Practice Essentials
Surgery is considered the primary treatment for breast cancer, with many early stage patients being cured with surgery alone. The goals of breast cancer surgery include the complete resection of the primary tumor, with negative margins to reduce the risk of local recurrences, and pathologic staging of the tumor and axillary lymph nodes to provide necessary prognostic information. Several different types of operations are available for the treatment of breast cancer. See the image below.
For more information, see Breast Cancer and Adjuvant Therapy for Breast Cancer.
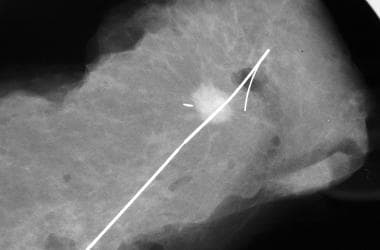 This specimen radiograph shows the wire and the localized speculated mass in situ, with a good excision margin.
This specimen radiograph shows the wire and the localized speculated mass in situ, with a good excision margin.
ASCO guidelines on lymph node dissection and biopsy in early stage breast cancer
The American Society of Clinical Oncology (ASCO) released updated guidelines on the use of lymph node dissection and biopsy for patients with early stage breast cancer. Recommendations included the following [1, 2] :
-
Women without sentinel lymph node (SLN) metastases should not undergo axillary lymph node dissection (ALND)
-
In most cases, ALND should not be performed on women with 1-2 metastatic SLNs who are planning to undergo breast-conserving surgery with whole-breast radiotherapy
-
ALND should be offered to women with SLN metastases who will be undergoing mastectomy
-
SLN biopsy may be offered to women who have operable breast cancer and multicentric tumors, women with ductal carcinoma in situ who will be undergoing mastectomy, women who have had previous breast and/or axillary surgery, and women who have been treated with preoperative/neoadjuvant systemic therapy
-
SLN biopsy should not be performed on women with large or locally advanced invasive breast cancer (tumor size T3/T4), inflammatory breast cancer, or ductal carcinoma in situ (when breast-conserving surgery is planned) or who are pregnant
Lumpectomy and mastectomy
Lumpectomy (partial or segmental mastectomy) is defined as complete surgical resection of a primary tumor with a goal of achieving widely negative margins (ideally 1 cm). It may be performed with palpation guidance or with image guidance and is applicable in most patients with stage I or II invasive carcinomas.
Relative contraindications include the following:
-
Small breast size
-
Large tumor size (>5 cm)
-
Collagen vascular disease
Absolute contraindications include the following:
-
Multifocal disease
-
History of previous radiation therapy to the area of treatment
-
Inability to undergo radiation therapy for invasive disease
-
First or second trimester of pregnancy
-
Persistent positive margins after attempts at conservation
Options for breast reconstruction after partial mastectomy include the following:
-
Fasciocutaneous local tissue advancement flaps
-
Breast parenchymal local flaps
-
Latissimus dorsi myocutaneous flaps
A total mastectomy involves complete removal of all breast tissue to the clavicle superiorly, the sternum medially, the inframammary crease inferiorly, and the anterior axillary line laterally, with en bloc resection of the pectoralis major fascia. The following variants are performed:
-
Modified radical mastectomy – A total mastectomy with axillary lymph node dissection (ALND)
-
Radical mastectomy – A total mastectomy plus en bloc resection of the pectoralis major and ALND
-
Extended radical mastectomy – A radical mastectomy with resection of the internal mammary lymph nodes
-
Skin-sparing total mastectomy (SSM)
-
Nipple-sparing total mastectomy (NSM)
Complications after total mastectomy include the following:
-
Risk of local recurrence (5-10%)
-
Wound infection
-
Seroma
-
Mastectomy skin flap necrosis
-
Hematoma
-
Chronic pain
-
Incisional dog ears
-
Fibrosis
Postmastectomy reconstruction may be immediate or delayed. Options are as follows:
-
Implant-based methods – Expanders and saline or silicone implants
-
Autologous tissue-based methods – Transverse rectus abdominis myocutaneous (TRAM) flap, latissimus dorsi flap, deep inferior epigastric perforator (DIEP) flap
-
A combination of the 2 methods
The following complications may be encountered:
-
Infected or ruptured prosthetic implant
-
Capsular contracture
-
Flap necrosis or loss
-
Fat necrosis
-
Asymmetry
-
Scarring
Sentinel lymph node biopsy and axillary lymph node dissection
Sentinel lymph node (SLN) biopsy is currently preferred for axillary staging, because it offers accuracy equivalent to that of ALND with less morbidity. [3] The American College of Breast Surgeons (ACBS) states the following:
-
SLN biopsy is suitable for virtually all patients with clinically node-negative T1-2 invasive breast cancers
-
Limited data are available regarding the suitability of SLN biopsy for patients with T3 cancers, multifocal/multicentric disease, prior radiation therapy, or prior breast/axillary surgery
-
SLN biopsy appears to be feasible in patients who have had minimal axillary surgery; the decision to use it in these situations requires individualized surgical judgment and an unequivocally successful mapping procedure
-
SLN biopsy is also indicated in patients with ductal carcinoma in situ (DCIS) in whom mastectomy is required or invasive disease is suspected
ALND is a complete en bloc removal of the level I and level II lymph nodes; the level III nodes are not removed unless suspicious or palpable adenopathy is present. All nodal tissue defined by the borders of the axillary vein superiorly, the latissimus dorsi laterally, the medial border of the pectoralis minor medially, and the subscapularis posteriorly is removed.
