lid gold weight is performed as a static procedure. [49] For lower-lid ectropion, wedge excision of the lateral lower eyelid and lateral canthopexy are possible static procedures. Cosmetic impairment as found in ptosis of the eyebrow can be corrected with partial forehead and brow lifts.
The midthird of the face is probably the most challenging region in facial reanimation. In this area, staple static procedures such as slings (either temporalis sling or Gore-Tex sling) and cervicofacial rhytidectomy, or more complex reconstruction such as tissue transfers or cross-facial reinnervation procedures, can be performed. Base decision making on the patient's personal needs of restoration and, most importantly, on the patients' general health.
Lower lip paralysis can be corrected with a static sling. [50] The sling usually is harvested from the anterior thigh fascia and adapted to the lateral orbicularis oris muscle and the zygomatic arch. Gore-Tex slings are described in the literature as well. They also yield excellent results in static facial reanimation. [51]
Moreover, "common" aesthetic procedures, such as partial cervicofacial rhytidectomies, cheiloplasty, and brow lift, can be offered to the patient as ancillary or "touch-up" procedures for the restoration of the paralyzed face.
Downward deviation of the lateral lip aspect often requires lateral lip wedge resection. This procedure often must be combined with static sling procedures or deep plane face lift to gain acceptable results in facial symmetry. [52]
Follow-up
For static procedures, clinical examination is the basis of follow-up care. Closely monitor overcorrection, since gravity and skin laxity should equalize facial hemispheres by approximately 6 months postoperatively.
Crossover facial reinnervation
The crossover facial reinnervation technique most often is performed when only the distal stump of the facial nerve is viable. Traditionally, the hypoglossal nerve has been used as the donor. However, the patient may experience swallowing and speech difficulties due to the lack of hypoglossal nerve motor input.
Some studies have shown that these complications can be minimized by transposition of only approximately one half of the hypoglossal nerve. [14, 53, 54] Also, jump grafting (interposition), with the use of the hypoglossal nerve to coapt the facial nerve by interposition of a nerve graft, has been described in the literature with good functional results (thus reducing the occurrence of complications of the hypoglossal-facial nerve transfer). [14] However, studies have demonstrated the masseter nerve to be a superior donor nerve. [55]
A study by Tanbouzi Husseini et al supported the use of hypoglossal-to-facial nerve anastomosis for facial nerve injury repair. In the study, anastomosis was performed on 40 patients suffering from facial nerve paralysis for a mean period of 11.3 months and with a House-Brackman (HB) facial nerve grading system score of VI. The investigators found that by 20 months’ mean follow-up time, all patients had attained an HB grade of III or IV, with just four patients having trouble eating and drinking. [56]
Follow-up
For interposition or cross-facial nerve grafts, the Tinel sign can be observed along the course of the regenerating nerve, approximately 1mm/day. Nerve conduction studies also can be used as adjunct studies.
VII-VII transfer
A well-known technique is the coaptation of the contralateral buccal branch to the ipsilateral facial nerve, a procedure that is termed VII-VII transfer. This technique requires 2 operations.
Primarily, a sural nerve graft is coapted to the contralateral buccal branch, then tunneled through the upper lip and usually left in the subcutaneous tissue. This is performed to allow axonal regeneration in the sural nerve.
Closely monitor the patient for axonal regeneration using the Tinel sign. The patient experiences dysesthesia upon tapping on the estimated end of the regenerated axons. However, the results of this technique are not favorable; additionally, the patient is at risk of losing innervation of the contralateral midface. [53]
Muscle transposition
Muscle transposition also can be offered for the dynamic restoration of the paralyzed face. The temporalis muscle often is used for this transposition technique. Another muscle for possible transposition is the masseter muscle, which is not used frequently due to the concomitant complications in mastication and speech. [57] (Some authors describe useful masseter muscle transposition in the case of massive buccal volume loss.)
The temporalis muscle transposition operation can be performed when the distal stump and the corresponding motor end plates are found electrophysiologically not to be viable. Advantage of this procedure is the adjacent volume gain.
Elevate a temporofascial flap using a hemi-coronal skin incision. Incise a strip of approximately 2cm and rotate it distally through a preformed subcutaneous tunnel down to the mesiolabial fold. Here, connect the flap to the upper lateral part of the orbicularis oris muscle. Overcorrection is of the utmost importance because of possible postoperative tissue laxity.