ALND carries a high rate of surgical morbidity, including the following:
-
Lymphedema (~25%)
-
Shoulder dysfunction
-
Wound infection
-
Seroma
-
Nerve damage
-
Numbness
-
Chronic pain
-
Brachial plexus injury (rare)
Postoperative care
Immediate postoperative care involves the following:
-
Assessment for appropriate wound healing
-
Evaluation and treatment of postoperative complications, such as seroma, wound infection, bleeding, and nerve damage
-
Follow-up of the pathologic specimen
-
Encouragement of early patient mobility and range-of-motion exercises
Recommendations for longer-term follow-up are as follows:
-
Baseline postoperative mammography of both breasts or of the remaining breast at 6 months
-
Clinical assessment every 4 months during the first 2 years, every 6 months up to the fifth year, and then annually for the remainder of the patient’s lifetime
-
Annual mammography and chest radiography
-
No further workup except as indicated by the development of suggestive symptoms such as bone pain, headache, or abnormal findings on annual routine laboratory chemistry panels
Lumpectomy
Lumpectomy is defined as complete surgical resection of a primary tumor with a goal of achieving widely negative margins (ideally a 1-cm margin around the lesion). It is applicable in most patients with stage I and stage II invasive carcinomas.
Other terms synonymous with lumpectomy include partial mastectomy, segmental mastectomy, and tylectomy. A quadrantectomy is a type of lumpectomy that is defined as complete removal of the entire affected breast quadrant; it is less cosmetically satisfactory than lumpectomy.
An observational longitudinal analysis by Nelson et al revealed that lumpectomy rates in the United States fell between 2005 and 2013 but rose again between 2013 and 2017. Using data from the National Surgical Quality Improvement Program (NSQIP); Surveillance, Epidemiology, and End Results (SEER) program; and National Cancer Database (NCDB), the investigators found the annual decrease in the lumpectomy rate from 2005-2013 to be 1.31%, 0.07%, and 0.15%, respectively by database. Between 2013 and 2017, however, the lumpectomy rate increased by 0.96%, 1.60%, and 1.66%, respectively by database. In contrast, a significant reduction in overall mastectomy rates occurred between 2013 and 2017. [4]
Contraindications
Relative contraindications to lumpectomy include small breast size, large tumor size (>5 cm), and collagen vascular disease. Absolute contraindications include the following:
-
Multifocal disease
-
History of previous radiation therapy to the area of treatment
-
Inability to undergo radiation therapy for invasive disease
-
First or second trimester of pregnancy
-
Persistent positive margins following attempts at conservation
Factors that are often considered but should not be deterrents include axillary node involvement and tumor location. Consideration of cosmesis, while important, should never outweigh the clinical priority of obtaining negative surgical margins. For instance, lesions involving Paget disease of the nipple may be treated with excision of the nipple-areolar complex and reconstruction. Larger lesions in patients with concerns regarding cosmesis may be better served by standard modified radical mastectomy and concurrent reconstruction.
Lumpectomy versus mastectomy
The National Surgical Adjuvant Breast and Bowel Project’s B-06 (NSABP-B06) was a landmark study that established breast-conserving surgery with radiation therapy to be equivalent to modified radical mastectomy. This was a prospective trial in which 2163 breast cancer patients were randomized to modified radical mastectomy (the standard of care at that time), lumpectomy and whole-breast radiation therapy, or lumpectomy without radiation. All patients underwent axillary lymph node dissection.
At 20-year follow-up, no significant difference was seen in overall survival, disease-free survival, or distant disease–free survival among the 3 treatment groups. However, the researchers did find a significant difference in the rate of local recurrence between the 3 treatment arms.
Patients in the group that received therapy with lumpectomy alone without radiation had a significantly higher local recurrence rate (39.2%) than patients undergoing lumpectomy plus radiation therapy (14.3%). Patients who underwent modified radical mastectomy had a 10.2% risk of chest wall recurrence. [5]
A study by Vaidya et al indicated that in patients who undergo lumpectomy, those who are treated with targeted intraoperative radiotherapy demonstrate no statistically significant differences from those who undergo external beam radiotherapy (EBRT) with regard to local recurrence–free, distant disease–free, mastectomy-free, or overall survival. Moreover, non-breast cancer–related mortality was much lower in the targeted intraoperative radiotherapy group. Patients the study, who were aged at least 45 years, had invasive ductal carcinoma up to 3.5 cm in size and were cN0-N1. [6]
Intraoperative fluorescence guidance
Pegulicianine was approved by the US Food and Drug Administration (FDA) in April 2024; it is a fluorescent dye used in a visualization system for adults with breast cancer. During lumpectomy, after the primary specimen has been removed, pegulicianine is employed to detect cancerous tissue within the resection cavity.
Approval was supported by a pilot feasibility trial and a prospective margin-status trial. [7, 8]
Technique
Lumpectomies may be performed with palpation guidance or with image guidance. Variations on the theme of image guidance include the following:
-
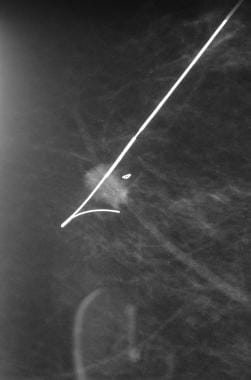
Wire localization of nonpalpable image-detected lesions via ultrasonographic, stereotactic, or magnetic resonance imaging (MRI) guidance (see images below) (Go to Breast Biopsy With Needle Localization for complete information on this topic.) [9]
-
Hematoma ultrasonographic guidance by the operating surgeon
-
Radioactive seed localization; the specimen should be evaluated radiographically to confirm excision of the intended lesion before completion of the operation
See the images below.
 This orthogonal (mediolateral) projection confirms the position of the needle to be placed beyond the cluster of microcalcification.
This orthogonal (mediolateral) projection confirms the position of the needle to be placed beyond the cluster of microcalcification.
 This specimen radiograph shows the wire and the localized speculated mass in situ, with a good excision margin.
This specimen radiograph shows the wire and the localized speculated mass in situ, with a good excision margin.
Patients who undergo a lumpectomy for calcifications should always be advised to have a mammogram following their lumpectomy to establish definitively that all calcifications were removed successfully. This mammogram should be performed before the administration of any radiation therapy.
In general, 2 mm or greater is a reasonable definition of a clear margin. Patients with margin widths less than 2 mm are often advised to return to the operating room for reexcision to improve local recurrence rates. The rate of surgical reexcision after lumpectomy ranges from 20-60% in the published literature.
In a 2018 update of the American Society of Breast Surgeons’ guidelines for lowering lumpectomy reoperation rates, McEvoy et al reached the following conclusions [10] :
-
Patients require complete preoperative imaging, but the choice of imaging modality depends on patient factors, the surgeon’s comfort level, and communication with the radiologist
-
Minimally invasive breast biopsy is appropriate for breast cancer diagnosis
-
The authors recommend that following the confirmation of breast cancer via minimally invasive biopsy and prior to the performance of a breast-conserving procedure, a multidisciplinary plan be devised
-
With several methods of localizing nonpalpable cancers available, including the increasingly popular nonwire localization techniques, surgeons should employ the modality with which they are most comfortable
-
Studies (primarily consisting of unadjusted, nonrandomized, retrospective reviews) indicate that in patients undergoing initial breast-conserving surgery, those who are treated with oncoplastic lumpectomy require reoperation for margin-associated reasons less often than do patients who undergo nononcoplastic lumpectomy; however, the oncoplastic patients may have higher rates of postoperative surgical site infection and reoperations for nonmargin-related causes
-
It is essential to orient a specimen via any technique that enables the breast pathologist to orient all of the specimen’s sides during margin evaluation
-
Multiple modalities exist for intraoperative specimen review, which requires communication between the breast surgeon and radiologist
-
A 50% decrease in the reexcision rate can be achieved by using cavity shaves for margins
-
Facilities that evaluate margins via routine intraoperative frozen section or imprint cytology have significantly lower reoperation rates following initial lumpectomies for cancer
-
Evidence strongly indicates that reoperation rates can be reduced through compliance with the Society of Surgical Oncology (SSO)–American Society for Radiation Oncology (ASTRO) margin guidelines
-
The achievement of higher breast conservation rates, lower reoperation rates, and better cosmetic outcomes may be encouraged though the use, in eligible patients, of oncoplastic lumpectomy (even in cases of large tumors) and/or neoadjuvant chemotherapy
Breast reconstruction after partial mastectomy
Oncoplastic surgery is a rapidly advancing field that uses local tissue rearrangement to reconstruct a partial mastectomy defect. Options include fasciocutaneous local tissue advancement flaps, breast parenchymal local flaps, or latissimus dorsi myocutaneous flaps. The selection of aesthetically appropriate incisions also impacts the overall cosmetic result after lumpectomy.
Silverstein and Lagios reported a variety of options for oncoplastic approaches to breast conservation. [11, 12] Kronowitz et al reported that partial mastectomy reconstruction produces superior aesthetic results and lower complication rates when performed before radiation therapy. [13]
Mastectomy
A total mastectomy is defined as complete removal of all breast tissue to the clavicle superiorly, the sternum medially, the inframammary crease inferiorly, and the anterior axillary line laterally, with en bloc resection of the fascia of the pectoralis major. The nipple-areolar complex (NAC) is resected along with a skin paddle to achieve a flat chest wall closure when performing a total mastectomy. A total mastectomy does not refer to removal of any axillary nodes but may be performed in conjunction with a sentinel or axillary node dissection.
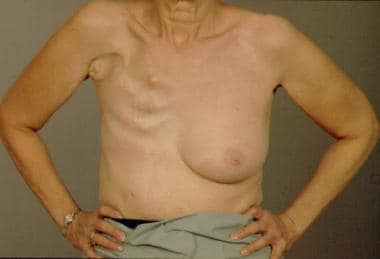
A modified radical mastectomy is defined as a total mastectomy with axillary lymph node dissection. In contrast, a radical mastectomy is defined as a total mastectomy plus en bloc resection of the pectoralis major and axillary lymph node dissection. Extended radical mastectomy refers to a radical mastectomy with resection of the internal mammary lymph nodes. Historically, radical (Halsted) mastectomy was the most commonly performed procedure for breast cancer. See the image below.
Two modern variations of the total mastectomy include the skin-sparing total mastectomy (SSM) and the nipple-sparing total mastectomy (NSM). [14] These operations refer to surgical approaches designed for patients who elect to have immediate reconstruction.
Both SSM and NSM are minimally invasive surgical approaches that are technically more difficult and, thus, more time-consuming than traditional mastectomy. SSM and NSM result in preservation of the patient's skin envelope and maintain the position of the inframammary fold. However, both SSM and NSM are intended to be complete total mastectomies with the same extent of resection as a traditional total mastectomy.