Microneurovascular free tissue transfer
Microneurovascular free tissue transfer frequently is used after tumor surgery of the face, thus restoring voluntary facial movement and at the same time reconstructing and/or filling the soft tissue defect. Generally, initial microneurovascular free tissue transfer yields excellent results in postoperative movement of the face, mastication, and speech. [57, 58]
The advantage over muscle transposition is the possibility of reconstruction of spontaneous facial musculature movement by coaptation to the proximal facial nerve stump. Free tissue transfer also can be useful to restore large volume defects that could not be reconstructed easily with the comparable "low-volume" temporalis muscle transposition. The gracilis muscle free flap and the latissimus dorsi muscle flap are frequently used muscles for microneurovascular free tissue transfer. [59, 60]
After dissection of 2 muscle paddles originating from 1 neurovascular paddle of the thoracodorsal vessels and nerves, the graft is transferred up to the face and fixed to the zygomatic arch, lateral corner of the mouth, and second paddle from the lateral canthus to the medial, thus reanimating both the middle part of the face (smile reconstruction) and the upper part (eye closure). [61]
Harvest the gracilis muscle using a groin-to-middle thigh incision running medially to the tibia. After identification of the gracilis muscle, separate the adductor longus muscle. The vascular pedicle usually is found when the adductor muscle is retracted laterally. Then make an incision on the superior part of the gracilis muscle as needed for reconstruction of the recipient site. Thereafter, transect the vascular pedicle and bring the muscle flap up to the face.
After suturing the inferior part of the gracilis muscle, the lateral aspect of the orbicularis oris muscle, pull the superior part cranially, giving until the desired muscle tension is reached, and consecutively suture it to the zygomatic arch. Coapt the donor nerve (anterior obturator nerve) to the recipient proximal stump of the facial nerve or the end of the sural crossover nerve graft. Other donor nerves, such as the ansa hypoglossi or the motor branch of the masseter muscle, also are used.
Complications with speech and swallowing must be accepted by the patient; he or she also needs to learn to activate the transferred muscle by clenching the teeth or swallowing.
The rectus abdominis muscle free flap can be harvested in a standard fashion as needed, either with or without a skin paddle. Muscle transfer and preparation depend on the needed tissue volume and length. Donor vessels are the inferior epigastric artery and vein. Place the muscle flap in an oblique position and afterward suture it to the lateral aspects of the orbicularis oris muscle and the zygomatic arch, as previously described.
Overcorrection is essential in the gracilis muscle free flap and the rectus abdominis muscle free flap. The gracilis muscle flap and the rectus abdominis muscle flap can be harvested with a skin island, thereby providing coverage of even large tissue defects after radical tumor surgery.
Another excellent muscle for free microneurovascular transfer is the pectoralis minor muscle. This muscle, originating from the third, fourth, and fifth ribs, has the major advantage in that it has a strong tendinous insertion, which makes it ideal for a "pull-up" muscle for the restoration of a smile. Moreover, the pectoralis minor muscle has a dual nerve supply (the lateral and medial pectoral nerves), making single-stage smile and eye closure restoration possible by splitting the flap. Excellent functional results for smile reconstruction using the pectoralis minor muscle have been described in the literature. [62, 63]
Follow-up
In microneurovascular transfers, follow-up care involves close monitoring in the hospital with Doppler signals and thorough examination of the flap. Capillary refill and the pinprick test can be used to assess flap viability. As mentioned before, thermocouple devices can be used for free tissue transfer follow-up care, but this technique is available only at some hospitals.
Physical Rehabilitation
The basis of physical rehabilitation is physical therapy. The physical therapist should teach the patient how to innervate the facial muscle efficiently after nerve transfer and grafting. Also, the patient should be encouraged to exercise the facial musculature to gain maximum strength of muscle pull. Nerve stimulation can be used postoperatively; however, electrical stimulation does not constantly demonstrate evident improvements.
A literature review by Khan et al indicated that physical therapy can be beneficial early in recovery from Bell palsy as well as in cases of chronic facial palsy. Two of the studies in the review evaluated the effect of facial exercise therapy alone, with both finding the result to be better functional recovery in comparison with no such therapy. All of six studies that assessed the outcome of combining physical therapy with biofeedback reported patient improvement, while nine of 10 studies determining the value of facial exercise therapy combined with corticosteroids, botulinum toxin, electrical stimulation, or laser treatment also noted a positive effect. However, the review found less clarity in determining the benefits of physical therapy in facial palsy cases of increased severity. All of the review’s studies looked at patients with Bell palsy, with three also including patients with Ramsey Hunt syndrome. [64]
Surgical Complications
Terzis studied 100 free muscle transfers (93 patients total) for facial paralysis. The results were as follows:
-
Complications observed in 11%
-
Arterial thrombosis in 5%
-
Venous thrombosis in 3%
-
Complete arterial and venous occlusion in 1 patient (1%)
-
Hematoma (3%)
-
Failure of muscle transplantation (4%)
-
Muscle necrosis (1%)
-
No signs of reinnervation noted in 3 patients; improvement after reexploration
-
Treatment algorithm according to facial region involvement.
-
Facial nerve anatomy.
Tables
What would you like to print?
- Practice Essentials
- Anatomy
- Pathophysiology
- Etiology
- Prognosis
- Patient History and Physical Examination
- Imaging and Electrophysiologic Studies
- Histologic Studies and Other Tests
- Botulinum Toxin Therapy
- Surgical Options and Overview
- Surgery for Acute Facial Nerve Palsy
- Surgery for Chronic Facial Nerve Paralysis
- Physical Rehabilitation
- Surgical Complications
- Show All
- Media Gallery
- Tables
- References