These operations may not be appropriate for cancers near the skin or nipple. Additionally, SSM or NSM are not appropriate for locally advanced or inflammatory breast cancer. Multiple retrospective single-institution studies have reported excellent results with SSM and NSM. [15] No randomized clinical trials have compared survival results for SSM, NSM, and total mastectomy. One study analyzed breast cancer recurrence data on patients who had undergone mastectomy with immediate reconstruction versus those who had not undergone reconstruction after mastectomy and concluded that neither incidence nor time to detection of recurrent disease was impacted by reconstruction. [16]
However, most surgical oncologists accept that as long as SSM and total mastectomy are performed carefully and patients are selected carefully, these are reasonable oncologic choices for prophylactic mastectomy and for the treatment of selected early stage breast cancers. [17]
A relative contraindication to modified radical mastectomy is locally advanced cancer requiring neoadjuvant therapy before surgical intervention.
Mastectomy complications
Complications after total mastectomy include the following:
-
Risk of local recurrence (5-10%)
-
Wound infection
-
Seroma
-
Mastectomy skin flap necrosis
-
Hematoma
-
Chronic pain
-
Incisional dog ears
-
Fibrosis
A literature review by Joo et al found that in patients with breast cancer who underwent skin- or nipple-sparing mastectomy and reconstruction, local recurrence most often arose in the skin and/or subcutaneous tissue. In skin- or nipple-sparing mastectomy patients who underwent autologous reconstruction, 1.8% experienced local recurrence in the skin/subcutaneous tissue; 0.7%, in the chest wall; and 0.4%, in the nipple-areolar complex. In those who underwent implant reconstruction, 4.7% suffered local recurrence that included the skin/subcutaneous tissue, 0.4% had local recurrence in the chest wall, and 0.4% experienced local recurrence in the nipple-areolar complex. [18]
Preoperative Details
Preoperative preparation of the patient for breast surgery should include attention to psychosocial as well as surgical issues. Patients may have unexpressed concerns regarding risk of recurrence, need for adjuvant radiation or chemotherapy, surveillance, length of rehabilitation, and particularly cosmesis. In discussing treatment options, it is important not to neglect the options of immediate versus delayed reconstruction and/or augmentation.
From a surgical standpoint, routine preoperative laboratory testing should be performed based on the patient's age, presence of symptoms, and comorbid conditions. Preoperative administration of a first-generation cephalosporin is a common practice, albeit with no proven benefit.
General Intraoperative Details
The keys to successful surgery of the breast include a thorough knowledge of anatomy, accurate assessment of the extent of disease, and recognition of the potential for future operations.
All biopsy incisions should be placed carefully with consideration for the placement of a future mastectomy incision. For instance, a radial incision in the upper inner quadrant does not incorporate into an elliptical mastectomy scar with the same ease as a horizontal or curvilinear incision. However, clearly, adequate surgical margins should never be compromised for the sake of cosmesis. Circumareolar incisions are cosmetically favorable and generally adequate for most central parenchymal lesions.
The axillary incision, if done separately, can be made in a curvilinear or S-shaped fashion based on surgeon preference. Dissection begins with incision of the clavipectoral fascia and identification of the lateral border of the pectoralis minor and the inferior border of the axillary vein. The vein then is traced laterally to the thoracodorsal complex. Once this has been identified with careful preservation of the nerve, attention is turned directly medially to the chest wall where the long thoracic nerve descends to the serratus.
Often, several branches of the intercostobrachial nerve can be identified superficially during axillary dissection. These can be divided if preservation means compromise of the extent of dissection. level I and II lymphatic tissue is resected with a combination of blunt and careful sharp dissection. Use of electrocautery should be avoided during deep dissection. Hemoclips or sutures are used to divide small vessels or lymphatics to reduce the risk of seroma and/or hematoma formation. Next, an axillary drain, if placed, is brought through a separate stab incision inferiorly.
For a mastectomy, the standard elliptical incision includes the nipple-areolar complex and extends from the lateral border of the sternum to the latissimus dorsi. An umbilical tape or suture may be helpful in measuring the upper and lower sides of the ellipse to ensure even lengths and avoid dog ears, particularly at the lateral corner. Cat's paw retractors or rakes are used to elevate the skin edges, and flaps are raised superiorly and inferiorly using electrocautery. Ideally, the thickness of the flaps should be approximately 1.0 cm.
This relatively avascular plane is readily identifiable with adequate flap traction perpendicular to the chest wall. The breast parenchyma is removed from medial to lateral either sharply or with electrocautery in continuity with the pectoral fascia. Care should be taken to ligate or cauterize any major perforating vessels. The axillary dissection then should proceed as described above through the same incision.
Some authors routinely place 2 drains through separate stab incisions inferior and lateral just above the inframammary fold. One is placed in the axilla and the other in the parenchymal defect. Fine subcuticular suture is used to close the skin.
In a randomized, double-blinded, parallel-group, placebo-controlled study of 66 women who had undergone ambulatory breast tumor resection, Abdallah et al found that multilevel, ultrasonographically guided paravertebral blocks and total intravenous anesthesia improved the quality of recovery and postoperative analgesia and expedited discharge in comparison with inhalational gas- and opioid-based general anesthesia. [19]
Breast Reconstruction
Breast reconstruction after mastectomy may be performed in the immediate or the delayed setting. Most patients undergoing mastectomy for prophylaxis or early stage breast cancer are candidates for reconstruction. [20]
Immediate reconstruction, when feasible, generally provides superior cosmetic results, because a skin-sparing total mastectomy (SSM) or nipple-sparing total mastectomy (NSM) may be offered to selected patients, resulting in preservation of the native skin envelope and inframammary crease. [21] However, when postmastectomy radiation is likely or a reconstructive surgeon is unavailable, delayed reconstruction following all adjuvant therapies may be recommended.
Reconstruction may be performed using implant-based methods, autologous tissue-based (termed flaps) methods, or a combination of the two. Implant-based approaches include tissue expanders and saline or silicone implants. Tissue-based approaches include the transverse rectus abdominis myocutaneous (TRAM) flap, latissimus dorsi flap, and the deep inferior epigastric perforator (DIEP) flap.
A study by Knoedler et al, using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, indicated that in patients who undergo post-mastectomy breast reconstruction, the short-term complication rate is greater among individuals in whom immediate breast reconstruction is carried out than in those in whom the reconstruction if performed after 30 days. The investigators reported that in patients who underwent implant-based reconstruction, complications occurred in 11% of the immediate-reconstruction patients, versus 4.4% of those treated with delayed reconstruction. In the autologous reconstruction patients, these figures were 25% and 21%, respectively. Using propensity score weighting analyses, the report found that the greater complication rate for immediate reconstruction held true for both implant-based and autologous reconstruction. [22]
Although federal law protects the rights of patients to have reconstruction by mandating that insurance companies support the procedure, most patients undergoing mastectomy do not undergo breast reconstruction. Reasons for this include provider biases, patient preferences, and lack of available specialty services.
Patients and physicians should have realistic expectations for breast reconstruction. Although excellent results may be achieved, often multiple operations are required for revisions, symmetry procedures, and nipple reconstruction. Complications related to reconstruction include an infected prosthetic implant, implant rupture, capsular contracture, flap necrosis, flap loss, fat necrosis, asymmetry, and scarring.
Using multivariate analysis, a study by Ito et al of patients who underwent either SSM, NSM, or total mastectomy, followed by immediate breast reconstruction, determined that NSM and weight of breast resection (WBR) are significant risk factors for skin flap necrosis, while WBR is associated with nipple-areolar complex necrosis. [23]
Management of the Contralateral Breast
In the United States, an increased rate of contralateral prophylactic mastectomies (CPMs) has been found among patients with unilateral breast cancer. [24]
Patients diagnosed with breast cancer who are not known carriers of a deleterious BRCA mutation are predicted to have a 0.7% annual risk of contralateral breast cancer. Patients who are known BRCA mutation carriers have a 3% annual risk of a contralateral breast cancer.
The decision for contralateral prophylactic mastectomy (CPM) is a personal decision for the patient and is impacted by cancer stage, the patient’s desire for symmetry, comorbidities, histologic risk factors, family history, potential difficult surveillance, and degree of risk aversion. Patients with locally advanced breast cancers should be discouraged from a contralateral prophylactic mastectomy, as potential surgical complications could compromise their oncologic treatments. Mastopexy and reduction mammoplasty for the contralateral breast are potential alternatives to contralateral prophylactic mastectomy as symmetry procedures.
One study sought to identify factors predictive of high-risk lesions and/or occult contralateral breast cancer in women undergoing CPM for newly diagnosed breast cancer. The study found that while the diagnosis of multifocality/multicentricity invasive index cancer was the only factor associated with occult malignancy, patient age and progesterone receptor positivity of the index cancer were associated with finding either malignancy or a high-risk lesion in the CPM. The findings suggest that because lack of standardized definitions and differences in pathologic evaluation may limit the use of this data in the preoperative setting, the use of CPM in an average-risk woman with newly diagnosed breast cancer is not supported. [25]
A study by ElSherif et al indicated that following implant-based reconstruction in patients with unilateral breast cancer, the incidence of complications requiring reoperation did not significantly differ between individuals who underwent unilateral nipple-sparing mastectomy and those who chose contralateral prophylactic nipple-sparing mastectomy. The overall rates of complications requiring reoperation at 30 and 120 days were 11% and 13%, respectively. The investigators did find that the rate of postmastectomy radiation treatment was significantly greater in the unilateral patients, but they also reported that the rate of adjuvant chemotherapy did not differ between the two groups. [26]
The Society of Surgical Oncology (SSO) released a 2024 position statement on CPM, with the society stating that reasons why patients consider the procedure include (mirroring the reasons outlined above) “concerns regarding the risk of contralateral breast cancer (CBC), desire for improved cosmesis and symmetry, and preferences to avoid ongoing screening.” Among surgeons, according to the position statement, recommendation for a CPM is made primarily after consideration of the contralateral breast cancer risk. The society acknowledges that, while the likelihood of new breast cancer in high-risk patients is reduced via CPM, whether an overall survival benefit is derived from the procedure is not known. [24]
The position statement, although recommending that the patient undergo mammography within the year prior to CPM, does not recommend routine preoperative magnetic resonance imaging (MRI) of the breast. Also, according to the SSO, no evidence exists supporting routine imaging surveillance following the prophylactic mastectomy. In addition, the society does not recommend routine sentinel lymph node surgery, stating that it is unlikely that an occult malignancy will be identified during CPM. [24]
Sentinel Lymph Node Biopsy
Sentinel lymph node (SLN) biopsy is a minimally invasive procedure designed to stage the axilla in breast cancer patients who have clinically negative nodes. Sentinel nodes are the first node or first group of nodes that drain from the breast to the axilla.
SLN biopsy has become the preferred SLN technique for axillary staging, because it offers accuracy equivalent to that of axillary lymph node dissection with less morbidity. [27] According to the American College of Breast Surgeons (ACBS), SLN biopsy is suitable for virtually all clinically node-negative T1-2 invasive breast cancers. Limited data are available regarding the suitability of SLN biopsy for patients with the following conditions [27] :
-
T3 cancers
-
Multifocal/multicentric disease
-
Prior radiation therapy
-
Prior breast/axillary surgery
SLN biopsy appears to be feasible in patients who have had minimal axillary surgery, especially previous SLN surgery and radiotherapy. The ACBS advises that the decision to use SLN biopsy in these situations requires individualized surgical judgment and an unequivocally successful mapping procedure.
SLN biopsy is also indicated in patients with ductal carcinoma in situ (DCIS) in whom mastectomy is required or in whom invasive disease is suspected. The role of SLN biopsy in patients who have had neoadjuvant therapy remains controversial and is currently under study.
There is no role for SLN biopsy in inflammatory breast cancer. [27]
SLN biopsy technique
The best results with SLN biopsy are achieved with the combination of careful intraoperative digital examination and lymphatic mapping. [27] The latter technique involves injecting radioisotope (technetium-99m sulfur colloid) alone or radioisotope plus a patent blue dye (Lymphazurin or methylene blue) into the tissues of the breast.
Several techniques of injection are available, including subareolar, peritumoral, intradermal, or intraparenchymal. The technique of injection may not be as important as the skill and experience of the surgeon with the chosen technique and with SLN identification in general. With SLN dissection, typically 1-3 lymph nodes are removed and tested for nodal metastasis with hematoxylin and eosin (H&E) stain and immunohistochemistry (IHC) with an anticytokeratin cocktail.
SLNs may be checked intraoperatively by imprint touch preparation, frozen section, or real-time polymerase chain reaction (RT-PCR). Intraoperative evaluation allows for immediate axillary lymph node dissection if an SLN is unequivocally positive for nodal metastasis. The American Society of Clinical Oncology (ASCO) guideline recommendations for SLN biopsy in early stage breast cancer include axillary lymph node dissection after detection of a positive SLN. [28] However, isolated tumor cells detected by specialized techniques such as IHC and RT-PCR remain of uncertain significance.
Success and failure in SLN mapping
Most groups would agree that success in SLN mapping improves with surgeon experience. In their initial series, Krag et al demonstrated a mapping failure rate of 18% with the solitary use of radiocolloid and a failure rate of 35% based on the solitary use of blue dye. [29] Subsequent reports by Giuliano have demonstrated a failure rate of 5%. [30] In the initial series by Cox et al, a failure rate of 6.6% was noted with a combination technique, [31] which subsequently decreased to 5% in a later study. [32]
Factors that are thought to contribute to mapping failure include the following:
-
Medial lesions that map to the intramammary nodes without concomitant drainage to the ipsilateral axillary lymph nodes
-
Injection into a cavity with a mature lining
-
Inflammation around a biopsy site that occludes lymphatic channels
-
Obstruction of lymphatic channels by tumor cells
-
Complete replacement of lymph nodes by tumor
In addition, in older patients, the capacity of lymph nodes to retain the radioactive colloid or dye may be reduced because lymph nodes in the elderly tend to be replaced by fat.
When SLN mapping is not successful, complete axillary lymph node dissection is recommended. Absolute contraindications for SLN dissection include clinically suspicious axillary nodes, which should be evaluated by ultrasound-guided fine-needle aspiration, and biopsy-proven, node-positive disease.
One study evaluated the accuracy of 4 nomograms in patients with sentinal lymph node–positive breast cancer. [33] The authors found the Memorial Sloan-Kettering Cancer Center nomogram to be more predictive than the other nomograms.
Axillary Lymph Node Dissection
Axillary lymph node dissection for breast cancer is a complete en bloc removal of the level I and level II lymph nodes. level I nodes are lateral to the pectoralis minor, level II nodes are beneath the pectoralis minor, and level III nodes are medial to the pectoralis minor.
The level III nodes are not removed surgically unless there is suspicious or palpable adenopathy present. Skip metastasis to the axillary apex of level III without lower axillary involvement is very rare.
Axillary lymph node dissection removes all nodal tissue defined by the borders of the axillary vein superiorly, the latissimus dorsi muscle laterally, the medial border of the pectoralis minor muscle medially, and the subscapularis muscle posteriorly.
Care is taken to preserve the long thoracic and thoracodorsal nerves along their course through the axilla. Injury to the long thoracic nerve results in a winged scapula, whereas injury to the thoracodorsal nerve compromises internal rotation and abduction of the arm beyond 90°.
The median and lateral pectoral nerves may also be injured during axillary lymph node dissection. The intercostobrachial nerves run directly through the resection specimen and are typically sacrificed, resulting in a predictable pattern of cutaneous numbness in the inner arm region for most patients after this procedure.
The American College of Surgeons Oncology Group Z0011 trial, a phase 3 randomized noninferiority trial, found that overall 5-year survival was similar for women with sentinel lymph node metastatic breast cancer who had undergone sentinel lymph node dissection alone versus axillary lymph node dissection, 92.5% and 91.8% survival rate, respectively. Five-year disease-free survival rate was also similar; 83.9% for sentinel lymph node dissection alone compared with 82.2% for axillary lymph node dissection. [34]
Axillary lymph node dissection was previously considered the standard of care for all patients diagnosed with invasive breast cancer. However, axillary lymph node dissection carries a high rate of surgical morbidity, including the following:
-
Lymphedema (rates of about 25%)
-
Shoulder dysfunction
-
Wound infection
-
Seroma
-
Nerve damage
-
Numbness
-
Chronic pain
-
Brachial plexus injury (rare)
Lymphedema is the abnormal accumulation of protein-rich edema fluid in the upper extremity following axillary lymph node dissection. This occurs because a portion of the lymphatics that drain from the breast to the axilla and those that drain from the arm are shared within the axilla. [35]
Early detection of lymphedema is paramount, as lymphedema is potentially reversible when treated in its earliest stage. Compression garments and physical therapy with lymphatic massage remain the backbone for the treatment of lymphedema.
Patients who have an axillary lymph node dissection should be cautioned about the risk of lymphedema and should take precautions to avoid breaks in the skin or infections in the affected extremity. Lymphedema may develop at any time after lymph node dissection but most commonly occurs within the first 2 years after the surgery.
Risk factors for developing lymphedema include obesity and radiation therapy. Although patients are commonly advised to avoid having blood pressure measurements taken or intravenous catheters placed in the affected arm after axillary lymph node dissection, no level I or level II evidence supports these recommendations.
Postoperative Details
Immediate postoperative care involves assessment for appropriate wound healing and evaluation and treatment of postoperative complications, such as seroma, wound infection, bleeding, and nerve damage. Drains are routinely removed when output is less than 30 mL/d. Seromas that develop following drain removal are usually best managed with repeat aspiration. Follow-up of the pathologic specimen should be routine to determine adequacy of margins in the resection of the primary tumor.
Early patient mobility and range of motion exercises should be encouraged postoperatively, although the timing and degree should be tailored to the extent of the procedure performed (ie, lumpectomy vs skin-sparing mastectomy with immediate reconstruction).
Postsurgical Surveillance
Recommendations for surveillance include baseline postoperative mammography of both breasts or of the remaining breast at 6 months and tapered clinical visits. The suggested frequency of clinical assessment during the first 2 years is every 4 months; every 6 months up to the fifth year; then annually for the remainder of the patient's lifetime. Mammography and chest radiographs should also be performed annually. Further workup is not indicated in the absence of suggestive symptoms such as bone pain, headache, or findings on annual routine laboratory chemistry panels.
Go to Postsurgical Breast Imaging for more complete information on this topic.
COVID-19–related Guidelines
COVID 19 Pandemic Breast Cancer Consortium
On March 24, 2020, the American College of Surgeons published COVID-19 Guidelines for Triage of Breast Cancer Patients. [36] The guidelines were developed by the COVID 19 Pandemic Breast Cancer Consortium, which comprises representatives from the National Accreditation Program for Breast Centers (NAPBC), the Commission on Cancer (CoC), the American Society of Breast Surgeons (ASBrS), and the National Comprehensive Cancer Network (NCCN). The guidelines are divided into the following three phases:
- Semi-urgent setting (preparation phase)
- Urgent setting
- Hospital resources have all been routed to COVID-19 patients, there is no ventilator or ICU capacity, or supplies have been exhausted
As a general recommendation, the guidelines advise that determination of patients’ case status (ie, risk of death time frame) be made by a multidisciplinary team, ideally in a multi-clinician setting (breast tumor board conference). This multidisciplinary discussion should be documented in the medical record.
Phase I
In this setting, the hospital has few COVID-19 patients, its resources are not exhausted, it still has ICU ventilator capacity, and the COVID-19 trajectory is not in rapid escalation phase. Here, the guideline recommends restricting surgery to patients whose survival is likely to be compromised if their procedure is not performed within the next 3 months.
In phase I, cases that need to be done as soon as feasible (recognizing that the hospital’s status is likely to progress over next few weeks) include the following:
-
Patients finishing neoadjuvant treatment
-
Patients with clinical stage T2 or N1 estrogen receptor(ER)–positive/progesterone receptor(PR)–positive/HER2-negative tumors
-
Patients with triple negative or HER2 positive tumors
-
Patients with discordant biopsies likely to be malignant
-
Excision of malignant recurrence
Note that in some cases of ER+/PR+/HER2-, triple negative, or HER2 positive tumors, institutions may decide to proceed with surgery versus subjecting a patient to an immunocompromised state with neoadjuvant chemotherapy; those decisions will depend on institutional resources. The guidelines encourage use of breast conserving surgery whenever possible and recommend deferring definitive mastectomy and/or reconstruction until after the COVID-19 pandemic resolves provided radiation oncology services are available.
Cases that should be deferred include the following:
-
Excision of benign lesions (eg, fibroadenomas, nodules)
-
Duct excisions
-
Discordant biopsies likely to be benign
-
High-risk lesions (eg, atypia, papillomas)
-
Prophylactic surgery for cancer and noncancer cases
-
Delayed sentinel node biopsy for cancer identified on excisional biopsy
-
cTisN0 lesions - ER positive and negative
-
Re-excision surgery
-
Tumors responding to neoadjuvant hormonal treatment
-
Clinical stage T1N0 ER+/PR+/HER2- tumors (these patients can receive hormonal therapy)
-
Inflammatory and locally advanced breast cancers (these patients should receive neoadjuvant therapy)
Alternative treatment approaches to be considered (assuming resources permit) include the following:
-
Patients with clinical stage T1N0 ER+/PR+/HER2- tumors can receive hormonal therapy.
-
Patients with triple negative and HER2+ tumors can undergo neoadjuvant therapy prior to surgery
-
Some women with clinical stage T2 or N1 ER+/PR+/HER2- tumors can receive hormonal therapy
-
Patients with inflammatory and locally advanced breast cancers should receive neoadjuvant therapy prior to any surgery
The guidelines note that many women with early-stage, ER+ breast cancers do not benefit substantially from chemotherapy. In general, these include women with stage 1 or limited stage 2 cancers, particularly those with low-intermediate grade tumors, lobular breast cancers, low OncotypeDX scores (< 25), or “luminal A” signatures. High-level evidence supports the safety and efficacy of 6 to 12 months of primary endocrine therapy before surgery in such women, which may enable the deferral of surgery.
Phase II
In this setting, hospitals have many COVID-19 patients, ICU and ventilator capacity are limited, or supplies are limited or the COVID-19 trajectory within the hospital is in a rapidly escalating phase. The guidelines recommend restricting surgery to patients whose survival is likely to be compromised if their procedure is not performed within next few days.
Cases that need to be done as soon as feasible (recognizing that the hospital’s status is likely to progress over the next few days) include the following:
-
Incision and drainage of breast abscess
-
Evacuation of a hematoma
-
Revision of an ischemic mastectomy flap
-
Revascularization/revision of an autologous tissue flap (however, autologous reconstruction should be deferred)
All breast procedures should be deferred. Neoadjuvant therapy should be considered for eligible cases; observation is safe for the remaining cases
Phase III
In this setting, hospital resources are all routed to COVID-19 patients, there is no ventilator or ICU capacity, or supplies have been exhausted. The guidelines recommend restricting surgery to patients whose survival is likely to be compromised if their procedure is not performed within next few hours.
Cases that need to be done as soon as feasible (recognizing that the hospital’s status is likely to progress in hours) include the following:
-
Incision and drainage of breast abscess
-
Evacuation of a hematoma
-
Revision of an ischemic mastectomy flap
-
Revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred)
All other cases should be deferred. Alternate treatment recommendations are the same as for phase II.
Society of Surgical Oncology
On March 23, 2020, the Society of Surgical Oncology recommended deferring breast cancer surgery for at least 3 months for atypia, prophylactic/risk reducing surgery, reconstruction, and benign breast disease. [37] Additional recommendations were as follows:
Ductal carcinoma in situ (DCIS):
-
Defer surgery for 3-5 months
-
Treat ER+ DCIS with endocrine therapy
-
Monitor monthly for progression
-
Untreated DCIS is high priority for surgery when it becomes safe and operating rooms are available
ER+ invasive breast cancer (Stage I-III) :
-
Treat with endocrine or chemotherapy in a neoadjuvant fashion as deemed appropriate by multidisciplinary tumor board recommendations.
Triple negative/HER2+ invasive breast cancer:
-
Treat with neoadjuvant chemotherapy for T2+ and/or N1+ disease.
-
Consider primary surgery as urgent if the patient is unable to undergo chemotherapy or the tumor is small and surgical information could inform chemotherapy decisions.
Post-neoadjuvant chemotherapy:
-
Delay post-chemotherapy surgery for as long as possible (4-8 week window) in those patients for whom adjuvant systemic therapy is unclear/not indicated.
Unusual cases/surgical emergencies/special considerations
-
Patients with progressive disease on systemic therapy, angiosarcoma, and malignant phyllodes tumors should be considered for urgent surgery; their treatment should not be delayed.
-
This mammogram shows a spiculated mass to be transfixed by the guidewire
-
Grid technique of localization.
-
This orthogonal (mediolateral) projection confirms the position of the needle to be placed beyond the cluster of microcalcification.
-
This specimen radiograph shows the wire and the localized speculated mass in situ, with a good excision margin.
-
Radical mastectomy defect.
-
Inflammatory breast cancer.
-
Advanced breast cancer.
-
Metastatic breast cancer to the back.
-
Nipple retraction due to breast cancer.
-
Breast cancer. Lobular carcinoma in situ. Enlargement and expansion of lobule with monotonous population of neoplastic cells.
-
Breast cancer. Invasive lobular carcinoma.
-
Breast cancer. Infiltrating ductal carcinoma. Low-grade carcinoma with well-developed glands invading the fibrous stroma.
-
Breast cancer. Intraductal carcinoma, comedo type. Distended duct with intact basement membrane and central tumor necrosis.
-
Breast cancer. Intraductal carcinoma, noncomedo type. Distended duct with intact basement membrane, micropapillary, and early cribriform growth pattern.
-
Breast cancer. Papillary carcinoma. Solid papillary growth pattern with early cribriform and well-developed thin papillary fronds.
-
Breast cancer. Inflammatory carcinoma. Nests of tumor cells plugging dermal lymphatics.
-
Breast cancer. Colloid (mucinous) carcinoma. Nests of tumor cells in pool of extracellular mucin.
-
Breast cancer. Estrogen receptor, immunostain. Positive staining of tumor cell nuclei with monoclonal antibody to estrogen receptor.
-
Breast cancer. Her-2/neu overexpression. Infiltrating carcinoma with strong membrane immunoreactivity representing Her-2/neu overexpression.
Tables
What would you like to print?
- Practice Essentials
- Lumpectomy
- Mastectomy
- Preoperative Details
- General Intraoperative Details
- Breast Reconstruction
- Management of the Contralateral Breast
- Sentinel Lymph Node Biopsy
- Axillary Lymph Node Dissection
- Postoperative Details
- Postsurgical Surveillance
- COVID-19–related Guidelines
- Show All
- Media Gallery
- References